It is well known that lipid metabolism is influenced by sex hormones in animals and human subjects(Reference Gevers Leuven1). Males do not have a menopause equivalent, but sex hormones fall with advancing age. Studies in animals and human subjects suggest that androgen deficiency is associated with increased plasma TAG, total cholesterol and LDL-cholesterol.
The type of dietary carbohydrates also influences lipid metabolism. It is well known that lipid metabolism is influenced by high fructose intake(Reference Girard, Madani and Boukortt2). Studies based on high-fructose v. high-glucose diets have shown that high-fructose diets produce an increase in the concentrations of plasma TAG, total cholesterol, VLDL-cholesterol and LDL-cholesterol(Reference Bantle, Raatz and Thomas3). The availability of fructose increased substantially when it became possible in the 1960s to produce high-fructose syrup economically from maize starch and other starches(Reference Guthrie and Morton4). The most recent available data suggest that fructose consumption is increasing worldwide(Reference Bantle5, Reference Tappy and Lê6). The world average per capita sugar consumption, which does not include high-fructose syrup, has increased from 56 g/d in 1986 to 65 g/d in 2007(Reference Reeves, Nielsen and Fahey7). The average per capita hydroxypropyl starch consumption in Europe has increased from 40 g/d in 1985 to 52·4 g/d in 2005(Reference Reeves, Nielsen and Fahey7). As described earlier, both male sex hormone and fructose influence lipid metabolism. However, less information is available concerning the relationship between male sex hormones and fructose in lipid metabolism. The aim of the present study was to consider the effects of male sex hormones and fructose on endogenous cholesterol metabolism. Therefore, we compared the effects of fructose on lipid metabolism in sham-operated (sham) and orchiectomised (ORX) rats fed a semi-purified diet with or without sucrose. Sucrose was used as a fructose source.
Materials and methods
Animals and diets
The present study was approved by the Laboratory Animal Care Committee of Ehime University. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
Sprague–Dawley male rats (6 months old, n 36) were housed individually in screen-bottomed stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (lights on, 07.00–19.00 hours). The rats were acclimatised by feeding them a commercial solid diet (Roden Lab, Diet EQ; PMI International, Brentwood, MO, USA) for 7 d. After acclimatisation, half of the rats were bilaterally ORX under sodium pentobarbital (30 mg/kg body weight; Nembutal; Abbott Laboratories, Chicago, IL, USA) and the other half of the rats were sham-operated. Orchiectomy was done through an anterior median incision in the scrotum. Each ductus deferens was isolated, ligated and severed, allowing the testicle to be removed. The rats were fed the commercial solid diet during the 7 d recovery period, after which ORX and sham-operated rats were randomly divided into three groups (n 6), respectively, and allowed free access to one of the experimental diets for 28 d. The compositions of each diet are shown in Table 1(Reference Reeves, Nielsen and Fahey7). Body weight and food intake were recorded daily for each rat in the morning before the food was replaced.
Table 1 Composition of the experimental diets
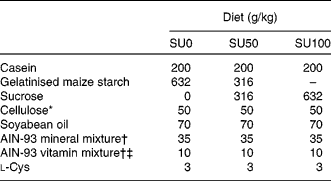
SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose.
* PC200 (Danisco Japan Limited, Tokyo, Japan).
† Based on AIN-93G(Reference Reeves, Nielsen and Fahey7).
‡ The AIN-93 vitamin mixture used in the present study contained 20 g choline bitartrate/100 g.
Sampling and analytical procedures
Before the rats were killed, faeces were collected from each rat over the final 4 d of the experimental period. The faeces were freeze-dried, weighed and milled. On the last day of the experimental period, diet was deprived after recording food intake.
A blood sample was collected from the jugular vein using a light diethyl ether anaesthesia on the rats at night. Blood was collected into a blood collection tube (Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA) that contained heparin as an anticoagulant. Plasma was separated by centrifugation at 1400 g at 4°C for 15 min and stored at − 50°C until analysed. After blood collection, the liver was immediately perfused with cold saline (NaCl, 9 g/l), removed, washed with cold saline, blotted dry on filter paper, weighed and stored at − 50°C until analysed. After the liver had been removed, the small intestine was removed. The contents of the small intestine were transferred into a preweighed tube by washing out with physiological saline, freeze-dried and weighed. The washed small intestine was wiped with a paper towel and returned to the carcass. The stomach, caecum and colon were then removed and opened, and the contents were washed away with physiological saline and returned to the carcass after wiping with a paper towel.
White adipose tissues (perirenal, epididymal and mesenteric adipose tissues) and interscapular brown adipose tissue were removed and weighed. White adipose tissues were returned to the carcass after weighing. The whole carcass was weighed and immediately preserved at − 50 °C for further analysis.
Biochemical analysis
The concentrations of total cholesterol, TAG and phospholipids in the plasma were determined enzymatically using commercial diagnostic kits (Cholesterol E-Test Wako, TAG E-Test Wako and Phospholipid C-Test Wako; Wako Pure Chemical Industries, Osaka, Japan). The level of liver total lipids was determined gravimetrically after extraction in accordance with the method of Folch et al. (Reference Folch, Lees and Sloane Stanley8). Liver TAG and cholesterol levels were determined enzymatically as described previously(Reference Ebihara, Shiraishi and Okuma9). Steroids were extracted from the faeces and digestive tract contents (small intestine and caecum) with a chloroform–methanol mixture (1:1, v/v) at 70°C for 60 h. The concentrations of bile acids in the small-intestinal contents and faeces were determined enzymatically by the 3-dehydrogenase assay method of Sheltaway & Losowsky(Reference Sheltaway and Losowsky10) using taurocholic acid as a standard. Cholesterol 7α-hydroxylase (CYP7A1) activity in the liver was determined according to the method of Ogishima & Okuda(Reference Ogishima and Okuda11).
RNA extraction from the liver and adipose tissue and RT-PCR analysis of gene expression
Total RNA was extracted from frozen livers and interscapular fat pad for brown adipose tissue in accordance with the method described by Chomczynski & Sacchi(Reference Chomczynski and Sacchi12). RNA integrity was verified by agarose gel electrophoresis after purification of mRNA using Oligotex-dT30 (Takara Bio, Shiga, Japan). Then, 1 μg of mRNA was used for complementary DNA synthesis with 10 units of avian myeloblastosis virus RT (Takara Bio) and 2 μl of oligo(dT) primer (Novagen, Inc., Madison, WI, USA) in accordance with the manufacturers' instructions. Expression of mRNA for acyl-coenzyme A:cholesterol acyltransferase 1, acyl-coenzyme A:cholesterol acyltransferase 2, β3-adrenergic receptor, ApoB, ApoE, cholesterol 27-hydroxylase, cholesterol 12α-hydroxylase, fatty acid synthase, farnesoid X receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, LDL-receptor, liver X receptor, microsomal TAG transfer protein (MTP), sterol-regulatory element-binding protein-1a (SREBP-1a), sterol-regulatory element-binding protein-1c (SREBP-1c), sterol-regulatory element-binding protein-2 (SREBP-2), uncoupling protein-1 (UCP-1) and β-actin (as a housekeeping gene for normalisation) was determined by real-time monitoring of PCR using a Light Cycler instrument (Roche Diagnostics, Mannheim, Germany). Then, 2 μl of complementary DNA were amplified in a total volume of 20 μl using the 2 × QuantiTect SYBR Green RTPCR Master Mix (Qiagen, Hilden, Germany) and specific primers each at 0·5 m. After initial denaturation and activation of the polymerase at 95°C for 15 min, cycling was performed for fifty cycles with annealing at the temperatures shown in Table 2 for 25 s, synthesis was performed at 72°C for 30 s and denaturation was performed at 94°C for 15 s. Fluorescence was measured at the end of the elongation step at 72°C. The sequences of the gene-specific primers (Carl Roth, Karlsruhe, Germany) used in the present study are listed in Table 2. Relative gene expression was calculated by using the crossing point of each target gene, with the crossing point of the β-actin gene used as the reference.
Table 2 Primer sequences, product sizes and annealing temperatures

ACAT, cholesterol acyltransferase; CYP7A1, cholesterol 7α-hydroxylase 1; CYP8B1, cholesterol 12α-hydroxylase; CYP27, cholesterol 27-hydroxylase; FAS, fatty acid synthase; FXR, farnesoid X receptor; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; LXRα, liver X receptor-α; MTP, microsomal TAG transfer protein; RXRα, retinoid X receptor-α; SREBP, sterol-regulatory element-binding protein; UCP-1, uncoupling protein-1.
Body composition analysis
The frozen carcasses were minced using an electric mincing machine (#22GM-D, Nippon Career Industry Company Limited, Matsuyama, Japan), ground and thoroughly mixed. The moisture content of the carcass was analysed by drying to constant weight at 90°C in an oven. Then, minced carcasses were freeze-dried pending the determination of their fat and N contents. Carcass N was determined in 250–300 mg samples of dehydrated carcasses using the micro Kjeldahl procedure. Carcass protein content was computed by multiplying the carcass N content by 6·25. Carcass fat content was in approximately 1·0 g samples of dehydrated carcasses using a Soxhlet apparatus with chloroform–methanol (2:1).
Statistical analyses
Data are expressed as means with their standard errors (n 6). To test the significance of the effects of sucrose and ORX, and their interaction, two-way ANOVA (StatView version 4.5; Abacus Concepts, Berkeley, CA, USA) was used. When significant F ratios were found, individual comparisons were made by the Tukey–Kramer test using the Super ANOVA statistical software package (Abacus Concepts). Differences were considered to be significant at P < 0·05.
Results
Body-weight gain, food intake, plasma lipids, liver lipids and carcass composition
Body-weight gain, food intake and feed efficiency were increased by sucrose ingestion, but decreased by ORX (Table 3). The weights of perirenal and mesenteric white adipose tissue and interscapular brown adipose tissue were not affected by sucrose ingestion and ORX. The weight of epididymal white adipose tissue was not affected by sucrose ingestion, but decreased by ORX. The body fat percentage was increased by sucrose ingestion, but the body water percentage was decreased by sucrose ingestion, while they were not affected by ORX. The body protein percentage was not affected by sucrose ingestion and ORX.
Table 3 Effects of sucrose ingestion and orchiectomy (ORX) on body-weight gain, food intake, adipose tissue weight and body composition†
(Mean values with their standard errors, n 6)

C, control; SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
† Sham-operated and ORX rats were fed the experimental diets for 28 d.
‡ Significant effect (P < 0·05) of dietary sucrose ingestion (sucrose) and ORX, and significant effect of the interaction between dietary sucrose ingestion and ORX (sucrose × ORX).
Plasma total cholesterol and phospholipid concentrations were increased by sucrose ingestion and ORX (Table 4). Plasma TAG concentration was increased by sucrose ingestion, but was decreased by ORX. In sham-operated rats, plasma TAG and phospholipid concentrations were significantly (P < 0·05) higher in SU100 diet-fed rats than in SU0 diet-fed rats. Plasma glucose concentration was increased by sucrose ingestion, but was not affected by ORX. Plasma insulin concentration was decreased by ORX, but was not affected by sucrose ingestion. Liver weight was increased by sucrose ingestion, but was not affected by ORX. In sham-operated rats, liver weight was significantly greater in SU100 diet-fed rats than in SU0 diet-fed rats. The concentrations of total lipids, cholesterol and TAG in the liver were not affected by sucrose ingestion and ORX; however, the concentration of cholesterol in the liver tended to increase by ORX (P = 0·085).
Table 4 Effects of sucrose ingestion and orchiectomy (ORX) on body-weight gain, food intake, adipose tissue weight and body composition†
(Mean values with their standard errors, n 6)

C, control; SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose; BW, body weight.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
† Sham-operated and ORX rats were fed the experimental diets for 28 d.
‡ Significant effect (P < 0·05) of dietary sucrose ingestion and ORX, and significant effect of the interaction between dietary sucrose ingestion and ORX (sucrose × ORX).
Hepatic cholesterol 7α-hydroxylase 1 activity, bile acids in intestinal contents and faecal excretion of bile acids
The activity of CYP7A1 in the liver and dry weight of small-intestinal contents were not affected by diet and ORX (Table 5). The amount of bile acids in the small-intestinal contents was decreased by sucrose ingestion, but was not affected by ORX. Faecal dry weight was increased by sucrose ingestion, but decreased by ORX. Faecal excretion of bile acids was decreased by sucrose ingestion and ORX.
Table 5 Effects of sucrose ingestion and orchiectomy (ORX) on cholesterol 7α-hydroxylase 1 (CYP7A1) activity, the dry weight of the small intestine, the amount of bile acids in the small-intestinal contents and faecal excretion*
(Mean values with their standard errors, n 6)

C, control; SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Sham-operated and ORX rats were fed the experimental diets for 28 d.
† Significant effect (P < 0·05) of dietary sucrose ingestion and ORX, and significant effect of the interaction between dietary sucrose ingestion and ORX (sucrose × ORX).
Hepatic gene expression
The levels of cholesterol 12α-hydroxylase, LDL-receptor, SREBP-2, liver X receptor and fatty acid synthase mRNA in the liver were not affected by sucrose ingestion and ORX (Table 6). The levels of ApoB, cholesterol acyltransferase 2, farnesoid X receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, retinoid X receptor-α, SREBP-1a and SREBP-1c mRNA in the liver were increased by sucrose ingestion, and they were significantly higher in SU100 diet-fed rats than in SU0 diet-fed rats, but they were not affected by ORX. The level of cholesterol acyltransferase 1 mRNA in the liver was increased by sucrose ingestion and ORX, and was significantly higher in SU100 diet-fed rats than in SU0 diet-fed rats. The level of MTP mRNA in the liver was increased by sucrose ingestion and ORX, and was significantly higher in SU100 diet-fed rats than in SU0 and SU50 diet-fed rats. The levels of cholesterol 27-hydroxylase mRNA in the liver tended to increase by ORX, but were not affected by sucrose ingestion.
Table 6 Effects of sucrose ingestion and orchiectomy (ORX) on the hepatic mRNA levels of genes upon lipid metabolism*
(Mean values with their standard errors, n 6)

C, control; SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose; ACAT, cholesterol acyltransferase; CYP7A1, cholesterol 7α-hydroxylase 1; CYP8B1, cholesterol 12α-hydroxylase; CYP27, cholesterol 27-hydroxylase; FAS, fatty acid synthase; FXR, farnesoid X receptor; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; LXRα, liver X receptor-α; MTP, microsomal TAG transfer protein; RXRα, retinoid X receptor-α; SREBP, sterol-regulatory element-binding protein.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Sham-operated and ORX rats were fed the experimental diets for 28 d.
† Significant effect (P < 0·05) of dietary sucrose ingestion and ORX, and significant effect of the interaction between dietary sucrose and ORX (sucrose × ORX).
Brown adipocyte gene expression
The level of UCP-1 mRNA tended to decrease by ORX, but was not affected by sucrose ingestion (Table 7). The levels of β3-adrenergic receptor were not affected by sucrose ingestion and ORX.
Table 7 Effects of dietary sucrose and orchiectomy (ORX) on the brown adipose tissue mRNA levels of genes upon lipid metabolism*
(Mean values with their standard errors, n 6)
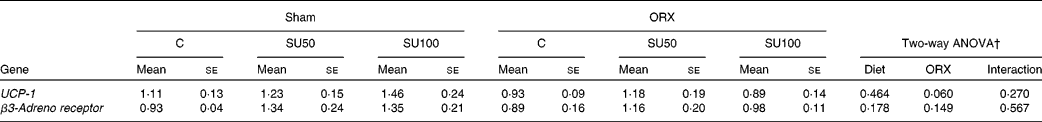
C, control; SU0, maize starch-based diet without sucrose; SU50, diet by which half of maize starch was replaced by sucrose; SU100, diet by which all of maize starch was replaced by sucrose; UCP-1, uncoupling protein-1.
* Sham-operated and ORX rats were fed the experimental diets for 28 d.
† Significant effect (P < 0·05) of dietary sucrose and ORX, and significant effect of the interaction between dietary sucrose and ORX (sucrose × ORX).
Discussion
In the present study, ORX caused a significant reduction in food intake, which is in agreement with the results of other researchers(Reference Borst and Conover13, Reference Erben, Eberle and Stahr14). ORX decreases food intake by decreasing meal frequency(Reference Asarian and Geary15). The mechanisms underlying this effect are not well understood(Reference Woods, Gotoh and Clegg16). However, the effects of gonadectomy on eating in rats and mice are apparently due to testosterone in males(Reference Chai, Blaha and Meguid17). Anabolic steroids, which have testosterone-like effects, have been shown to increase appetite and food intake in human subjects(Reference Hengge, Stocks and Wiehler18). The decline in testosterone levels in older males leads to increased leptin levels and this may explain the greater decline in food intake with ageing in males(Reference Morley19). Leptin inhibits food intake by acting in the appetite control centres of the brain. Therefore, the proposed mechanism for appetite decreased by ORX may be through an increase in circulating leptin. Leptin is manufactured primarily in the adipocytes of white adipose tissue, and the level of circulating leptin is directly proportional to the total amount of fat in the body. Testosterone is a fat-reducing hormone(Reference De Pergola20). Plasma leptin concentrations have a strong positive correlation with body fat(Reference Song, Sheu and Lee21). However, in the present study, the weight of bilateral epididymal fat pads to be an important white adipose tissue was decreased by ORX, and the body fat mass tended to be decreased by ORX (P = 0·078). Moreover, the concentration of plasma insulin was significantly decreased by ORX. It has been shown that fasting serum leptin and insulin concentrations are highly correlated(Reference Suga, Hirano and Kageyama22). We did not measure plasma leptin concentration in the present study; however, in our previous study on rats fed the SU0 diet, plasma leptin concentration was significantly increased by ORX (8·83 (se 0·23) ng/ml for ORX rats and 7·21 (se 0·42) ng/ml for sham rats; K Ebihara and S Makino, unpublished results), which agrees with the result of Pinilla et al. (Reference Pinilla, Seoane and Gonzalez23). On the other hand, plasma leptin half-life was shortened by ORX(Reference Castrogiovanni, Perelló and Gaillard24). Leptin is also secreted by several other tissues such as the stomach, placenta and brain as well as white adipose tissue(Reference Margetic, Gazzola and Pegg25). Though the reason why plasma leptin concentration was increased by ORX with decreasing abdominal fat tissues, and shortened half-life of leptin is at present unclear, an increased leptin concentration might depend on leptin secreted from several other tissues except white adipose tissue. It is certain that leptin plays an important role in the regulation of food intake; however, factors other than leptin also might take part in the food intake decreased by ORX.
ORX also caused a significant reduction in body-weight gain. Body-weight gain tended to be a positive correlation with the weight of body fat (r 0·402, P = 0·073) and the body fat mass:body protein mass ratio (r 0·462, P = 0·053). Testosterone stimulates lipolysis by increasing the number of lipolytic β3-adrenergic receptor(Reference De Pergola20). However, in the present study, the level of β3-adrenergic receptor mRNA of brown adipose tissue was not affected by ORX. UCP-1 is responsible for dissipating energy as heat instead of generating ATP from the oxidation of NEFA, and implicates for the regulation of total body fat. The level of UCP-1 mRNA of brown adipose tissue was decreased by ORX. Therefore, the decrease in body weight depends on the decrease in body fat mass, and it might depend on the increase in energy expenditure and the decrease in food intake.
Body-weight gain, food intake and body fat mass were increased by the ingestion of sucrose not only in sham-rats but also in ORX rats. There are many studies in rats reporting that sucrose-induced overeating led to obesity(Reference Hirsch and Walsh26, Reference Kanarek and Marks-Kaufman27), which might occur because sucrose stimulates the appetite and leads to excess consumption of dietary energy. High consumption of sucrose led to the accumulation of adipose tissue(Reference Bouchard, Després and Mauriège28). Body-weight gain was a strong positive correlation with food intake (r 0·919, P = 0·0025), which shows that the increases in body-weight gain and body mass fat depend on an increase in food intake. Sucrose is a disaccharide made of glucose and fructose. The consumption of diets high in fructose increased de novo lipogenesis(Reference Basciano, Federico and Adeli29). Lane & Cha(Reference Lane and Cha30) explored the suggested link between the consumption of fructose and increased food intake in rats. Fructose was shown to result in decreased circulating leptin(Reference Teff, Elliott and Tschöp31). Therefore, the increases in food intake and body fat mass by sucrose ingestion might depend on fructose in sucrose.
Plasma TAG concentration was increased by sucrose ingestion, but was decreased by ORX. MTP plays a critical role in the secretion of VLDL from the liver and is an important factor for the assembly of TAG-rich lipoproteins(Reference Haug and Hostmark32, Reference Ginsberg33). The level of SREBP-1c mRNA tended to decrease by ORX (P = 0·075); however, fatty acid synthase mRNA level and liver TAG concentration was not affected by ORX. Therefore, a decreased synthesis of fatty acid in the liver might explain the decreased plasma TAG concentration in ORX rats. However, some studies in animals and human subjects have suggested that androgen deficiency is associated with increased plasma TAG(Reference Wetterau, Aggerbeck and Bouma34, Reference Simon, Charles and Nahoul35).
Plasma total cholesterol concentration was increased by ORX, which agrees with the results of Haug & Høstmark(Reference Haug and Hostmark32) and Traish et al. (Reference Traish, Abdou and Kypreos36). The levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase and SREBP-2 were not affected by ORX, but liver cholesterol concentration tended to be increased by ORX (P = 0·085). ApoE plays a major role in systemic cholesterol metabolism by serving as a ligand for removal of cholesterol-laden plasma lipoproteins by hepatic receptors. The level of apoE mRNA was increased by ORX. The activity of hepatic CYP7A1 was not affected by ORX; however, the amount of bile acids in the intestinal contents and the faecal excretion of bile acids tended to decrease (P = 0·131) and was significantly decreased by ORX. Therefore, the increase in plasma cholesterol by ORX might depend on the decreased conversion from cholesterol to bile acids in the liver.
It is known that growth hormone (GH) plays an important role in the regulation of lipid metabolism(Reference Rudling, Eggertsen and Angelin37, Reference Améen and Oscarsson38). ORX decreased GH levels(Reference Sinha, Selby and Lewis39). Fructose stimulates GH secretion(Reference Strauch, Pandos and Bricaire40). SREBP-1c is a possible mediator of the action of GH(Reference Améen, Lindén and Larsson41). Fructose is a stronger inducer of SREBP-1c(Reference Miyazaki, Dobrzyn and Man42). In the present study, the concentration of MTP mRNA was increased by ORX and sucrose ingestion. The level of SREBP-1c mRNA was increased by sucrose ingestion and tended to decrease by ORX. On the other hand, GH stimulates both CYP7A1 activity and faecal bile acid excretion not only in GH-deficient animals, but also in normal young rats(Reference Parini, Angelin and Rudling43). However, in the present study, CYP7A1 activity was not affected by ORX and sucrose ingestion, and faecal bile acid excretion was decreased by ORX and sucrose ingestion. Therefore, ORX and sucrose ingestion might influence each other through GH, and regulate lipid metabolism. However further studies are necessary to discuss a relationship or interaction between the role of male hormone and fructose ingestion in lipid metabolism.
In both sham-operated and ORX rats, plasma TAG and cholesterol concentrations increased by sucrose ingestion, and liver TAG and cholesterol concentrations tended to be increased by sucrose ingestion (P = 0·106 and P = 0·101). Sucrose is a dimer of fructose and glucose. Numerous studies have reported that dietary fructose induces hyperlipidaemia(Reference Hallfrisch44–Reference Fields and Lewis46). Therefore, the increases in plasma TAG and cholesterol concentrations by sucrose ingestion would depend on fructose in sucrose.
In conclusion, lipid metabolism of rats was affected by ORX, sucrose ingestion and the amount of ingested sucrose. Plasma total cholesterol concentration was increased by ORX and dose-dependently by sucrose ingestion. Plasma TAG concentration was decreased by ORX, but was increased dose-dependently by sucrose ingestion. Liver TAG was increased by sucrose ingestion and ORX; however, liver cholesterol concentration was not affected by sucrose ingestion and ORX. The changes in lipid metabolism were influenced by sucrose ingestion more than ORX. Further studies are necessary to discuss a relationship or interaction between the role of male hormone and fructose ingestion in lipid metabolism.
Acknowledgements
The present study was supported by a research fund for students and teachers from Ehime University and a research grant from the Japanese Ministry of Education, Science and Culture (16580101). There are no conflicts of interest. The contribution of each author is as follows: S. M. carried out the experimental plan, summarised the experimental results and discussed with the other researchers about the experimental results; T. K. advised all aspects of the experiment; K. E. designed the experiment and was involved in manuscript preparation.









