Carbohydrates (CHO) are the most economical non-protein energy substitute in fish diets, due to their high abundance and low cost(Reference Hemre, Mommsen and Krogdahl1). However, most fish have a relatively low tolerance for CHO(Reference Enes, Panserat and Kaushik2,Reference Kamalam, Medale and Panserat3) and often display a prolonged postprandial hyperglycaemia to high-carbohydrate (HC) diets. Generally, maintaining blood glucose concentrations in a relatively narrow range involves the active assistance of several tissues. Among them, the intestine plays an essential role in the regulation of glucose homoeostasis, since it is responsible for the digestion and absorption of CHO(Reference Chen, Eslamfam and Fang4,Reference Mithieux, Rajas and Gautier-Stein5) . All cells need adenosine triphosphate (ATP) as a source of energy(Reference Pi, Liu and Shi6), while mitochondria are the principal organelles producing ATP(Reference Marcu, Zheng and Hawkins7). A previous study reported that impaired mitochondrial biogenesis and function would result in oxidative stress of fish and could consequently inhibit the mitochondrial respiratory chain complex activities and ATP production, thus impairing nutrient metabolism(Reference Li, Wang and Xu8). Hence, maintaining the normal intestinal mitochondrial function is of great significance, as it may facilitate non-protein energy utilisation.
In the cell, mitochondrial function is closely related to gene expression(Reference Tang, Luo and Chen9). Indeed, a previous study indicated that intracellular mitochondrial biogenesis is a complex physiological process that includes replication of mitochondrial DNA (mtDNA) and multiple mitochondrial genes(Reference Bartolák-Suki and Suki10). In this regard, adenosine monophosphate (AMP)-activated protein kinase (AMPK) has attracted considerable attention as an energy sensor. AMPK can be activated by an increased cellular AMP:ATP ratio(Reference Fogarty and Hardie11). Once activated, AMPK can enhance the activity of the mitochondrial master switch, PPAR γ coactivator-1α (PGC-1α), thus stimulating the expression of mitochondrial genes and other proteins involved in mitochondrial biogenesis(Reference Leick, Fentz and Biensø12). However, these studies focused primarily on mammals, while related information in aquatic animals is quite limited. Recently, differences have been identified between fish and mammals related to the mitochondrial biogenesis pathway. In fish, PGC-1β has been demonstrated to be more effective in inducing mitochondrial biogenesis than PGC-1α (Reference Lu, Tomas and Song13,Reference Bremer, Kocha and Snider14) . In addition, mitochondrial function in fish can be affected by various factors in aquaculture practices, such as genetics, water temperature and dietary composition(Reference Papa and Skulachev15–Reference Eya, Ashame and Pomeroy17). However, the connection between mitochondrial function and CHO utilisation is poorly understood in fish, thus warranting further in-depth studies.
Thiamine is a member of the vitamin B family. It plays a fundamental role in regulating energy metabolism, specifically in coordinating mitochondrial and cytosolic biochemical processes(Reference Depeint, Bruce and Shangari18). As a mitochondrial nutrient, thiamine serves as an important cofactor in regulating the activities of the respiratory chain complex enzymes, thereby enhancing mitochondrial function(Reference Gangolf, Wins and Thiry19). In addition, it also enhances glucose oxidation in mitochondria by increasing the activities of dehydrogenase enzyme complexes, thus improving glucose homoeostasis(Reference Depeint, Bruce and Shangari18,Reference Mehta, Shangari and O’Brien20) . In fish, thiamine has been demonstrated to improve the integrity, function and health of the intestine(Reference Huang, Feng and Liu21). However, the underlying mechanisms are still poorly understood. Whether it could be attributed to the enhanced mitochondrial biogenesis and function is still unknown.
Blunt snout bream (Megalobrama amblycephala) is an important culture fish in China. At present, diets formulated for this species commonly contain relatively high concentrations of CHO. However, reduced growth rates, metabolic disorders and compromised health status have been reported and linked to dietary CHO concentrations(Reference Li, Wang and Xu8,Reference Li, Lu and Liu22) . This study explored the interactive effects of dietary CHO levels and thiamine supplementation on growth performance, feed utilisation, and intestinal mitochondrial biogenesis and function in bream. The findings could shed light on how dietary thiamine might impact CHO metabolism and utilisation by fish, thereby advancing the development of high-energy feed known as ‘the low-pollution diet’ for aquatic species.
Materials and methods
Ethics statement
All procedures were performed in accordance with the guide of the National Institute of Health for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). This study was approved by the Institutional Animal Care and Use Committee of South China Agricultural University.
Experimental diets and the feeding trial
Thiamine was acquired from Kang Yuan Biomedical Company (Wuhan, China). Four diets including two dietary CHO levels (30 and 45 %) and two thiamine doses (0 and 1·5 mg/kg) were formulated (C, CT, HC and HCT). C diets included 30 % CHO, which is considered to be the optimal dietary CHO level for juvenile blunt snout bream according to our previous study(Reference Li, Lu and Liu22); HC diets included 45 % CHO. CT and HCT diets were both supplemented with 1·5 mg/kg thiamine compared with their C and HC base formulation. Maize starch was added as the main CHO source and substituted at the expense of microcrystalline cellulose. The dietary thiamine met the requirement for this species(Reference Li, Wang and Jiang23). All ingredients were first mixed thoroughly and then pelleted by an automatic pellet-making machine (MUZL 180; Jiangsu Muyang Group Co. Ltd). Finally, pellets were air-dried and stored at −20°C in bags until use. Proximate compositions of the diets were analysed using standard procedures(24). The actual thiamine contents of the experimental diets were measured by HPLC. The dietary ingredients and proximate compositions are shown in online Supplementary Table 1.
Juvenile M. amblycephala were acquired from a local fish hatchery at Ezhou (Hubei province, China). This feeding trial was performed using a re-circulating aquaculture system in the laboratory. Prior to the start of the experiment, fish were acclimated to the experimental facilities and were fed a commercial diet (32 % protein, 6 % lipids and 33 % CHO) for 2 weeks. After acclimation, a total of 320 fish (initial weight 24·73 (sem 0·45) g) were randomly allocated to one of sixteen tanks (300 litres). Dietary treatment was assigned to quadruplicate tanks. Fish were hand-fed to apparent satiation thrice daily at 07.30, 11.30 and 16.30 hours for 12 weeks. During this period, water temperature was maintained at 27·4 (sem 0·6)°C, pH was 7·4–7·5 and dissolved oxygen was maintained above 5 mg/l. These parameters were measured with YSI Proplus multiparameter meter (YSI). Photoperiod was kept at 12 h light–12 h dark.
Sample collection
At the beginning of the experiment, four fish were randomly collected for chemical analysis. After 12 weeks, following a 24-h fast, all fish within each tank were counted and weighed. A total of four fish per tank were randomly collected and stored at −20°C for analysis of N concentrations and calculation of N retention efficiency (NRE). An additional four fish from each tank were immediately euthanised with MS-222 at 100 mg/l. Blood was collected from the caudal vein, centrifuged (3000 g , 10 min, 4°C) and kept at −70°C until analysis. Samples from the middle intestine from four fish per tank were collected and fixed in 2·5 % glutaraldehyde for histological analysis. In addition, the whole intestine from the other eight fish per tank was sampled, frozen in liquid N2 and stored at −80°C.
Analysis of plasma parameters and intestinal glycogen and lipid contents
Plasma glucose, lactate, TAG and total cholesterol levels were analysed according to the methods described in the corresponding assay kits (Nanjing Jiancheng Bioengineering Co. Ltd). Plasma pyruvate level was assayed as described by Nigam (1962)(Reference Nigam25). Intestinal glycogen and lipid content were determined following the method detailed by Keppler et al. (1974)(Reference Keppler, Decker and Bergmeyer26) and Folch et al. (1957)(Reference Folch, Lees and Sloane-Stanley27).
Analysis of intestinal histology and enzymatic activities
Transmission electron microscopy (TEM) analysis of the middle intestine was performed by the protocol described(Reference Merrifield, Dimitroglou and Bradley28). Briefly, the samples were fixed with 2·5 % glutaraldehyde and 1 % osmium tetroxide. Then, four tissue blocks were randomly selected from each sample. Tissue blocks were cut into 70 nm thickness and stained with 0·2 % lead citrate. Eight micrographs were obtained from each tissue block with a total of at least thirty-two in each group. Mitochondrial areas were measured with a disk planimeter. The total number of mitochondrial profiles was counted; partial mitochondria were included in the count provided; their average area was >50 % of the wholly included organelles. Intestinal microvilli length (ML) was also photographed and analysed by the ImageJ 1.44p software (National Institutes of Health).
As for the analysis of intestinal enzyme activities, the whole intestine from four fish per tank was homogenised with nine volumes (v/w) of chilled physiological saline and centrifuged at 3500 g at 4°C for 10 min. The soluble protein concentration present in the enzymatic crude extract was determined according to the method of Bradford (1976)(Reference Bradford29), using bovine serum albumin as a standard. Amylase activity was determined by hydrolysis of the starch based on the method of Bernfeld (1955)(Reference Bernfeld and Colowich30). Lipase activity was determined from the hydrolysis of the synthetic substrate p-nitrophenyl myristate, according to Iijima et al. (1998)(Reference Iijima, Tanaka and Ota31) with a modified method of Gjellesvik et al. (1992)(Reference Gjellesvik, Lombardo and Walther32). The alkaline protease activity was determined by the method of Mukhopadhyay et al. (1978)(Reference Mukhopadhyay, Dehadrai and Banerjee33). Intestinal activities of alkaline phosphatase, creatine kinase (CK) and Na+, K+-ATPase were analysed following the method described by Engstad et al. (1992)(Reference Engstad, Robertsen and Frivold34), Weng et al. (2002)(Reference Weng, Chiang and Gong35) and McCormick (1993)(Reference McCormick36), respectively.
Analysis of intestinal adenosine triphosphate and adenosine monophosphate contents
The intestine from four fish per tank was homogenised in a perchloric acid buffer. The homogenate was centrifuged at 10 000 g for 10 min. The supernatant was separated and neutralised with 0·5 volumes of a 2 mol/l potassium hydroxide cocktail. The concentrations of ATP and AMP were measured as described by Bergmeyer (1983)(Reference Bergmeyer37) and Adam (1965)(Reference Adam38), respectively.
Analysis of intestinal reactive oxygen species levels, mitochondrial membrane potential and mitochondrial respiratory chain complex enzyme activities
The intestinal mitochondria isolation was performed using a commercial mitochondria isolation kit (G006, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. Briefly, after the last feeding, four fish per tank were immediately anaesthetised by MS-222 and intestinal samples were collected. Approximately, 2 g of intestinal sample was homogenised in 5 volumes of the mitochondria isolation reagent A. The homogenate was centrifuged at 1500 g for 10 min at 4°C. The sediment was re-suspended in the mitochondrial isolation reagent A containing 1 mg/l of bovine serum albumin. Finally, the mitochondrial suspensions were frozen in liquid N2 and stored at −80°C until assayed.
Intestinal reactive oxygen species (ROS) concentrations were determined by measuring the oxidative conversion of cell permeable 2′, 7′dichlorofluorescein diacetate to fluorescent dichlorofluorescein in a fluorescence microplate (Fluoroskan Ascent FL; Thermo)(Reference Liu, Cui and Brown39). The mitochondrial membrane potential (ΔΨm) was assayed using fluorescent rhodamine 123 dye (Beyotime Institute of Biotechnology), which preferentially localises to active mitochondria based on highly negative ΔΨm(Reference Liu, Cui and Brown39). Mitochondrial protein concentration was assayed as described by Bradford & Dodd (1977)(Reference Bradford and Dodd40). The activities of NADH-ubiquinone oxidoreductase, succinate-ubiquinone oxidoreductase and ubiquinone-ferricytochrome-c oxidoreductase (complexes I–III) were all measured according to the methods detailed by Jeejeebhoy (2002)(Reference Jeejeebhoy41). The activities of cytochrome c oxidase and F1F0-ATP synthase (complex IV) were analysed according to Kirby et al. (2007)(Reference Kirby, Thorburn and Turnbull42).
Analysis of western blot and RT-PCR
Western blot analysis (20 μg of intestine protein) was performed using anti-AMPKα (no. 2532, Cell Signaling Technology), anti-phospho-AMPKα (no. 2535, Cell Signaling Technology), anti-PGC-1α (no. 2178, Cell Signaling Technology), anti-PGC-1β (22378-1-AP; Proteintech) and anti-β-actin (BM3873; Boster) antibodies. These antibodies have all been shown to successfully cross-react with M. amblycephala proteins. The signals of Western blot were quantitatively assayed by ImageJ 1.44p software.
Total RNA extraction and cDNA synthesis in the frozen intestine (about 2 g) were performed as described in our previous study(Reference Xu, Liu and Remø43,Reference Xu, Liu and Zhang44) . The transcriptional levels were determined for the following genes, including AMP-activated protein kinase α 1 and 2 (AMPKα 1 and 2), PPAR-γ coactivator-1α and 1β (PGC-1α and 1β), mitochondrial transcription factor A (TFAM), mitofusin-1, optic atrophy-1 (opa-1), dynamin-related protein-1 (drp-1), fission-1, mitochondrial fission factor (mff); NADH dehydrogenase-1 (ND-1), cytochrome B, cytochrome c oxidase-1 and 2 (COX-1 and 2), sodium/glucose cotransporter-1 (SGLT-1) and GLUT-2. The forward and reverse primers of these genes are shown in online Supplemental Table 2. To select the reference genes with the most stable expression, the relative stability measure (M) of three genes, namely β-actin, glyceraldehyde-3-phosphate dehydrogenase and elongation factor 1α, was calculated by GeNorm (http://medgen.ugent.be/ge norm/). The value M represents an average pairwise variation of a reference gene with all other reference genes. A lower M value corresponds to high expression stability(Reference Tang, Dodd and Lai45). All the M values ranged from 1·2 to 0·6 (online Supplementary Fig. S1), and elongation factor 1α had the most stable expression. The relative transcriptions of these genes were calculated by elongation factor 1α according to the 2−ΔΔCT method(Reference Livak and Schmittgen46).
Analysis of mitochondrial content
Mitochondrial content in the intestine was assayed by the PCR quantitation of mtDNA copies(Reference Song, Samad and Zhou48,Reference Lawn, Efstratiadis and O’Connell49) . The mtDNA copy number was determined by: mtDNA copy number = ND − 1/β-globin(Reference Livak and Schmittgen46). The β-globin gene was selected as a reference since it is an example of a single copy gene of known sequence(Reference Lawn, Efstratiadis and O’Connell49) that conveniently acts as a marker of diploid genome content. However, repetitive nuclear genes (such as elongation factor 1α) may vary in number from one individual to another and thus are not attractive as markers of genome content.
Statistical analysis
All the results in this study were expressed as mean values with their standard errors. The normality of distribution and the homogeneity of variances were both checked prior to two-way ANOVA followed by Tukey’s HSD test (IBM SPSS Statistics 22.0). If significant (P < 0·05), differences were found in the interaction; each factor (namely dietary CHO levels and thiamine dosages) was further analysed using one-way ANOVA followed by Tukey’s HSD test.
Results
Growth performance and feed utilisation
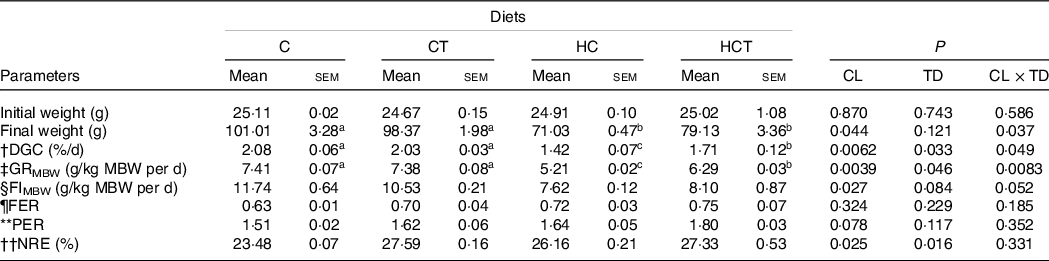
No statistical difference (P > 0·05) was found in either feed efficiency ratio or protein efficiency ratio (Table 1). However, the final weight, daily growth coefficient (DGC), growth rate per metabolic body weight (GRMBW) and feed intake per metabolic body weight (FIMBW) of fish all decreased significantly (P < 0·05) with increasing dietary CHO levels. In terms of thiamine supplementation, the DGC, GRMBW and NRE all increased significantly (P < 0·05) with increasing dietary thiamine levels. In addition, a significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also found in final weight, DGC and GRMBW with the maximised values all obtained in the C group.
Table 1. Growth performance and feed utilisation of blunt snout bream subjected to different dietary treatments
(Mean values with their standard errors)
CL, carbohydrate levels; TD, thiamine dosages.
Mean metabolic body weight (MBW) = [(Wi/1000)0·75 + (Wf/1000)0·75]/2.
† Daily growth coefficient (DGC) = 100 × (Wf1/3–Wi1/3)/D.
‡ Growth rate per metabolic body weight (GRMBW) = (Body average weight gain/MBW)/D.
§ Feed intake per metabolic body weight (FIMBW) = DI/MBW/D.
¶ Feed efficiency ratio (FER) = total weight gain (g)/total feed intake (g).
** Protein efficiency ratio (PER) = fish weight gain (g)/total protein fed (g).
†† Nitrogen retention efficiency (NRE) (%) = [(Wf × Cf)−(Wi × Ci)] × 100/(Cdiet × feed intake). Where Wi and Wf are the initial and final fish average weights (g), respectively. D is the feeding days. DI is the dry feed intake per fish (g/fish) during the feeding period. Ci and Cf are the initial and final contents in whole body, respectively, and Cdiet is the content in the diets.
a,bMean values in the same line with unlike superscript letters were significantly different (P < 0·05).
Plasma and intestinal biochemistry parameters
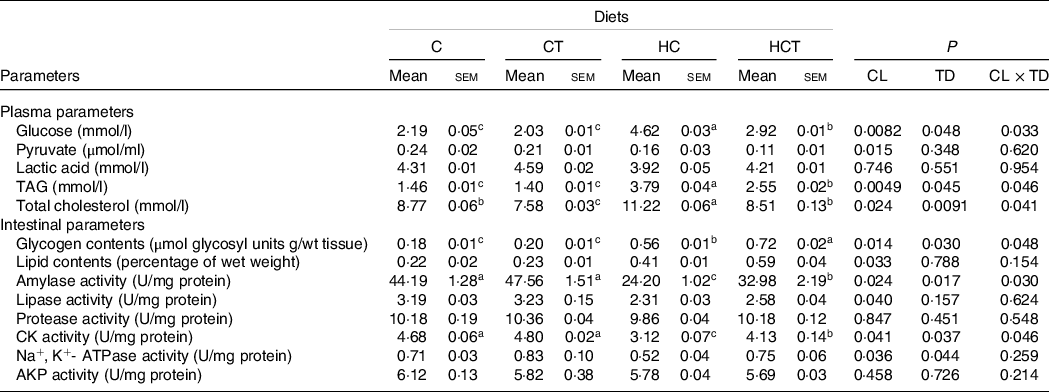
Plasma lactic acid concentrations, intestinal protease and alkaline phosphatase activities all showed no statistical difference (P > 0·05) among treatments (Table 2). However, plasma pyruvate concentrations and intestinal activities of amylase, lipase, Na+, K+-ATPase and CK all decreased significantly (P < 0·05) with increasing dietary CHO levels, whereas the opposite was true for plasma levels of glucose, TAG and total cholesterol as well as the intestinal concentrations of glycogen and lipid. In terms of thiamine supplementation, intestinal amylase, CK and Na+, K+-ATPase activities and glycogen concentrations all increased significantly (P < 0·05) with increasing dietary thiamine levels, while the opposite was true for plasma concentrations of glucose, TAG and total cholesterol. In addition, a significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also found in plasma glucose, TAG and total cholesterol concentrations, intestinal amylase and CK activities as well as the glycogen concentration.
Table 2. Plasma and intestinal parameters of blunt snout bream fed different dietary treatments
(Mean values with their standard errors)
CL, carbohydrate levels; TD, thiamine dosages; CK, creatine kinase; AKP, alkaline phosphatase.
a,b,cValues in the same line with unlike superscript letters were significantly different (P < 0·05).
The transcriptions of glucose absorption-related genes
As can be seen from Fig. 1, the transcription of GLUT-2 increased significantly with increasing dietary CHO levels. As for thiamine supplementation, the transcriptions of SGLT-1 and GLUT-2 both increased significantly (P < 0·05) with increasing dietary thiamine levels. In addition, they were also significantly (P < 0·05) affected by the interaction between dietary CHO levels and thiamine supplementation with the highest values found in the CT and HCT groups, respectively.

Fig. 1. The transcription of glucose absorption-related genes in the intestine of blunt snout bream fed different treatments. The transcriptions of sodium/glucose cotransporter-1 (SGLT-1) (a) and GLUT-2 (b) were both evaluated using real-time RT-PCR. Each data point represents the mean values with their standard error of four replicates (CL, carbohydrate levels; TD, thiamine dosages. Bars assigned with different superscripts were significantly different (P < 0·05). The same below).
Intestinal histological analysis
Mitochondrial area showed no statistical difference (P > 0·05) among treatments (Fig. 2). However, intestinal ML and number of mitochondrial per field both decreased significantly (P < 0·05) with increasing dietary CHO levels. In terms of thiamine supplementation, intestinal ML and number of mitochondrion per field both increased significantly (P < 0·05) with increasing dietary thiamine levels. In addition, a significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also found in the number of mitochondrion per field with the highest value observed in the CT group.
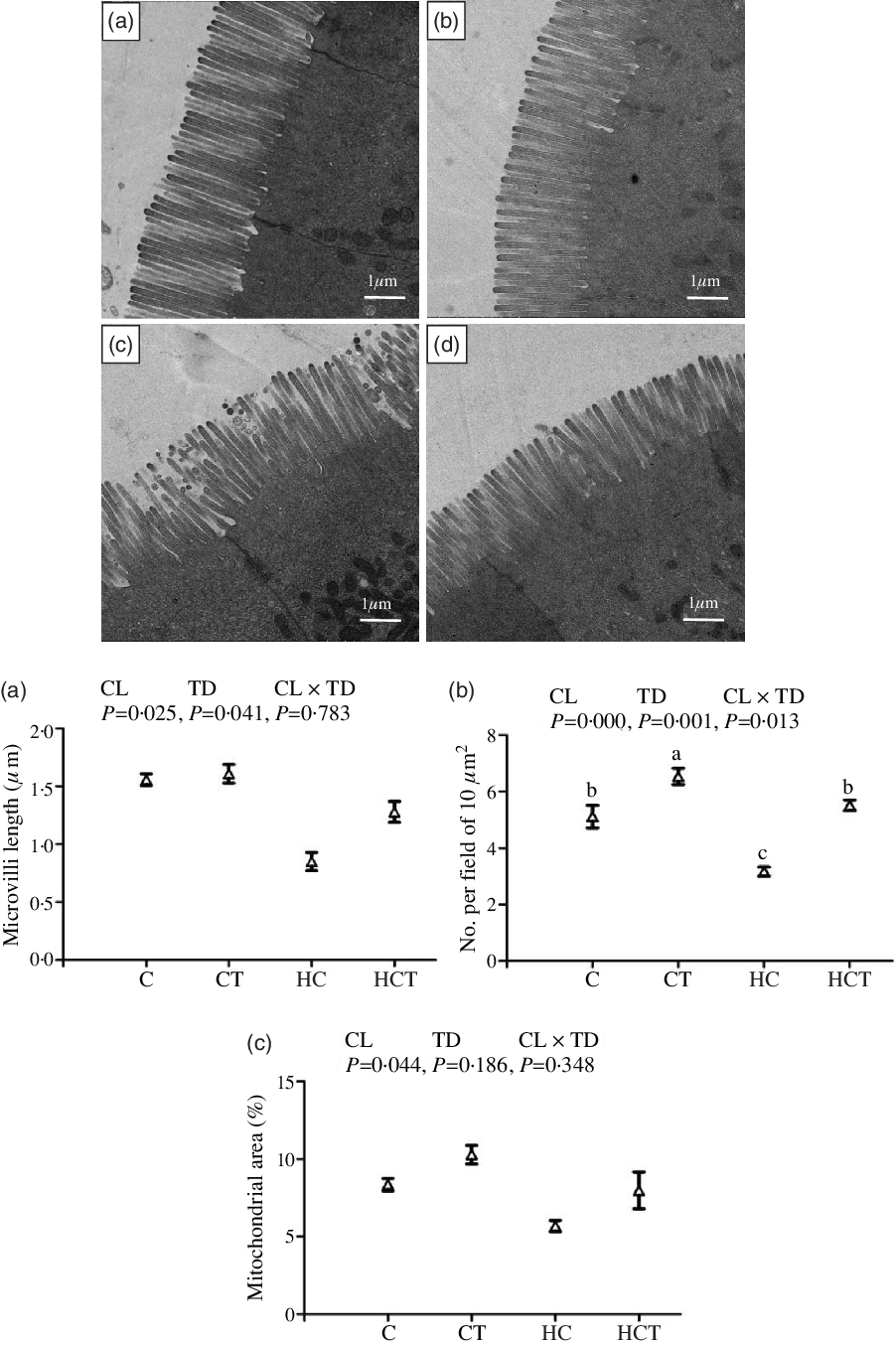
Fig. 2. Comparative micrographs of the transmission electron microscope (TEM) (5000×) of the intestine of Megalobrama amblycephala fed different treatments. A: the control diet; B: the C diet supplemented with 1·5 mg/kg thiamine; C: the high-carbohydrate (HC) diet; D: the HC diet supplemented with 1·5 mg/kg thiamine; a: microvilli length; b: No. per field of 10 μm2; c: mitochondrial area.
Intestinal biochemistry parameters and mitochondrial DNA copies per nucleus
Intestinal ROS, ATP and AMP concentrations all increased significantly (P < 0·05) with increasing dietary CHO levels, while the opposite was true for both ΔΨm, mtDNA copies per nucleus and TFAM (Figs. 3 and 4). In terms of thiamine supplementation, intestinal ΔΨm, mtDNA copies per nucleus, AMP content and the AMP:ATP ratio all significantly (P < 0·05) increased with increasing dietary thiamine levels, whereas the opposite was true for intestinal ROS and ATP concentrations. In addition, a significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also observed in intestinal ROS and ATP concentrations and the AMP:ATP ratio with the lowest value of the AMP:ATP ratio was observed in the HC group.
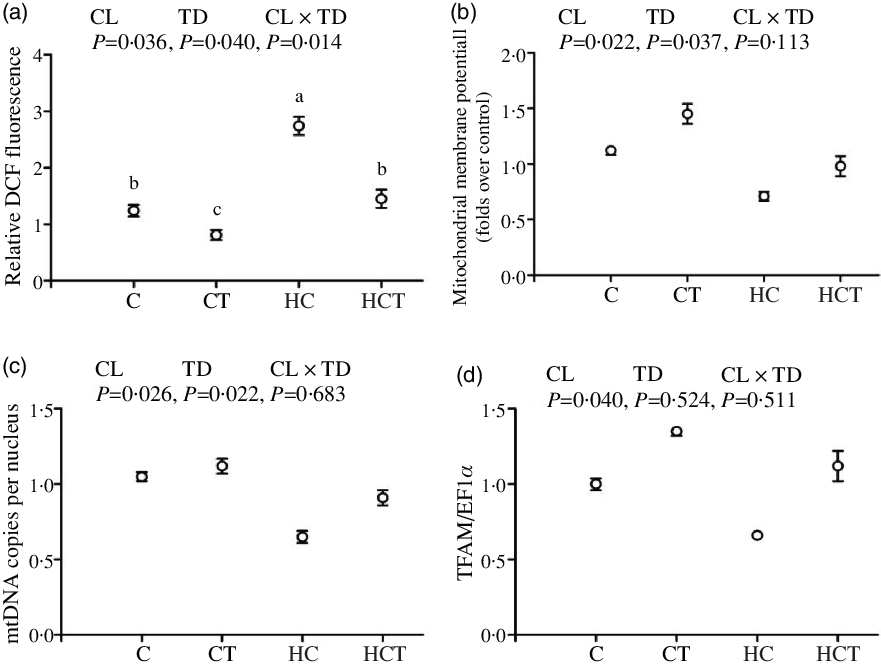
Fig. 3. Intestinal reactive oxygen species (ROS) contents (a), mitochondrial membrane potential (ΔΨm) (b), mitochondrial DNA (mtDNA) copies per nucleus (c) and mitochondrial transcription factor A (TFAM) (d) of Megalobrama amblycephala fed different treatments. Each data point represents the mean values with their standard errors of four replicates.

Fig. 4. Intestinal adenosine triphosphate (ATP) (a) and adenosine monophosphate (AMP) (b) contents and the AMP:ATP ratio (c) of Megalobrama amblycephala fed different treatments. Each data point represents the mean values with their standard errors of four replicates.
Protein concentrations and transcriptions of adenosine monophosphate-activated protein kinase and PPAR γ coactivator-1
Protein concentrations and transcription of PGC-1α both showed no statistical difference (P > 0·05) among treatments (Fig. 5). However, the P-AMPK:T-AMPK ratio, PGC-1β protein concentrations and the transcriptions of AMPKα1, AMPKα2 and PGC-1β all decreased significantly (P < 0·05) with increasing dietary CHO concentrations. In terms of thiamine supplementation, they all increased significantly (P < 0·05) with increasing dietary thiamine levels. A significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also observed in the P-AMPK:T-AMPK ratio, PGC-1β protein contents as well as the transcriptions of AMPKα1, AMPKα2 and PGC-1β with the lowest value in the HC group.

Fig. 5. Intestinal P-adenosine monophosphate-activated protein kinase α (AMPKα):T-AMPKα ratio (a), protein contents of PPAR γ coactivator-1α (PGC-1α) (b) and PGC-1β (c) and the transcriptions of AMPKα1 (d), AMPKα2 (e), PGC-1α (f) and PGC-1β (g) of blunt snout bream fed different treatments. Gels were loaded with 20 mg total protein per lane. Protein contents were normalised to β-actin levels.
Transcriptions of mitochondrial dynamics-related genes
The transcription of mitofusin-1 was not significantly different (P > 0·05) among treatments (Fig. 6). However, transcriptions of Opa-1 decreased significantly (P < 0·05) with increasing dietary CHO concentrations, while the opposite was true for Drp-1, fission-1 and Mff. In terms of thiamine supplementation, transcriptions of Opa-1 increased significantly (P < 0·05) with increasing dietary thiamine levels, while the opposite was true for Drp-1 and Mff. A significant (P < 0·05) interaction between dietary CHO levels and thiamine supplementation was also found in the transcriptions of Opa-1, Drp-1 and Mff, with the lowest value of Opa-1 observed in the HC group.

Fig. 6. Transcription of mitochondrial dynamics-related genes in the intestine of Megalobrama amblycephala fed different treatments. The transcriptions of mitofusin-1 (Mfn-1) (a), optic atrophy-1 (Opa-1) (b), dynamin-related protein-1 (Drp-1) (c), fission-1 (Fis-1) (d) and mitochondrial fission factor (Mff) (e) were all evaluated by real-time RT-PCR. Each data point represents the mean values with their standard errors of four replicates.
Intestinal activities of mitochondrial respiratory chain complexes
The activities of complex II showed no statistical difference (P > 0·05) among treatments (Fig. 7). However, the activities of complexes I, III and IV all decreased significantly (P < 0·05) with increasing dietary CHO concentrations. As for thiamine supplementation, the activities of complexes I and IV both increased significantly (P < 0·05) with increasing dietary thiamine concentrations. Furthermore, the activities of complexes I and IV were both significantly (P < 0·05) affected by the interaction between dietary CHO levels and thiamine supplementation with the highest values found in the CT group.

Fig. 7. Activities of mitochondrial respiratory chain complexes (Complexes I (a), II (b), III (c) and IV (d)) in the intestine of blunt snout bream fed different treatments. Complex I: NADH–ubiquinone oxidoreductase; Complex II: succinate–ubiquinone oxidoreductase; Complex III: ubiquinone–ferricytochrome-c oxidoreductase; Complex IV: cytochrome c oxidase. Each data point represents the mean values with their standard errors of four replicates.
Transcriptions of mitochondrial function-related genes
No statistical difference (P > 0·05) was found in the transcription of cytochrome B among treatments (Fig. 8). However, the transcriptions of ND-1, COX-1 and COX-2 all decreased significantly (P < 0·05) with increasing dietary CHO concentrations. As for thiamine supplementation, the transcriptions of ND-1, COX-1 and COX-2 all increased significantly (P < 0·05) with increasing dietary thiamine concentrations. Moreover, the transcriptions of COX-1 and COX-2 were both significantly (P < 0·05) affected by the interaction between dietary CHO levels and thiamine supplementation with the highest values found in CT and HCT, respectively.

Fig. 8. Transcription of mitochondrial function-related genes in the intestine of blunt snout bream fed different treatments. The transcriptions of NADH dehydrogenase-1 (ND-1) (a), cytochrome B (CYT B) (b) and cytochrome c oxidase-1 and 2 (COX-1 and 2) (c and d) were all evaluated using real-time RT-PCR. Each data point represents the mean values with their standard errors of four replicates.
Discussion
After the 12-week feeding trial, HC intake led to a decrease in final weight, DGC, GRMBW and FIMBW in blunt snout bream, while the opposite was true for both protein efficiency ratio and NRE. CHO-rich diets can cause persistent hyperglycaemia of fish, which might lead to metabolic disorders, thus reducing growth rate(Reference Kamalam, Medale and Panserat3). In addition, high dietary CHO levels can depress appetite in fish, resulting in decreased feed consumption(Reference Hemre, Mommsen and Krogdahl1). As for protein efficiency ratio and NRE, high dietary CHO concentrations can reduce catabolism of protein for energy, thereby improving its retention and utilisation efficiency (protein-sparing effect)(Reference Sanchez-Muros, Garcia-Rejon and Lupianez50). In this study, thiamine supplementation increased final weight, DGC, GRMBW, FIMBW, protein efficiency ratio and NRE in blunt snout bream. These results might be due to the following facts: (1) dietary thiamine improves the digestive and absorptive capabilities of the intestine and might correspondingly promote CHO utilisation by fish, thereby increasing the growth(Reference Huang, Feng and Liu21) and (2) thiamine could activate hypothalamic AMPK and consequently up-regulate the transcriptions of neuropeptide Y, leading to increased appetite and feed consumption(Reference Liu, Alimov and Wang51).
In this study, high dietary CHO levels increased plasma levels of glucose, TAG and total cholesterol, and the intestinal concentrations of glycogen and lipid, while the opposite was true for pyruvate and lactic acid concentrations. The results were in line with previous studies, in which the long-term intake of CHO-enriched diets led to elevated plasma glucose levels and enhanced glycogenesis and lipogenesis pathways of fish, thus resulting in the accumulated glycogen and lipid contents in both plasma and tissues(Reference Li, Lu and Liu22,Reference Xu, Liu and Zhang44) . As for pyruvate and lactic acid levels, high dietary CHO could enhance the glycolysis pathway and tricarboxylic acid cycle of the organism(Reference Hemre, Mommsen and Krogdahl1,Reference Zakim, Pardini and Herman52) . These pathways need pyruvate for normal functioning, as might consequently reduce the plasma pyruvate and lactic acid levels in the HC group. In addition, thiamine supplementation decreased plasma glucose, TAG, total cholesterol and pyruvate levels, but increased intestinal glycogen and lipid contents. A previous study suggested that thiamine could promote the pentose phosphate pathway of animals and enhance fatty acid synthesis, thus leading to increased tissue lipid accumulation(Reference Babaei-Jadidi, Karachalias and Kupich53). Meanwhile, thiamine could also promote insulin synthesis, thus decreasing plasma glucose levels(Reference Bakker, Hoogeveen and Nijpels54). The increased insulin levels could result in the dephosphorylation of both GPase and GSase by inhibiting adenylyl cyclase activity and decreasing the intracellular cAMP formations, thus inhibiting glycogen breakdown, and increasing tissues glycogen contents(Reference Moon55).
The ability of fish to utilise ingested nutrients is closely related to intestinal enzymes activities and morphology(Reference Zhou, Zhao and Lin56). In this study, high dietary CHO levels resulted in relatively low intestinal activities of amylase, lipase, protease, CK, Na+, K+-ATPase and alkaline phosphatase, and ML, indicating a decreased digestive and absorptive function of the intestine in fish fed the HC diets. This could be supported by the following facts that: (1) amylase, lipase and protease are three key enzymes involved in the digestion of CHO, lipid and protein, respectively(Reference Suzer, Çoban and Kamaci57); (2) the absorption and transportation of nutrients in piscine intestines are positively correlated with the activities of gut basolateral enzymes like alkaline phosphatase and Na+, K+-ATPase(Reference Tengjaroenkul, Smith and Caceci58); (3) CK directly participates in ATP metabolism, thereby providing energy needed for the absorption of nutrients(Reference Wallimann and Hemmer59) and (4) intestinal ML positively reflects the intestinal function of animals(Reference Zhou, Zhao and Lin56). According to a previous study, the intake of high-energy diets usually induces an overload of the intestine(Reference Harpaz, Hakim and Barki60), which could negatively affect the intestinal health of fish, thus reducing intestinal enzyme activities and ML. In addition, the decreased enzymatic activities might also be ascribed to the mitochondrial dysfunction in the intestine of fish due to HC feeding. In fact, excessive CHO intake could result in mitochondrial damage by inducing the over-production of ROS, thus reducing the activities of mitochondrial respiratory chain complexes and ATP production(Reference Garcia-Berumen, Alejandre-Buitron and Montoya-Perez61). This could aggravate the intestinal injuries induced by HC feeding, thus resulting in decreased intestinal enzymatic activities and ML. In this study, the intake of HC diets up-regulated the transcriptions of both SGLT-1 and GLUT-2, indicating the enhanced intestinal glucose absorption of fish due to excessive CHO consumption. This was explained by the fact that intestinal glucose is actively transported by SGLT-1 and passively by GLUT-2(Reference Roder, Geillinger and Zietek62). As for thiamine supplementation, the activities of amylase, lipase, protease, CK and Na+, K+-ATPase and ML all increased with increasing dietary thiamine contents. This might be due to the fact that thiamine can promote intestinal growth and development of fish(Reference Huang, Feng and Liu21), thus enhancing intestinal enzymatic activities and ML. In addition, thiamine supplementation has also been demonstrated to increase the activities of mitochondrial enzyme complexes, such as α-ketoglutarate and branched-chain ketoacid dehydrogenases(Reference Depeint, Bruce and Shangari18). This could accelerate the oxidative phosphorylation of glucose to generate ATP and promote intestinal self-renewal, thus resulting in increased intestinal enzymatic activities and ML. Furthermore, the transcriptions of SGLT-1 and GLUT-2 both increased with increasing dietary thiamine contents, suggesting a beneficial effect of thiamine on intestinal glucose absorption in fish.
In this study, HC intake resulted in increased intestinal ROS concentrations, while the opposite was true for the number of mitochondrion per field, ΔΨm and mtDNA copies per nucleus, partially indicating a decreased mitochondrial concentration caused by excessive CHO consumption. Generally, high glucose can induce over-production of superoxide radical O2−1, resulting in mitochondrial damages(Reference Chen, Huang and Li63,Reference Al-Kafaji, Sabry and Skrypnyk64) . This might correspondingly decrease the mitochondrial contents and mtDNA copy numbers in the intestine. This was further supported by the fact that the loss of ΔΨm is an established indicator of mitochondrial damage in animals(Reference Kroemer65). As for thiamine supplementation, the number of mitochondrion per field, ΔΨm and mtDNA copies per nucleus all increased significantly, whereas the opposite was true for ROS concentrations. According to previous studies, thiamine activates AMPK, thereby enhancing mitochondrial function by modulating the gene networks controlling mitochondrial biogenesis(Reference Liu, Alimov and Wang51,Reference Miranda, Tovar and Palacios66) . In addition, the activated AMPK can also enhance anti-oxidative enzyme activities by activating the NF-E2-related factor 2-mediated antioxidant pathway, thus facilitating the clearance of ROS(Reference Xu, Liu and Remø43). This could further promote the mitochondrial biogenesis and function.
ATP is the primary energy currency of all living organisms. Therefore, its content could directly reflect the energy status of animals(Reference Nesci, Ventrella and Trombetti67). In this study, intestinal ATP and AMP contents both increased significantly with increasing dietary CHO levels, while the opposite was true for the AMP:ATP ratio. This result is justifiable since CHO-enriched diets can lead to increased energy state of organisms, which is reflected by increased ATP content. Then, the increased ATP can be hydrolysed to AMP, thereby leading to increased AMP content. Thiamine supplementation significantly increased intestinal AMP content and the AMP:ATP ratio, but the opposite was true for ATP content. This was also justifiable since thiamine can promote ATP hydrolysis by the following reaction: thiamine + ATP → thiamine diphosphate + AMP, leading to an increase in the AMP:ATP ratio(Reference Depeint, Bruce and Shangari18).
There is an increasing body of information, indicating that mitochondrial fusion and fission processes are closely related to mitochondrial biogenesis and function(Reference Marín-García and Akhmedov68,Reference Schmid and Frolov69) . In this respect, the AMPK/PGC-1 pathway plays an important role, since once activated it can promote mitochondrial biogenesis in animals(Reference Leick, Fentz and Biensø12). In the present study, high dietary CHO levels significantly decreased the P-AMPK:T-AMPK ratio, PGC-1β protein content and the transcriptions of AMPKα1, AMPKα2, PGC-1β, TFAM and Opa-1, while the opposite was true for the transcriptions of Drp-1, fission-1 and Mff. This indicates that HC diets increase mitochondrial fission in the intestine of blunt snout bream, but decrease mitochondrial fusion and mitochondrial biogenesis. The decreased P-AMPK:T-AMPK ratio, PGC-1β protein content and the transcriptions of AMPKα1, AMPKα2 and PGC-1β could be attributed to the decreased AMP:ATP ratio due to HC intake, since high AMP:ATP ratio could activate AMPK, thereby regulating the expression of its target proteins(Reference Rutter and Leclerc70). In addition, HC intake induces mitochondrial oxidative stress by promoting the over-production of superoxide radical O2−1, thus accelerating mitochondrial fission characterised by the up-regulation of fission-related genes including Drp-1, fission-1 and Mff(Reference Zhuang, Maimaitijiang and Li71). As for thiamine supplementation, the P-AMPK:T-AMPK ratio, PGC-1β protein expression, as well as the transcriptions of AMPKα1, AMPKα2, PGC-1β, TFAM and Opa-1 all increased with increasing dietary thiamine levels, but the opposite was true for the transcription of fission-related genes. This was due to thiamine accelerating ATP hydrolysis to elevate the AMP:ATP ratio, thereby activating AMPK(Reference Depeint, Bruce and Shangari18). Once activated, AMPK promotes mitochondrial biogenesis by activating PGC-1, which regulates the replication of mtDNA and the transcriptions of mitochondrial fusion-related genes (such as TFAM and Opa-1)(Reference Wu, Puigserver and Andersson72). In addition, activated AMPK enhances the cellular defence against oxidative stress by increasing the activity of the NF-E2-related factor 2-mediated antioxidant pathway(Reference Xu, Liu and Remø43). This could inhibit high glucose-induced mitochondrial fission.
In the present study, HC levels down-regulated the activities of complexes I, III and IV, suggesting a decreased mitochondrial oxidative phosphorylation capability of the intestine. Generally, the intake of HC diets induces oxidative stress of fish and could cause mitochondrial damages, thus reducing the activities of mitochondrial respiratory chain complexes(Reference Lin, Xue and Sha73). In addition, dietary thiamine supplementation increased the activities of complexes I–IV, suggesting a beneficial effect of it on the intestinal mitochondrial function of fish. According to a previous study, thiamine protects mitochondria from oxidative stress though the clearance of free radicals, and the inhibition of ROS over-production, thereby enhancing the activities of mitochondrial respiratory chain complexes in the intestine(Reference Zhou, Sun and Xing74). Another possible explanation might be that thiamine is the important cofactor of mitochondrial enzyme complexes(Reference Depeint, Bruce and Shangari18). Subsequently, we investigated the transcriptions of related genes to further assess the intestinal mitochondrial function. The transcriptions of ND-1, COX-1 and COX-2 all decreased significantly with increasing dietary CHO levels and were in line with the activities of mitochondrial complexes I, III and IV, respectively. The results further indicated that HC diets can impair intestinal mitochondrial function of M. amblycephala. According to a previous study, the decreased expressions of ND-1, COX-1 and COX-2 negatively influence the assembly of complexes I, III and IV, which in turn decreases the mitochondrial oxidative phosphorylation capability(Reference Eya, Ashame and Pomeroy75). Generally, excessive CHO intake accelerates mitochondrial fission by inducing the over-production of ROS, thus down-regulating the expression of mitochondrial function-related genes(Reference Al-Kafaji, Sabry and Skrypnyk64). As for thiamine supplementation, the transcriptions of ND-1, COX-1 and COX-2 all increased significantly with increasing dietary thiamine levels, reinforcing that thiamine can enhance intestinal mitochondrial function of fish.
Conclusions
Dietary thiamine supplementation can promote growth performance and intestinal mitochondrial biogenesis and function of blunt snout bream fed CHO-enriched diets. This might be achieved through the improved intestinal digestive and absorptive functions, the activation of the AMPK/PGC-1 axis, the up-regulation of the fusion-related genes, and the enhanced activities and transcriptions of mitochondrial enzyme complexes.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (32002361 and 31872576), the National Technology System of Conventional Freshwater Fish Industries of China (CARS-45-14) and the National Key R&D Program of China (2018YFD0900400).
C. X., X.-F. L., Y.-Y. L. and D.-Z. X. conceived and designed the experiments. C. X. analysed the data. C. X., L.-L. G. and Z.-R. D. performed the experiments and contributed reagents, materials and analysis tools. C. X., X.-F. L. and P. B. wrote the paper. All authors contributed to the revision of the manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452100101X












