Various stressful factors are associated with gastrointestinal disturbance, increased risk of enteric infections and accompanied inflammatory response in human and animals( Reference Tan, Li and Kong 1 ). The intestine is the predominant organ for nutrient digestion and absorption and the major site subjected to a high level of external stimulation( Reference Ren, Yin and Chen 2 ). During immune stimulation, the integrity of the intestinal mucosa may be damaged by the secretion of many inflammatory cytokines such as IL-1, IL-6 and TNF-α ( Reference Xiao, Wang and Liu 3 , Reference Xiao, Tang and Yin 4 ). Certain bacterial endotoxins, such as lipopolysaccharide (LPS), activate the innate immunity and induce inflammatory reactions through the NF- κ B signalling pathway, which induces the secretion of pro-inflammatory cytokines in enterocytes, compromising the mucosal layer and the intestinal barrier functions in piglets( Reference Harada, Ohira and Isse 5 , Reference Maeda and Omata 6 ). In addition, LPS transmits cellular signal after being recognised by toll-like receptor 4 (TLR4), the activation of which induces the expression of inflammatory cytokines, including IL-6, TNF-α and IL-1( Reference Beutler 7 ). Therefore, an LPS-challenged animal model and an intestinal epithelial cell model have been widely used to study the anti-inflammatory effect of various nutrients( Reference Liu, Huang and Hou 8 ).
NF- κ B is the main molecular target involved in inflammatory response( Reference Tak and Firestein 9 ). The activation of the NF- κ B signalling pathway can induce an intestinal immune response( Reference Schottelius and Baldwin 10 ). Owing to the presence of calcifications at sites of chronic inflammation or ischaemic necrosis, the Ca-sensing receptor (CaSR) is one of the key targets for the prevention of chronic inflammation( Reference Rossol, Pierer and Raulien 11 ). The activation of CaSR can inhibit the activity of NF- κ B, subsequently inhibiting the LPS-induced production of pro-inflammatory cytokines( Reference Wu, Wu and Du 12 ).
l-Amino acids are allosteric activators of CaSR( Reference Busque, Kerstetter and Geibel 13 ). It is known that aromatic l-amino acids have a higher affinity for CaSR than aliphatic and polar l-amino acids( Reference Conigrave, Mun and Lok 14 ). Recent studies have shown that l-tryptophan (Trp) can recruit β-arrestin2 by activating the CaSR signalling pathway in Caco-2 and HT-29 cell lines, therefore inhibiting the NF- κ B signalling pathway, blocking inflammatory signal transduction and subsequently exerting an anti-inflammatory effect( Reference Mine and Zhang 15 ).
Together, we hypothesise that appropriate ratio aromatic amino acids. In this study, LPS-induced intestinal inflammation piglet model and porcine jejunal epithelial cell line (IPEC-J2) were used to investigate the regulatory effects of dietary supplementation with aromatic amino acids – tryptophan, phenylalanine and tyrosine (TPT) – on the CaSR signalling pathway and intestinal inflammatory response in piglets.
Methods
Animals and experimental design
The animal trial was approved by the Institutional Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (2013020).
A total of thirty-two cross-bred (Duroc×Landrace) weanling gilts and barrows with an initial body weight (BW) of 6·66 (sem 0·31) kg were randomly assigned into four treatments (eight piglets/treatment). Each group consisted of an equal number of gilts and barrows. The experimental treatment was designed in a 2×2 factorial arrangement (diet and LPS challenge) in a complete randomised design. Piglets were fed either a basal diet or the basal diet supplemented with 0·16 % Trp, 0·41 % Phe and 0·22 % Tyr for a period of 21 d. The levels of TPT were determined based on the preliminary experiment, which found that piglets fed diets supplemented with 1·5-fold TPT recommended by the National Research Council( 16 ) showed better performance compared with those fed diets supplemented with 1- or 2-fold TPT based on standardised ileal amino acid digestibility. To balance the N level with the supplemented TPT diets, alanine (1·41 % of diet) was added into the basal diet. The basic dietary composition and nutrient levels are shown in Table 1. All piglets had free access to diets and water.
Table 1 Composition and nutrient levels of diets (as-fed basis)

TPT, tryptophan, phenylalanine and tyrosine.
* Providing the following amounts of vitamins and minerals per kg: Zn (ZnO), 50 mg; Cu (CuSO4), 20 mg; Mn (MnO), 55 mg; Fe (FeSO4), 100 mg; iodine (KI), 1 mg; Co (CoSO4), 2 mg; Se (Na2SeO3), 0·3 mg; vitamin A, 2·48 mg; vitamin D3, 0·5 mg; vitamin E, 26·72 mg; vitamin B1, 2 mg; vitamin B2, 4 mg; pantothenic acid, 15 mg; vitamin B6, 10 mg; vitamin B12, 0·05 mg; nicotinic acid, 30 mg; folic acid, 2 mg; vitamin K3, 1·5 mg; biotin, 0·2 mg; choline chloride, 800 mg and vitamin C, 100 mg. The premix did not contain additional Cu, Zn, antibiotics or probiotics.
On day 21 of the experiment, the piglets of each dietary treatment were intraperitoneally injected with either LPS (Escherichia coli strain O5:B55) at a dose of 100 μg/kg BW or the same volume of 0·9 % sterilised saline, respectively. All piglets were euthanised with 4 % pentobarbital sodium (40 mg/kg BW) 4 h post injection. Blood samples were collected from the jugular vein and allowed to stand at 4°C for 4 h. Serum samples were obtained by centrifugation at 2000 g for 15 min and stored at –80°C until further analysis. All piglets were dissected to collect mid-jejunum, mid-ileum, anterior colon and posterior colon segments. All tissue samples were snap-frozen in liquid N2 and stored at –80°C. Extra-intestinal samples (2 cm) of each segment were rinsed with pre-cooling saline and fixed in formaldehyde solution.
Analysis of serum concentrations of cytokines
Serum concentrations of IL-1β, IL-4, IL-6, IL-8, IL-12, granulocyte–macrophage colony-stimulating factor (GM-CSF), transforming growth factor (TGF)-β1 and TNF-α were determined by multiplex ELISA system according to the manufacturer’s protocol( Reference Yuan, Hussain and Tan 17 ). The signals were visualised using InnoScan 300 Microarray Scanner (Innopsys) at 532 nm and the quantitative data analysis was performed using the Quantibody® Q-Analyzer (QAP-CYT-1; RayBiotech Inc.).
Histopathological grading
Histopathological grading of jejunum, ileum, anterior colon and posterior colon samples was performed as described previously( Reference Huang, Xiao and Tan 18 ). Briefly, jejunal and ileal tissues were fixed in 10 % buffered formalin solution and embedded in paraffin after a macroscopic evaluation. The embedded specimens were then sectioned (6 μm), stained with haematoxylin–eosin and examined by light microscopy. Histological scoring was carried out by a veterinary and blinded pathologist using the methods that ranged from 0 (minimal injury) to 15 (maximal injury) corresponding to four grades that included mononuclear or polymorph nuclear cell infiltration, histological injury and erosion or epithelial hyperplasia( Reference Huang, Xiao and Tan 18 ).
Analysis of myeloperoxidase activity in the colon
Colon samples were homogenised in ten volumes of ice-cold potassium phosphate buffer (pH 6·0) containing 0·5 % hexadecyl-trimethyl ammonium hydroxide through high-pressure homogeniser at 4°C. The homogenate was centrifuged at 700 g at 4°C for 15 min and the supernatant was diluted to a total of 50 μl each in PBS (pH 6·0) containing 0·17 mg/ml 3,3′-dimethoxybenzidine and 0·0005 % H2O2. Myeloperoxidase (MPO) activity was assessed by measuring H2O2-dependent oxidation of 3,3′-imethoxybenzidine. One unit of enzyme activity is defined as the amount of MPO present that causes a change in absorbance per min at 460 nm and 37°C( Reference Barreau, Ferrier and Fioramonti 19 , Reference Maslowski, Vieira and Ng 20 ).
Real-time PCR
Total RNA was isolated from the jejunum, ileum, anterior colon and posterior colon samples using a Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. The extracted RNA was dissolved in diethylpyrocarbonate (DEPC)-treated water, and its concentration was assessed using an Eppendorf Biophotometer (Eppendorf AG) and its integrity verified by electrophoresis on a 1 % agarose gel. After DNase I treatment (Takara), 1 μg of total RNA was used as a template for complementary DNA (cDNA) synthesis using an oligo(dT) primer (Takara). The resultant cDNA was diluted and used for evaluating gene expression.
All primers were developed previously for amplification of mRNA sequences of pig (Sus scrofa) (Table 2). Quantitative real-time PCR (qPCR) were performed to analyse the mRNA levels for the target genes including IL-1α, IL-1β, IL-8, TNF-α, IL-2, IL-4, IL-6, IL-12, TGF-β1, GM-CSF, interferon-γ (IFN-γ), IL-10 and monocyte chemoattractant protein (MCP)-1/chemoattractant CC chemokine ligand 2 (CCL2) and a housekeeping gene, β-actin. A 10-μl reaction system was applied, which includes 0·5 μl of each forward and reverse primer, 2 μl of cDNA, 2 μl of DEPC-treated water and 5 μl of SYBR Premix Ex Taq (Takara Bio Inc.). The qPCR was carried out (Lightcycler-480I I; Roche Diagnostics GmbH) as previously described(
Reference Huang, Xiao and Tan
21
). The relative mRNA levels of target genes were expressed as
![]() $$2^{{-\Delta \Delta C_{{\rm t}} }} $$
, where ΔΔC
t=(C
t Target–C
t β-actin)treatment–(C
t Target–C
t β-actin)control. Data are expressed as the relative values to those in basal-diet-treated piglets injected with saline.
$$2^{{-\Delta \Delta C_{{\rm t}} }} $$
, where ΔΔC
t=(C
t Target–C
t β-actin)treatment–(C
t Target–C
t β-actin)control. Data are expressed as the relative values to those in basal-diet-treated piglets injected with saline.
Table 2 Primers used for real-time PCR
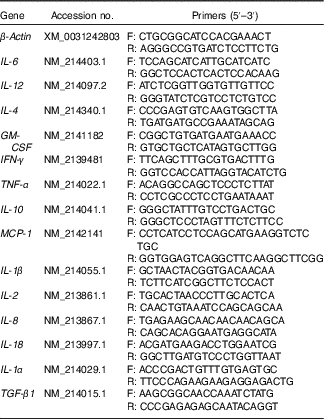
F, forward; R, reverse; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein-1; TGF-β1, transforming growth factor-β1.
Western blotting
Jejunum, ileum and anterior colon samples were collected and the protein levels of β-actin, CaSR, phospholipase C (PLC) β2, NF- κ B p65, phosphorylated NF- κ B (p-NF- κ B) p65, inhibitor of NF- κ B kinase (IKK)α/β, inhibitor of NF- κ B (I κ B) were determined as described previously( Reference Tan, Yin and Kong 22 ). The following antibodies were used for protein quantification: β-actin (1:1000; Cell Signaling Technology), CaSR (1:1000; Bioss Inc.), PLCβ2 (1:1000; Antibodies-Online), NF- κ B p65 (1:1000; Cell Signaling Technology), p-NF- κ B p65 (Ser536) (1:1000; Cell Signaling Technology), IKKα/β (1:400; Santa Cruz Biotechnology) and I κ B (1:1000; Cell Signaling Technology). Data are expressed relative to the values of piglets fed the basal diet without LPS challenge.
Cell culture and treatment
A porcine jejunal epithelial cell line, IPEC-J2, was seeded in 10-mm cell culture dishes and cultured with high-glucose (25 mm) Dulbecco’s modified Eagle’s medium (Hyclone) containing 10 % fetal bovine serum (Gibico) and 1 % antibiotic solution (P/S; Sigma) at 37°C in a 5 % CO2 incubator. After an overnight incubation, cells were incubated in medium containing the following: (1) normal TPT (0·08 mm Trp+0·4 mm Phe+0·4 mm Tyr), (2) normal TPT with CaSR agonist (R568, 0·05 μm), (3) normal TPT with CaSR inhibitor (NPS-2143, 0·08 μm), (4) supplemented TPT (0·16 mm Trp+0·8 mm Phe+0·8 mm Tyr) and (5) supplemented TPT (0·16 mm Trp+0·8 mm Phe+0·8 mm Tyr) with CaSR inhibitor (NPS-2143, 0·08 μm), respectively. After 20 h of incubation, 100 μg/ml of LPS (E. coli strain O5:B55) was added to induce inflammation for 4 h. The medium was removed and cells were washed with cold PBS and collected using Trizol reagent or lysis buffer for the analysis of relative cytokines mRNA and CaSR pathway protein level, as described in the animal experiment.
Statistical analysis
All data analysis was performed by ANOVA using the general linear model procedures of SPSS (SPSS Inc.). The statistical model included the effects of challenge (saline or LPS), diet (basal or TPT) and their interactions for the in vivo study. In the in vitro study, the statistical model included treatment as a fixed effect. The differences among treatments were evaluated using Tukey’s test. Data are presented as means with their standard errors. P values <0·05 were taken to indicate significance.
Results
Growth performance
There were no differences (P>0·05) in initial BW, final BW, average daily gain and daily feed intake between control and TPT treatments. The feed:gain ratio was decreased (P<0·05) by TPT supplementation (Table 3).
Table 3 Effects of dietary supplementation with tryptophan, phenylalanine and tyrosine (TPT) on growth performance of piglets (Mean values with their standard errors, n 16 per treatment)
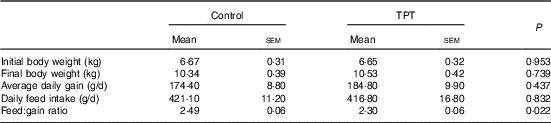
Control, basal diet; TPT, basal diet supplemented with 0·16 % tryptophan+0·41 % phenylalanine+0·22 % tyrosine.
Colonic myeloperoxidase activity
MPO activity was shown in Table 4. LPS injection increased MPO activity in the anterior colon when piglets were fed the control diet, whereas an opposite direction was observed in piglets fed the TPT diet (diet×LPS, P<0·05). In the posterior colon, a significant suppression on MPO activity was observed by diet rather (diet, P<0·05) than LPS (P>0·05).
Table 4 Effects of dietary supplementation with tryptophan, phenylalanine and tyrosine (TPT) on myeloperoxidase activity in the colon of piglets (Mean values with their standard errors, n 8 per treatment)

Control, basal diet; TPT, basal diet supplemented with 0·16 % tryptophan+0·41 % phenylalanine+0·22 % tyrosine; LPS, lipopolysaccharide.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Histopathological grading of the intestine
Intraperitoneal injection of LPS induced the higher score of histopathological grading in the jejunum, ileum and posterior colon (LPS, P<0·05). Dietary supplementation with TPT decreased (diet×LPS, P<0·05) the score of histopathological grading of ileum, anterior colon and posterior colon in LPS-treated piglets but not (P>0·05) in saline-injected piglets (Table 5).
Table 5 Effects of dietary supplementation with tryptophan, phenylalanine and tyrosine (TPT) on histopathological grading of the intestine in piglets (Mean values with their standard errors, n 8 per treatment)

Control, basal diet; TPT, basal diet supplemented with 0·16 % tryptophan+0·41 % phenylalanine+0·22 % tyrosine; LPS, lipopolysaccharide.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Serum cytokine concentrations
Intraperitoneal injection of LPS increased the serum concentrations of IL-1β, IL-6, IL-8, IL-12 and TNF-α, and decreased serum IL-4 and TGF-β contents in piglets fed the control diet. However, the effects induced by injection of LPS were reversed by dietary supplementation with TPT, which reduced the serum concentrations of IL-1β, IL-6, IL-8, IL-12 and TNF-α and increased serum IL-4 and TGF-β contents (diet×LPS, P<0·05) (Fig. 1).
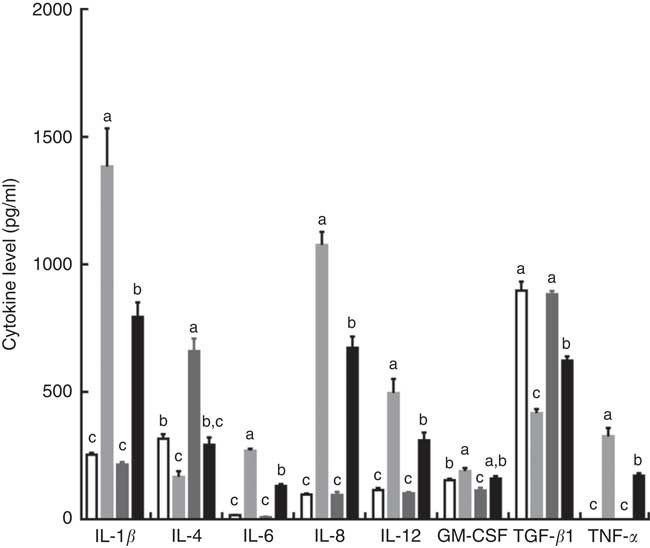
Fig. 1 Dietary supplementation with tryptophan, phenylalanine and tyrosine (TPT) affects serum concentrations of cytokines in piglets. Control, basal diet; TPT, basal diet supplemented with 0·16 % tryptophan+0·41 % phenylalanine+0·22 % tyrosine; LPS, lipopolysaccharide; GM-CSF, granulocyte–macrophage colony-stimulating factor; TGF-β, transforming growth factor-β. Values are means (n 8 independent experiments), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05). ![]() , Control diet+saline;
, Control diet+saline; ![]() , control diet+LPS;
, control diet+LPS; ![]() , TPT diet+saline;
, TPT diet+saline; ![]() , TPT diet+LPS.
, TPT diet+LPS.
Relative cytokine mRNA levels in the intestine
The relative cytokine mRNA levels in the jejunum are shown in the online Supplementary Table SA. LPS injection induced a significant increase in the mRNA levels of IL-8 and MCP-1/CCL2 in piglets fed both the control diet and TPT diet (LPS, P<0·05), as well as IL-6 level in pigs fed the control diet, suggesting the successful induction of enteric inflammation. TPT supplementation alleviated the inflammation as demonstrated by the moderate increase in the mRNA levels of IL-6, IL-8, IL-12, MCP-1/CCL2 and TNF-α in LPS-challenged piglets (diet×LPS, P<0·05). However, TPT did not affect mRNA level of cytokines in piglets fed the control diet.
In the ileum, significantly higher mRNA levels of IL-1α and MCP-1/CCL2 were observed in piglets infected with LPS compared with the pigs fed the control diet (LPS, P<0·05). TPT treatment reduced mRNA levels of IL-6, IL-12 and TNF-α in LPS-challenged piglets (diet, P<0·05). A significant interaction between diet and LPS was observed on the mRNA level of IL-1β, IL-8, IL-12, IL-18, GM-CSF, MCP-1/CCL2 and TNF-α (diet×LPS, P<0·05) (online Supplementary Table SB).
In the anterior colon, LPS induced a significant increase in the mRNA levels of IL-1β and MCP-1/CCL2 in piglets fed the control diet (LPS, P<0·05). Compared with the LPS-challenged treatment fed with the control diet, dietary supplementation with TPT reduced mRNA level of IL-1α, IL-2, IL-8 and IL-18 in piglets infected with LPS (diet, P<0·05). In addition, TPT supplementation decreased the mRNA level of IFN-γ in sham-challenged piglets (diet, P<0·05) (online Supplementary Table SC).
In the posterior colon, LPS injection increased the IFN-γ mRNA level in piglets fed the control diet (LPS, P<0·05), whereas TPT supplementation decreased IL-6, IL-12, IL-18 and TNF-α mRNA levels in LPS-challenged piglets (diet, P<0·05). In addition, dietary TPT supplementation decreased the mRNA levels of IL-1β, IL-12, IL-18 and TNF-α in the control group (diet, P<0·05) (online Supplementary Table SD).
Protein expression levels of calcium-sensing receptor and NF- κ B signalling pathway in the intestine
The CaSR and the NF- κ B signalling pathway in the jejunum, ileum and colon were determined by Western blotting, as shown in Fig. 2 and Table 6. In the jejunum, supplementation of TPT significantly increased the protein level of CaSR and PLCβ2 (diet, P<0·05). LPS injection increased NF- κ B protein level in the piglets fed the control diet, whereas TPT supplementation significantly reduced the protein levels of IKKα/β, I κ B and NF- κ B (diet×LPS, P<0·05). p-NF- κ B was also reduced by TPT supplementation in LPS-challenged piglets (P<0·05).
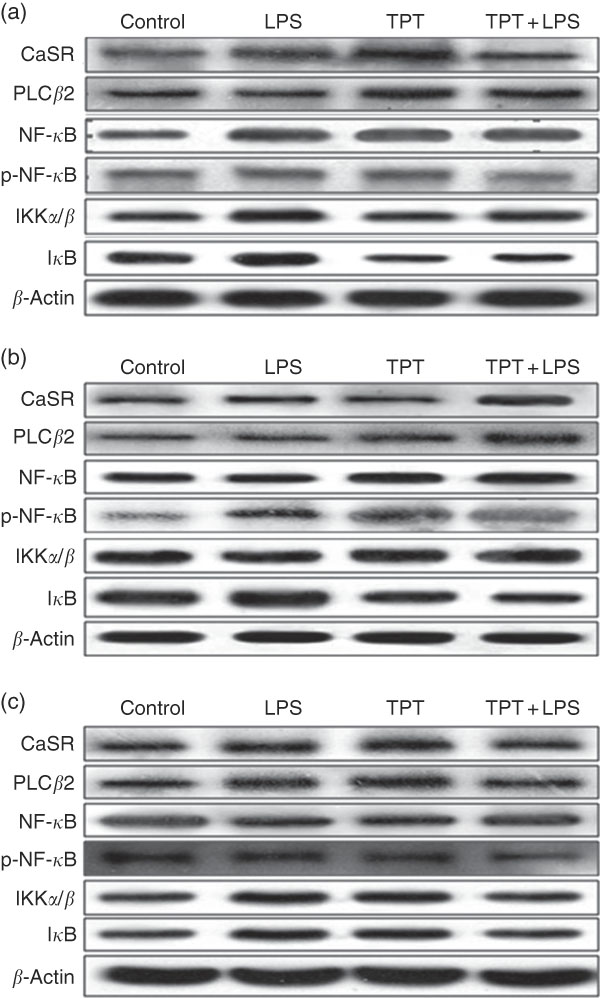
Fig. 2 Representive Western blot images of the calcium-sensing receptor (CaSR) pathway and the NF-κB pathway in the jejunum (a), ileum (b) and anterior colon (c). LPS, lipopolysaccharide; TPT, tryptophan, phenylalanine and tyrosine; PLC, phospholipase C; p-NF-κB, phosphorylated NF-κB; IKK, inhibitor of NF-κB kinase; IκB, inhibitor of NF-κB.
Table 6 Effects of dietary supplementation with tryptophan, phenylalanine and tyrosine (TPT) on the relative protein levels of calcium-sensing receptor (CaSR) and NF-κB signalling pathway in the jejunum, ileum and anterior colon of piglets(Mean values with their standard errors, n 8 per treatment)
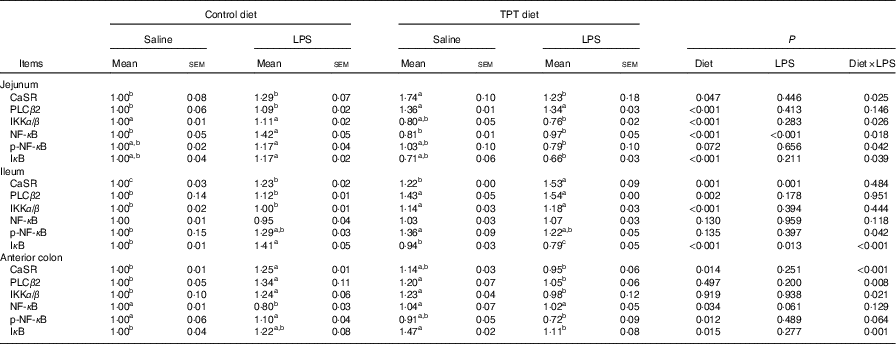
Control, basal diet; TPT, basal diet supplemented with 0·16 % tryptophan+0·41 % phenylalanine+0·22 % tyrosine; LPS, lipopolysaccharide; PLCβ, phospholipase Cβ; IKKα/β, inhibitor of NF-κB kinase α/β; p-NF-κB, phosphorylated NF-κB; IκB, inhibitor of NF-κB.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
In the ileum, injection of LPS significantly increased the protein level of CaSR and I κ B in the weaned piglets fed the control diet (LPS, P<0·05). Dietary supplementation of TPT significantly increased CaSR and PLCβ2, and down-regulated phosphorylation of NF- κ B and I κ B protein levels in piglets challenged with LPS (diet, P<0·05). We observed a significant diet and LPS interaction at the protein levels of I κ B and phosphorylation of NF- κ B (diet×LPS, P<0·05).
In the anterior colon, LPS injection increased the protein levels of CaSR, PLCβ2, IKKα/β and I κ B compared with the control group, but the opposite effect was observed in piglets fed the TPT diet (diet×LPS, P<0·05). p-NF- κ B decreased in TPT-supplemented LPS-treated piglets compared with TPT-supplemented saline-treated piglets but not compared with LPS-treated piglets fed the control diet (diet, P<0·05).
Relative cytokine mRNA and calcium-sensing receptor pathway protein level in the cells
In comparison with the control group, R568 and TPT treatments decreased the mRNA levels of IL-6 in IPCE-J2 cells treated with LPS (P<0·05), whereas NPS-2143 significantly increased the mRNA levels of TNFA, IL-1α, IL-8, IL-18 and GM-CSF (P<0·05). R568 treatment also reduced the mRNA levels of GM-CSF (P<0·05). Adding NPS-2143 in TPT-treated cells significantly increased the mRNA level of TNF-α and IL-6 (P<0·05) (Table 7).
Table 7 Effects of tryptophan, phenylalanine and tyrosine (TPT) supplementation and calcium-sensing receptor (CaSR) agitation or inhibition on the relative cytokine mRNA levels in lipopolysaccharide-challenged intestinal porcine epithelial cells-J2 (Mean values with their standard errors of at least four independent experiments)
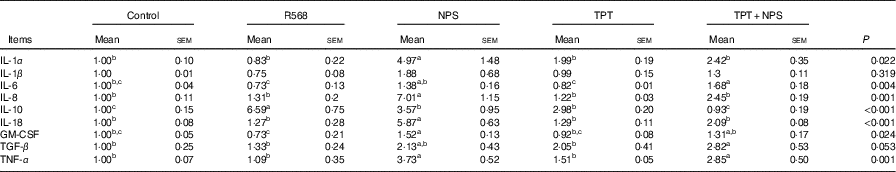
Trp, tryptophan; Phe, phenylalanine; Tyr, tyrosine; Control, normal TPT (0·08 mm Trp+0·4 mm Phe+0·4 mm Tyr); R568, normal TPT with CaSR agonist (R568, 0·05 μm); NPS, normal TPT with CaSR inhibitor (NPS-2143, 0·08 μm); TPT, supplemented TPT (0·16 mm Trp+0·8 mm Phe+0·8 mm Tyr); TPT+NPS, supplemented TPT (0·16 mm Trp+0·8 mm Phe+0·8 mm Tyr) with CaSR inhibitor (NPS-2143, 0·08 μm); GM-CSF, granulocyte–macrophage colony-stimulating factor; TGF-β, transforming growth factor-β.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
The protein levels of CaSR and NF- κ B signalling pathway in IPEC-J2 cells are shown in Figs. 3 and 4. In comparison with the control cells, R568 and TPT treatments significantly up-regulated CaSR level, whereas NPS-2143 reduced CaSR protein level (P<0·05). R568 decreased the protein level of p-NF- κ B and I κ B (P<0·05). TPT decreased the protein level of I κ B, whereas NPS-2143 significantly increased the levels of p-NF- κ B, IKKα/β and I κ B (P<0·05). NPS-2143 with TPT treatment up-regulated p-NF- κ B (P<0·05).
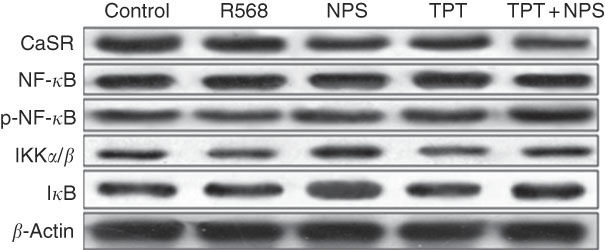
Fig. 3 Representive Western blot images of IPEC-J2 supplemented with tryptophan, phenylalanine and tyrosine (TPT) and calcium-sensing receptor (CaSR) agitation or inhibition. p-NF-κB, phosphorylated NF-κB; IKK, inhibitor of NF-κB kinase; IκB, inhibitor of NF-κB.
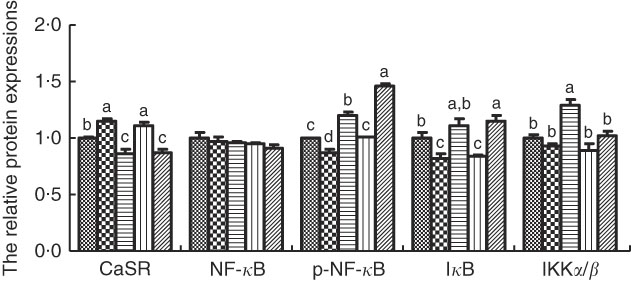
Fig. 4 Effects of tryptophan, phenylalanine and tyrosine (TPT) supplementation and calcium-sensing receptor (CaSR) agitation or inhibition on the protein levels of NF-κB signalling pathway in lipopolysaccharide (LPS)-challenged IPEC-J2. Values are means (n 4 independent experiments), with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , R568;
, R568; ![]() , NPS;
, NPS; ![]() , TPT;
, TPT; ![]() , TPT+NPS. p-NF-κB, phosphorylated NF-κB; IκB, inhibitor of NF-κB; IKK, inhibitor of NF-κB kinase.
, TPT+NPS. p-NF-κB, phosphorylated NF-κB; IκB, inhibitor of NF-κB; IKK, inhibitor of NF-κB kinase.
Discussion
CaSR plays a key role in modulating fluid secretion and absorption in the GI tract( Reference Geibel and Hebert 23 ). Recent studies have demonstrated that CaSR may serve as novel therapeutic targets for the treatment of inflammatory bowel diseases( Reference Huang, Xiao and Tan 21 , Reference Tang, Cheng and Sun 24 , Reference Cheng, Lightfoot and Yang 25 ). In this study, activation of CaSR through its ligands, aromatic amino acids, demonstrated anti-inflammatory effect in response to LPS challenge, which is mediated through the inhibition of the NF- κ B signalling pathway.
LPS has been widely used for induction of inflammatory response in numerous animal and in vitro models( Reference Walton, Collins and Sartor 26 ). Intraperitoneal LPS injection can trigger the systemic inflammation mirrored by increased circulation of pro-inflammatory cytokines through the activation of the NF- κ B signalling pathway( Reference Blackwell, Yull and Chen 27 ). It has been shown that intraperitoneal LPS injection aggravates the inflammatory response of the intestinal mucosa and causes tissue damage to the intestinal epithelium and the intestinal barrier functions in piglets( Reference Strober, Fuss and Mannon 28 ). In this study, intraperitoneal injection of LPS led to systemic and intestinal inflammation, increased the MPO activity in the proximal and distal colon and increased the serum concentrations of IL-1β, IL-6, IL-8, IL-12, GM-CSF and TNF-α.
However, dietary supplementation of aromatic amino acids alleviated intestinal inflammation. First, aromatic amino acids significantly improved histopathological grading of ileum and colon and MPO activity in the posterior colon in the LPS-challenged piglets. MPO was released into the extracellular space after phagocyte activation to induce damage to the host tissue at inflammatory sites. The increased intestinal MPO levels as a biomarker of oxidative stress were found in inflammatory bowel disease( Reference Algieri, Zorrilla and Rodriguez-Nogales 29 ).
Consistent with the result of MPO, supplementation of aromatic amino acids down-regulated the mRNA levels of pro-inflammatory cytokines in intestine tissue of LPS-treated piglets. During intestinal inflammation, mucosal immune dysfunction and innate immune cell activation result in the excess production of pro-inflammatory cytokines( Reference Wallach, Varfolomeev and Malinin 30 ). Elevated expression of pro-inflammatory cytokines is closely related to increased intestinal permeability( Reference Förster 31 ). Conversely, aromatic amino acids increased the levels of anti-inflammatory cytokines, such as IL-4 and TGF-β, which contribute to the repair of the intestinal epithelium. Collectively, feed supplementation of aromatic amino acids decreased mRNA levels of certain pro-inflammatory cytokines in the jejunum, ileum and colon and enhanced mRNA levels of anti-inflammatory cytokines.
The NF- κ B pathway plays a pivotal role in the modulation of the inflammatory response and cytokine gene expression. The activation of NF- κ B signalling can induce the mucosal immune response and early pathologic reactions of inflammation in segments of the intestine( Reference Atreya, Atreya and Neurath 32 ). This study demonstrates that additional aromatic amino acids in the diet could reduce the LPS-induced activation of the NF- κ B signalling pathway and decrease the level of IKKα/β, p-NF- κ B and I κ B from the jejunum to the anterior colon. The observed inhibitory effect was shown to be associated with the activation of the CaSR signalling pathway by aromatic amino acids( Reference Yousef, Pichyangkura and Soodvilai 33 ). In this study, dietary supplementation with aromatic amino acids increased the protein levels of PLCβ2 in the intestine from the jejunum to the anterior colon, whereas the protein levels of CaSR were elevated in the jejunum and ileum but not in the anterior colon. In the taste signalling pathway, activation of PLCβ2 results in a transient increase in intracellular Ca ions( Reference Clapp, Stone and Margolskee 34 ). Then, transient receptor potential melastatin channel subtype 5 (TRPM5) is activated by the changing intracellular Ca levels, causing depolarisation of taste receptor cells( Reference Behrens and Meyerhof 35 ). Interestingly, the colocalisation of PLCβ2 with TRPM5 in colon sections was not observed as in the other regions of the gut( Reference Bezencon, le Coutre and Damak 36 ). Thus, the diversity of PLCβ2 level affected by aromatic amino acids may stem from the limit of upstream and downstream signalling molecules in different segments of the intestine. l-Amino acids are allosteric activators of CaSR with the following order of affinity: aromatic amino acids>aliphatic amino acids>polar amino acids( Reference Conigrave and Brown 37 , Reference Conigrave, Franks and Brown 38 ). Recent studies have shown that the aromatic amino acid l-Trp in intestinal cells can recruit β-arrestin2 through the activation of the CaSR signalling pathway, which inhibits the NF- κ B signalling pathway, blocks inflammatory signal transduction and consequently exerts anti-inflammatory activity( Reference Zhang, Kovacs-Nolan and Kodera 39 , Reference Mine and Zhang 40 ).
As a nutrient-sensing receptor, CaSR plays an important role in the maintenance of immune homoeostasis in intestinal mucosa( Reference Cheng, Lightfoot and Yang 25 , Reference Macleod 41 ). Recent studies have proven that CaSR is an important target for the prevention of chronic inflammation and tumour progression( Reference Zhang, Kovacs-Nolan and Kodera 39 , Reference Cheng, Lightfoot and Yang 42 , Reference MacLeod 43 ). To further explore the role of the CaSR signalling pathway in the regulation of aromatic amino acids on the intestinal inflammatory response, the in vitro study was performed in the intestinal epithelial cell line. In IPEC-J2 cells, the mRNA levels of pro-inflammatory cytokines, such as IL-1β and IL-6, were significantly reduced by the addition of aromatic amino acids. The treatment of IPEC-J2 cells with R568, a CaSR specific activator( Reference Davey, Leach and Valant 44 ), also yielded similar results with TPT supplementation in this study. NPS-2143 can bind to specific amino acid binding sites on seven-transmembrane α-helices to inhibit the effect of CaSR activators on the CaSR signalling pathway( Reference Nemeth, Delmar and Heaton 45 , Reference Soudijn, Van Wijngaarden and IJzerman 46 ). As expected, the addition of the CaSR antagonist, NPS-2143, significantly reduced the protein levels of CaSR, p-NF- κ B and I κ B. Intriguingly, it also blocked the anti-inflammatory effects of aromatic amino acids, which further indicated involvement of the CaSR signalling pathway.
This study shows that dietary supplementation with aromatic amino acids could regulate the secretion of inflammatory cytokines induced by LPS in the intestine of piglets. The anti-inflammatory effect might be mediated via the activation of CaSR, which subsequently inhibits NF- κ B signalling pathways. Our study highlighted dietary supplementation of aromatic amino acids as a nutritional strategy to alleviate intestinal stress in a pig model. CaSR signalling may be a potential target to reduce enteric inflammation in the livestock industry. LPS-induced cell injury is predominantly mediated by the activation of TLR4( Reference Elner, Petty and Elner 47 ). The up-regulated mRNA expression of CaSR and decreasing level of IKKα/β-I κ B in the LPS-reduced pigs followed the supplementation of TPT. In the in vivo study, the inhibitor of CaSR activated I κ B expression, leading to appreciable cytokine production. Together, the regulatory effects of TPT on inflammation followed the cross talk between CaSR and TLR4 pathway (Fig. 5). Given that excessive activation of either I κ B or NF- κ B has been associated with inflammatory tissue injury, the new insight on dietary aromatic amino acid supplementation may have therapeutic implications.
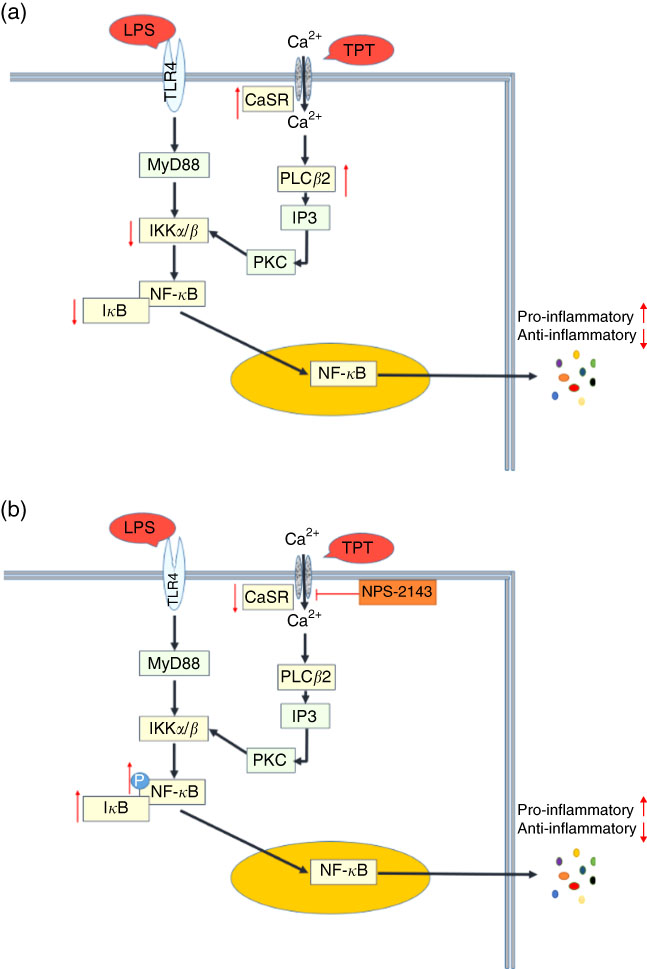
Fig. 5 Cross talk of toll-like receptor 4 (TLR4) and calcium-sensing receptor (CaSR) pathways. (a) Tryptophan, phenylalanine and tyrosine (TPT) supplementation in pig diet increased the mRNA level of CaSR and reduced the activity of the NF-κB pathway induced by lipopolysaccharide (LPS) challenge, leading to the inhibition of cytokine production; (b) in LPS challenged IPEC-J2 cells, CaSR inhibitor (NPS-2143) suppressed the activity of CaSR, resulting in the phosphorylation of NF-κB and the recovery of cytokine secretion in inflammation. Myeloid differentiation factor 88 (MyD88) is a key adaptor in the TLR signalling pathway. It mediates the activation of downstream NF-κB. Inositol 1,4,5-triphosphate (IP3) is a messenger to activate protein kinase C (PKC) and induce the cellular cascade response.
Acknowledgements
This study was supported by the National Key R&D Program (2017YFD0500503), Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), National Natural Science Foundation of China (nos 31330075, 31672433, 31501964 and 31560640), the Earmarked Fund for China Agriculture Research System (CARS-35) and Youth Innovation Team Project of ISA, CAS (2017QNCXTD_TBE).
B. T. and Y. Y. designed the research and analysed the data. H. L., B. H., J. L., J. W. and G. G. conducted the research. H. L., B. T., P. L. and P. J. wrote the article. B. T. and Y. Y. had primary responsibility for the final content, and all authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002891















