Vitamin D deficiency and insufficiency are worldwide problems. Among the vulnerable groups are pregnant and lactating women and their exclusively breastfed infants( Reference Brender, Burke and Glass 1 , Reference Saraf, Morton and Camargo 2 ). Low vitamin D status is associated with a wide range of risk factors/diseases in both pregnant women and their infants. Some of these associations have not been consistently found( Reference Camargo, Ingham and Wickens 3 ). Although recently more trials and meta-analyses have been published, the clinical relevance of higher serum 25-hydroxyvitamin D (25(OH)D) during pregnancy and/or supplementing pregnant women with vitamin D is still unclear( Reference De-Regil, Palacios and Lombardo 4 , Reference Roth, Leung and Mesfin 5 ). There is increasing evidence that adequate vitamin D status may reduce the risk of pre-eclampsia( Reference De-Regil, Palacios and Lombardo 4 , Reference Kiely, Zhang and Kinsella 6 ), low birth weight( Reference Aghajafari, Nagulesapillai and Ronksley 7 , Reference Miliku, Vinkhuyzen and Blanken 8 ) and preterm birth( Reference Aghajafari, Nagulesapillai and Ronksley 7 , Reference Wagner, Baggerly and McDonnell 9 ) and increase newborn length and head circumference( Reference De-Regil, Palacios and Lombardo 4 , Reference Wagner, Hollis and Kotsa 10 ).
Plasma 25(OH)D is the widely accepted vitamin D status parameter. Commonly employed cut-off values are <25–30 nmol/l for vitamin D deficiency, 25 or 30–50 nmol/l for vitamin D insufficiency and >50 nmol/l for vitamin D sufficiency( 11 , Reference Vinkhuyzen, Eyles and Burne 12 ). Many vitamin D experts consider 25(OH)D levels between 50 and 75 or 80 nmol/l as hypovitaminosis D and between 75 or 80 and 250 nmol/l as vitamin D sufficiency( Reference Dawson-Hughes, Heaney and Holick 13 – Reference Vieth 15 ). Dependent on definition, it is estimated that the worldwide prevalence of vitamin D deficiency and insufficiency during pregnancy ranges from 8 to 100 %( Reference Hossein-nezhad and Holick 16 ). The RDA for pregnant and lactating women is identical to that of non-pregnant adults up to 70 years. They range from 10 µg/d (Health Council of the Netherlands( 17 )) to 15 µg/d (Institute of Medicine (IOM)( 11 )). However, the median dietary vitamin D intake of Dutch women of 19–30 years is only 2·6 µg/d( 18 ). Since it is difficult to reach the RDA from unfortified dietary sources and because of the consequences for both mother and infant, the Health Council of the Netherlands advices pregnant women to take a 10 µg/d vitamin D supplement, starting preferably before conception( 17 ).
The current RDA of 10–15 µg might be insufficient to reach 50 nmol/l 25(OH)D in 97·5 % of the women by the end of pregnancy. This can be concluded from two recent randomised controlled trials (RCT) conducted in pregnant women in New Zealand( Reference Grant, Stewart and Scragg 19 ) and in Canada( Reference March, Chen and Karakochuk 20 ), with median baselines of 55 and 64–68 nmol/l 25(OH)D, respectively, at enrolment in the second trimester. The RCT used 2000 IU (50 µg), 1000 IU (25 µg) or 0 IU (placebo) supplemental vitamin D/d (New Zealand), or 2000 IU (50 µg), 1000 IU (25 µg) or 400 IU (10 µg) vitamin D/d (Canada). In all, 11, 9 and 50 %, respectively, of the women in New Zealand and 3, 12 and 7 % (analysis ‘as treated’) of the Canadian women exhibited vitamin D insufficiency (25(OH)D<50 nmol/l) at 36 GW. Thus, it seems that, dependent on baseline 25(OH)D and compliance, a vitamin D supplement of about 50 µg/d may be appropriate to reach 50 nmol/l 25(OH)D in about 97·5 % of women by the end of pregnancy.
Postnatal infant vitamin D sources include stores, exposure to sunlight and breast milk or formula. Early measurements of breast milk vitamin D conducted in the 80s showed that breast milk contains both the parent vitamin D and 25(OH)D, which are usually summed to the so-called antirachitic activity (ARA, in IU/l)( Reference Hollis, Roos and Draper 21 , Reference Reeve, Chesney and DeLuca 22 ). The IOM vitamin D adequate intake (AI) by infants of 0–6 months amounts to 10 µg/d( 11 ). This AI is based on some studies in Western countries, showing that 10 µg/d maintains infant serum 25(OH)D at 40–50 nmol/l in the first postnatal year and thereby supports normal bone accretion( 11 ). A 10 µg/d intake translates to a milk ARA of 513 IU/l at an average mature milk consumption of 780 ml/d( Reference Neville, Keller and Seacat 23 ). However, breast milk ARA of Western mothers ranges from 8 to 331 IU/l( Reference Dawodu and Tsang 24 – Reference við Streym, Hojskov and Moller 26 ). Even mature milk ARA of women with high vitamin D status from year-long abundant sunlight exposure does not reach the IOM AI( Reference Stoutjesdijk, Schaafsma and Nhien 27 ). Currently, in most Western countries it is recommended to supplement breastfed infants with 10 µg vitamin D/d( 17 , Reference Braegger, Campoy and Colomb 28 , Reference Wagner and Greer 29 ).
It is at present unclear what maternal vitamin D dose is needed to reach the IOM AI of 513 IU/l in milk. In the aforementioned New Zealand trial, published during the course of our study, Wall et al. ( Reference Wall, Stewart and Camargo 30 ) showed that 2000 IU (50 µg) vitamin D, administered during both pregnancy and lactation, results in a milk ARA of 64 (23–197) IU/l at 2 weeks PP. The only study successful in reaching the IOM AI was published by Wagner et al. ( Reference Wagner, Hulsey and Fanning 31 ). They showed that supplementation of lactating women with 6400 IU (160 µg) vitamin D/d for 6 months increases milk ARA to 873 IU/l at the study end. The corresponding mean plasma 25(OH)D of their exclusively breastfed infants was about 113 nmol/l and thereby similar to that of the offspring of unsupplemented mothers receiving 7·5 µg vitamin D/d from 1 month PP. This was confirmed in a large RCT in which a maternal intake of 6400 IU supplied the nursing infant with sufficient vitamin D to mimic the 25(OH)D concentrations to counterparts receiving a daily 400 IU oral vitamin D supplement( Reference Hollis, Wagner and Howard 32 ). However, although perfectly safe, with no adverse effects observed, a daily 160 µg vitamin D dose is well above the current upper limit of 100 µg/d( 33 ).
A strategy merely aiming at the postnatal period provides no benefits for the mother and her developing child during pregnancy. Vitamin D supplementation during pregnancy is likely to support the building of fetal vitamin D stores. Notwithstanding low milk ARA, we observed that exclusively breastfed infants of unsupplemented African mothers with lifetime abundant sunlight exposure have plasma 25(OH)D above 50 nmol/l. This suggested mobilisation of vitamin D from infant stores, since major sources from sunlight exposure and other vitamin D sources were unlikely at that stage( Reference Stoutjesdijk, Schaafsma and Nhien 27 , Reference Mbugua, Ogeda and Muthui 34 , Reference Scheper-Hughes 35 ). As the primary aim of our ‘ZOOG-MUM’ trial, we investigated what maternal ‘parent vitamin D’ (the sum of cholecalciferol and ergocalciferol) and 25(OH)D concentrations are needed to reach plasma 25(OH)D of 80 nmol/l by supplementing pregnant Dutch women with various vitamin D3 dosages from 20 GW up to 4 weeks PP. We were notably interested to see the corresponding milk ARA that will be reached.
Methods
Study design
This was a randomised trial called ZOOG MUM conducted in Groningen, the Netherlands. The study design has previously been detailed( Reference Stoutjesdijk, Schaafsma and Dijck-Brouwer 36 ). In brief, healthy pregnant women received increasing doses of vitamin D3, together with a multi-vitamin and increasing doses of fish oil from 20 GW until 4 weeks PP. The Ethics Committee of the University Medical Center Groningen (UMCG) (METc number 2014·263) approved the study. The study was registered in The Netherlands National Trial Register (Trial ID NTR4959). All women provided written informed consent. The study was in agreement with the Helsinki Declaration of 1975 as revised in 2013.
Power analysis
In the study of Grootheest et al. ( Reference van Grootheest, Milaneschi and Lips 37 ), non-pregnant adults in the Netherlands had 68·0 (sd 27·2) nmol/l plasma 25(OH)D. Vieth( Reference Vieth 15 ) and Wagner et al. ( Reference Wagner, Hulsey and Fanning 31 ) showed that 1 µg oral vitamin D3/d increases plasma 25(OH)D with 0·4–1·0 nmol/l. Based on these data, we expected a 23–75 nmol 25(OH)D/l difference between the lowest and highest supplemental groups. Earlier studies by Luxwolda et al. ( Reference Luxwolda, Kuipers and Kema 38 ) in Tanzania showed that non-pregnant adults had a 25(OH)D of 106·8 (sd 28·4)nmol/l. Combining the data of Grootheest et al. and Luxwolda et al., we performed a power analysis showing that differences in 25(OH)D between two groups of six subjects with 25(OH)D of 68·0 nmol/l (Grootheest et al.) and 107 nmol/l (Luxwolda et al.), respectively, should be detectable with 80 % power at P<0·05. We expected the dropout percentages to be <52 %, as observed in a previous study by Van Goor et al. ( Reference van Goor, Dijck-Brouwer and Hadders-Algra 39 ). We consequently aimed at an inclusion of 10–11 women per group.
Study population
From December 2014 until December 2015, forty-three apparently healthy women in the first trimester of a singleton pregnancy, all living in the Netherlands, agreed to participate in the trial. Their recruitment took place at six obstetric practices in the provinces of Groningen, Drenthe and Friesland (the Netherlands) via leaflets spread by ‘Moeders voor Moeders’ (translation: ‘Mothers for Mothers’) and posters in the city of Groningen. The women were randomly allocated to four groups (Fig. 1) using block randomisation. The participants were aware of the composition and the nutrient dosages in the multivitamins, fish oil capsules and vitamin D3 capsules (see ‘Supplements’ below). Exclusion criteria were as follows: hyperemesis gravidarum, vegetarian/vegan diet, not having the intention to exclusively breastfeed after delivery, pre-pregnancy BMI >29 kg/m2 and pregnancy complications or preterm delivery after inclusion.
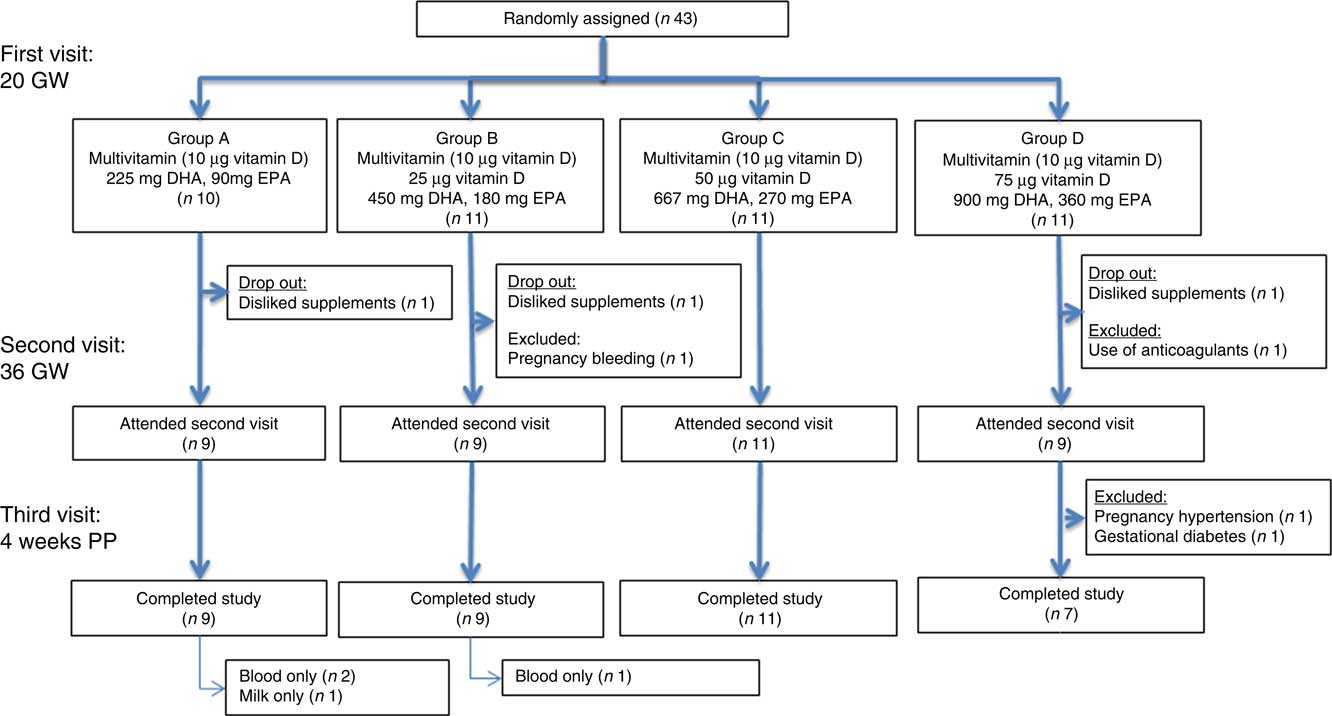
Fig. 1 Flow chart of the initially forty-three participating women. Pregnant women were supplemented from 20 gestational weeks (GW) to 4 weeks postpartum (PP). Blood samples were taken at 20 and 36 GW and at 4 weeks PP. A milk sample was taken at 4 weeks PP.
Supplements
The supplements, daily dosages and the number of capsules and tablets taken by women in the four dosage groups are shown in Table 1. All participants received a multivitamin supplement (Omega Pharma) providing 10 µg vitamin D3 and 12–125 % of the Dutch RDA/AI for vitamins and minerals for pregnant and lactating women. In addition, the participants took 0, 25, 50 and 75 µg vitamin D3 and 225+90, 450+180, 675+270 and 900+360 mg EPA and DHA in groups A, B, C and D, respectively. Taken together, we chose the daily total dosages of vitamin D3 of 10, 35, 60 and 85 µg. These are in between the vitamin D recommendation of 10 µg/d( 17 , Reference Braegger, Campoy and Colomb 28 , Reference Wagner and Greer 29 ) and the current upper limit of 100 µg/d( 33 ). The vitamin D3 and fish oil supplements were supplied by Bonusan. All mothers, allocated to groups A, B, C and D reported adherence to the protocol. They took >75 % of the supplements, as recorded by inquiry at appointment, by questionnaire or both.
Table 1 Dose of daily supplements per group

Sample collection and storage
Information on maternal and infant characteristics, socioeconomic status, supplement use and sunlight exposure was gathered by questionnaires at 20 GW and/or 4 weeks PP. We collected 24 h urine samples and non-fasting venous EDTA–blood and lithium-heparin-anticoagulated blood at the study start, 20 GW, 36 GW and 4 weeks PP. A milk sample was collected at 4 weeks PP. Blood samples were processed to plasma by centrifugation and stored at –20°C until analysis. The 24 h urine volume was measured and a sample was stored at –20°C until analysis. The participants were instructed to collect the full amount of breast milk from a single breast around noon (10·00–14·00 hours) on the day before, or on the day of, blood sampling, using a standardised protocol. The milk was collected either manually or using a breast milk pump. To ensure homogenisation, they were carefully swerved and subsequently transferred to two sampling tubes. The milk samples were stored in the participants’ freezer. On the sampling day, they were transported to the UMCG in a ‘cool transport container’ for frozen specimens (Sarstedt; mailing containers). Upon arrival, they were immediately stored at –20°C until analysis.
Analyses
The milk vitamin D profile (vitamin D3, vitamin D2, 25(OH)D3 and 25(OH)D2) was analysed by liquid chromatography–tandem MS (LC–MS/MS). The method includes saponification( Reference Corso, Rossi and De 40 ) and derivatisation with 4-(2-(3,4-dihydro-6,7-dimethoxy-4-methyl-3-oxo-2-quinoxalinyl)ethyl)-3H-1,2,4-triazole-3,5(4H)-dione( Reference Kamao, Tsugawa and Suhara 41 ). The inter- and intra-assay CV at 1·7–34·8 nmol/l were <15 and <10 %, respectively, for all four analytes. The quantification limit was 0·1 nmol/l for vitamin D and 0·2 nmol/l for 25(OH)D. Milk and EDTA plasma vitamin D3 and vitamin D2 were summed to vitamin D, and 25(OH)D3 and its 25(OH)D2 analogue were combined to 25(OH)D. For milk ARA calculation, we assumed that 1 IU/l equals 25 pg/ml vitamin D and 5 pg/ml 25(OH)D. EDTA plasma 25(OH)D was measured with isotope dilution online solid phase extraction LC–MS/MS, as described by Dirks et al. ( Reference Dirks, Vesper and van Herwaarden 42 ) EDTA plasma vitamin D was analysed using a modification of this method. These included the use of an additional derivatisation with 4-phenyl-1,2,4-triazoline-3,5-dione and the employment of a Supelco Ascentis Express F5; 2·7 µm; 2·1×50 mm column. The inter-assay and intra-assay CV for 25(OH)D were <15 and <10 % at 25–150 nmol/l, and <15 and <10 % for vitamin D at 11–57 nmol/l, respectively. The limits of quantification were 4·0 and 4·4 nmol/l for 25(OH)D and vitamin D, respectively. Plasma (lithium heparin) Ca and phosphate and urine Ca and creatinine were analysed with validated automatic routine laboratory methods (Roche Modular).
Employed cut-off values for vitamin D status
Cut-off values of 25, 50, 80 and 250 nmol/l 25(OH)D were employed for vitamin D deficiency (<25 nmol/l), vitamin D insufficiency (25–49 nmol/l), hypovitaminosis D (50–79 nmol/l), vitamin D sufficiency (80–249 nmol/l) and potential vitamin D toxicity (>250 nmol/l). We chose 25 over 30 nmol/l as this is a widely used cut-off for vitamin D deficiency( Reference Vinkhuyzen, Eyles and Burne 12 , Reference Zittermann 14 , Reference Basha, Rao and Han 43 ). We chose 80, rather than 50 nmol/l 25(OH)D since, based on lowest parathyroid hormone (PTH) and osteoporosis fractures, 25(OH)D>80 nmol/l is considered optimal by vitamin D experts( Reference Zittermann 14 , Reference Heaney 44 ).
Data analysis and statistics
The IBM PASW Statistics 22 and STATA, version 12 software were used. Since not all data were Gaussian distributed, we report medians and ranges. Total between-group differences were analysed with the Kruskal–Wallis test for continuous data and χ 2 test for nominal data. Between-group differences were analysed by Kruskal–Wallis pairwise comparison with post hoc Bonferroni correction for continuous data. A P value <0·05 was considered significant. Between time point differences were analysed using a Wilcoxon signed-rank test. Following Bonferroni correction, a P value <0·0167 was considered significant. The association between plasma 25(OH)D and other parameters was evaluated via generalised estimating equations (GEE) in a repeated and stepwise fashion. First, plasma 25(OH)D results at the various visits were subtracted from the result of the first visit (20 GW). These changes were analysed for normality using the Shapiro–Wilk test. Subsequently, the GEE model was constructed in which 25(OH)D changes were associated with the following independent parameters: visit, visit2 (to analyse a potential parabolic relationship in time), group (depending on the dosing of vitamin D3), month and month2 (to acknowledge the non-linear fit by month on the plasma 25(OH)D) concentration, baseline plasma 25(OH)D and also potential confounding factors being age, year, BMI, BMI at delivery, sex, birth weight, pregnancy duration (d), lactation duration (d), estimated time spent outside in direct sunlight during weekdays and time spent outside in direct sunlight during the weekend. A check was performed on the relationship between baseline 25(OH)D and month of year to avoid a potential interaction between these parameters. No such relationship was observed with the current data set (P>0·25). A P value <0·05 was considered significant.
Results
The flow chart of the included women is shown in Fig. 1. Of the forty-three included women, thirty-eight completed the study. In all, two were excluded due to late pregnancy complications. Of the remaining thirty-six, three discontinued breast-feeding before 4 weeks PP. Only one mother provided us with a breast milk sample but not with other samples at 4 weeks PP. Plasma parent vitamin D concentration below the limits of quantification were evaluated as such. Assigning these to zero did not alter our conclusions.
Baseline characteristics
Table 2 shows the characteristics of the investigated mothers and their infants. The median maternal age of the women at study start was 31 (range 21–38) years and their pre-pregnancy BMI was 24 (range 18–29) kg/m2. Most women had a high socio-economic status: 80 % went to college or university. Before the study start, twenty-six women (72 %) took a multivitamin, containing vitamin D3 or vitamin D3 supplements, containing 5–20 µg of vitamin D3/d. The women estimated to spend 1 h outside on a weekday, and 2 h on a weekend day, depending on the weather. We found no differences between 20 GW and 4 weeks PP (data not shown). A total of thirty-four women used a sunscreen with sun protection factor (SPF) 30.
Table 2 Basic characteristics of the investigated mothers and their infants, who completed the study (Medians and ranges; numbers and percentages)

* The total between-group differences were analysed with the Kruskal–Wallis test for continuous data and the χ 2 test for nominal data. A P value of 0·05 was considered significant.
† Missing data from five infants.
Maternal 25-hydroxyvitamin D concentration
Fig. 2 shows the dose–response curve for plasma 25(OH)D. Online Supplementary Table S1 contains the corresponding data. A median 25(OH)D of 85 (range 25–131) nmol/l was found for all women at 20 GW. There were no between-group differences (P=0·374). At 36 GW, 25(OH)D had increased in group C (P<0·017), while we noticed a trend in the others. The medians for 25(OH)D were 82 (range 55–143) nmol/l in group A, 111 (range 94–130) nmol/l in group B, 120 (range 88–157) nmol/l in group C and 118 (range 51–148) nmol/l in group D. There were between-group differences (P<0·050). Compared with 36 GW, 25(OH)D at 4 weeks PP had decreased in groups A, B and C (P<0·017), while there was a trend in group D. The medians were 65 (range 43–96) nmol/l in group A, 86 (range 76–105) nmol/l in group B, 84 (range 72–127) nmol/l in group C and 99 (range 52–122) nmol/l in group D. There were between-group differences (P<0·050). We did not observe 25(OH)D values of 250 nmol/l or above at any time. One woman assigned to the highest supplemental dose (group D; 85 µg vitamin D3/d) exhibited 25(OH)D concentrations of 61, 51 and 52 nmol/l at 20 GW, 36 GW and 4 week PP, respectively. She was also assigned to the highest fish oil intake, to which she also did not react.
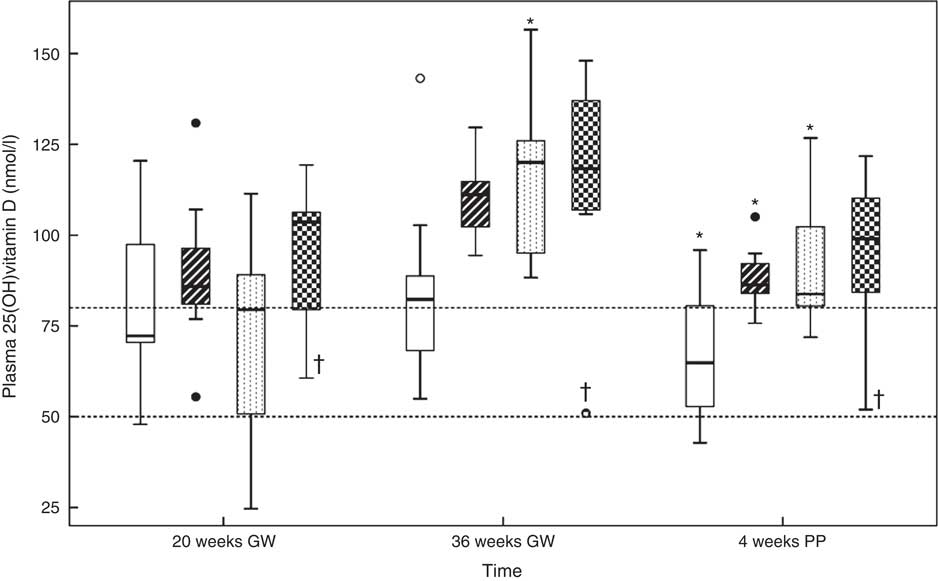
Fig. 2 Relations between the vitamin D dosages and plasma 25-hydroxyvitamin D (25(OH)D) concentration at 20 gestational weeks (GW), at 36 GW and at 4 weeks postpartum (PP). The participants took 10 (group A), 35 (group B), 60 (group C) or 85 (group D) µg vitamin D3/d from 20 GW to 4 weeks PP. The subgroups did not differ in plasma 25(OH)D at 20 GW. Horizontal lines indicate the cut-offs at 50 and 80 nmol/l. * Significance in that group compared with the previous outcome. † Potential non-user. Daily dose vitamin D –
![]() A: 10 µg (n 9) (missing data from one woman at 4 weeks PP),
A: 10 µg (n 9) (missing data from one woman at 4 weeks PP),
![]() B: 35 µg (n 9),
B: 35 µg (n 9),
![]() C: 60 µg (n 11) and
C: 60 µg (n 11) and
![]() D: 85 µg (n 7).
D: 85 µg (n 7).
Dose needed to reach vitamin D sufficiency (80–249 nmol 25-hydroxyvitamin D/l)
Fig. 3 shows the percentage of participants with plasma 25(OH)D above 80 nmol/l at 20 GW, 36 GW and 4 weeks PP. We found that 35 µg vitamin D3/d or higher was needed to increase 25(OH)D to adequacy (80–249 nmol/l) in >97·5 % of the participants at 36 GW, while >85 µg/d was needed to reach the same criterion at 4 weeks PP. Online Supplementary Table S2 shows the numbers of participants with plasma 25(OH)D within the employed categories of vitamin D status at 20 GW, 36 GW and 4 weeks PP. The parent vitamin D concentration of the one woman assigned to the highest supplemental dose and showing no 25(OH)D increment was below the limits of quantification at all sampling points.
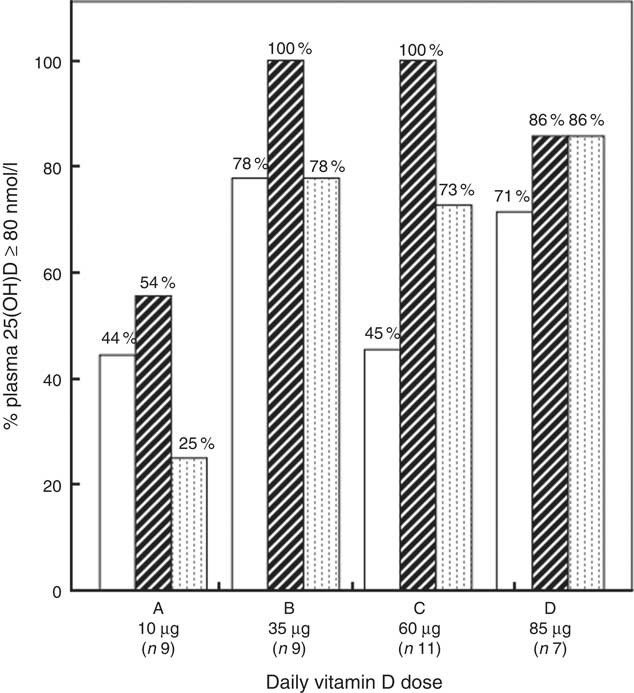
Fig. 3 Percentages participants with plasma 25-hydroxyvitamin D (25(OH)D) above the employed cut-off value for vitamin D adequacy at 80 nmol/l at 20 gestational weeks (GW), 36 GW and 4 weeks postpartum (PP). Time:
![]() , 20 GW;
, 20 GW;
![]() , 36 GW and
, 36 GW and
![]() , 4 weeks PP.
, 4 weeks PP.
Dependence of plasma 25-hydroxyvitamin D increments on baseline status and dose
Fig. 4(a) shows, for each of the supplemented groups A–D, the relation between plasma 25(OH)D at 20 GW and the plasma 25(OH)D increments from 20 GW to 36 GW. The increments were found to relate to 25(OH)D concentration at 20 GW. Independent of dose, there were higher 25(OH)D increments at low baseline status. The increments diminished gradually with dose, suggesting that plasma 25(OH)D saturation was reached at the higher dose. Although all participants reported compliance with the protocol, six women in group A, one woman in group B and two women in group D exhibited negative plasma 25(OH)D increments. Analogously, Fig. 4(b) shows the relation between baseline plasma 25(OH)D concentration and the plasma 25(OH)D increments from 20 GW to 4 weeks PP. Also here, the increments related to baseline 25(OH)D concentration, while the increments diminished with dose.
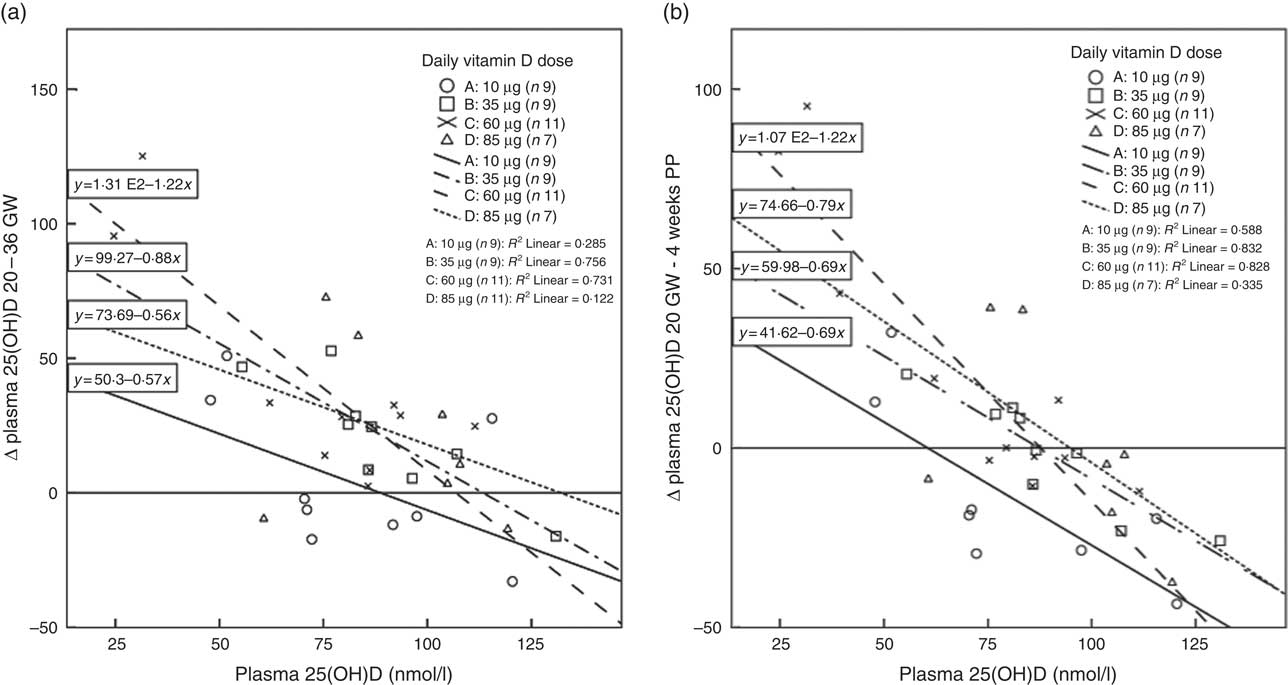
Fig. 4 (a) Relations between baseline plasma 25-hydroxyvitamin D (25(OH)D) concentrations at 20 gestational weeks (GW) and plasma 25(OH)D increments (∆ 25(OH)D in nmol/l) from 20 to 36 GW in the four dosage groups. (b) Relations between baseline plasma 25(OH)D concentrations at 20 GW and plasma 25(OH)D increments from 20 to 4 weeks postpartum (PP). Relations are given for groups A–D who received 10 (group A), 35 (group B), 60 (group C) and 85 (group D) µg vitamin D3/d from 20 GW to 4 weeks PP. The increments related negatively to baseline 25(OH)D concentration and diminished with dose. Zero increments occurred between 88 and 132 nmol 25(OH)D/l (a) and 60 and 95 nmol 25(OH)D/l (b).
Association between plasma 25-hydroxyvitamin D concentration and possible confounders
As shown in Fig. 4(a) and (b), GEE analysis confirmed that the increases in plasma 25(OH)D concentration were inversely related to the initial 25(OH)D concentration (P<0·001). GEE also showed that the month of year followed a second-order polynomial (P<0·001). Furthermore, GEE showed positive associations between the change in plasma 25(OH)D and visit (linear and quadratic combination, following a parabolic association P<0·001), vitamin D3 dosage (P<0·001) and age (P<0·007). Negative associations were found between increment in plasma 25(OH)D and BMI (P<0·006), time spent outdoor during the weekends (P<0·007) and duration of pregnancy (P<0·008). The model fit was significant: Wald χ 2 test was 248·29 (P<0·0001).
Maternal plasma parent vitamin D concentration
Fig. 5 shows the dose–response curves for the maternal plasma parent vitamin D. At the study start, the median for parent vitamin D for all women was ‘not detectable’ (nd) (range nd–40) nmol/l. There were no between-group differences (P=0·154). At 36 GW, parent vitamin D had increased in groups B and C (P<0·017). We noticed a trend in the other two groups. The medians of parent vitamin D at 36 GW were nd (range nd–9) nmol/l in group A, 11 (range nd–39) nmol/l in group B, 34 (range 19–76) nmol/l in group C and 88 (range nd–100) nmol/l in group D. There were between-group differences (P<0·050). Compared with 36 GW, parent vitamin D at 4 weeks PP had decreased in group C (P<0·017), while we noticed a trend in the others. At 4 weeks PP, parent vitamin D was 5 (nd–12) nmol/l in group A, 14 (nd–34) nmol/l in group B, 31 (8–52) nmol/l in group C and 38 (nd–71) nmol/l in group D. There were between-group differences (P<0·050). The corresponding data can be found in online Supplementary Table S1.
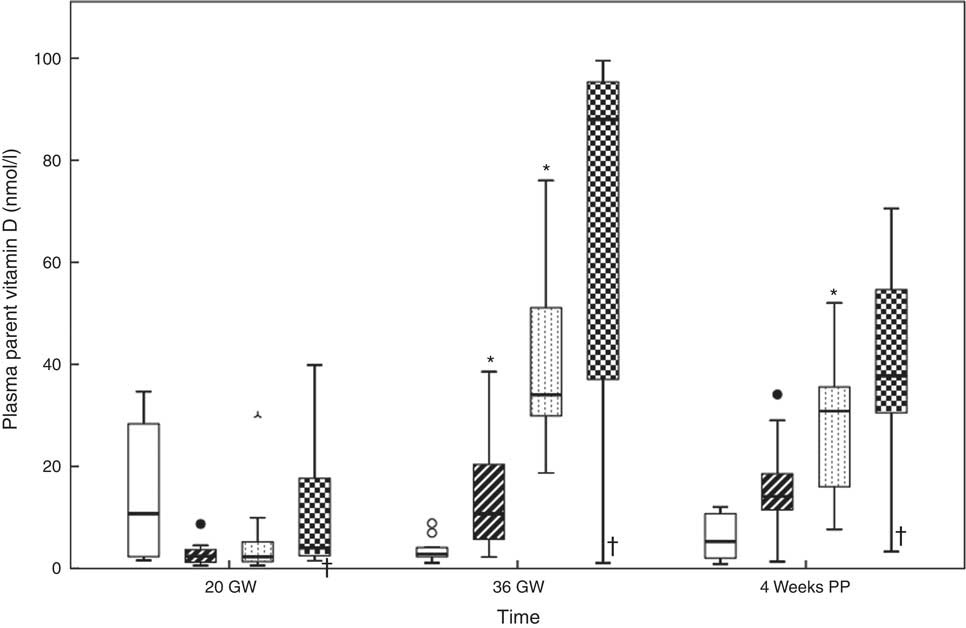
Fig. 5 Relations between the vitamin D dosages and plasma parent vitamin D at 20 gestational weeks (GW), at 36 GW and at 4 weeks postpartum (PP). The participants took 10 (group A), 35 (group B), 60 (group C) and 85 (group D) µg vitamin D3/d from 20 GW to 4 weeks PP. * Significance in the group compared with the previous group outcome. † Potential non-user. Daily dose vitamin D –
![]() 10 µg (n 9) (missing data from one woman at 4 weeks PP),
10 µg (n 9) (missing data from one woman at 4 weeks PP),
![]() 35 µg (n 9),
35 µg (n 9),
![]() 60 µg (n 11) and
60 µg (n 11) and
![]() 85 µg (n 7).
85 µg (n 7).
Milk antirachitic activity
Fig. 6 shows the dose–response curves for the milk ARA. Online Supplementary Table S3 presents the corresponding data. The medians of milk ARA at 4 weeks PP were 33 (range 20–57) IU/l in group A, 83 (range 48–145) IU/l in group B, 150 (range 45–1089) IU/l in group C and 156 (range 26–309) IU/l in group D. We found significant differences (P<0·050) for milk ARA between groups A and C, and groups A and D. None of the groups reached a median milk ARA of 513 IU/l, but there was one milk sample in group C that exceeded the IOM AI. Reanalysis of this sample confirmed the high concentration (1089 v. 1030 IU/l). The linear relation between vitamin D3 dosage and milk ARA at 4 weeks PP was milk ARA (IU/l)=29·5+2·27×vitamin D3 dosage (µg/d), R 2 0·109. Employing this relation, we estimate that the vitamin D3 dose needed to reach the 513 IU/l milk ARA target at 4 weeks PP amounts to 213 µg/d. We found relations (P<0·001) between both maternal plasma parent vitamin D (r 0·870) and 25(OH)D (r 0·685) with milk ARA.
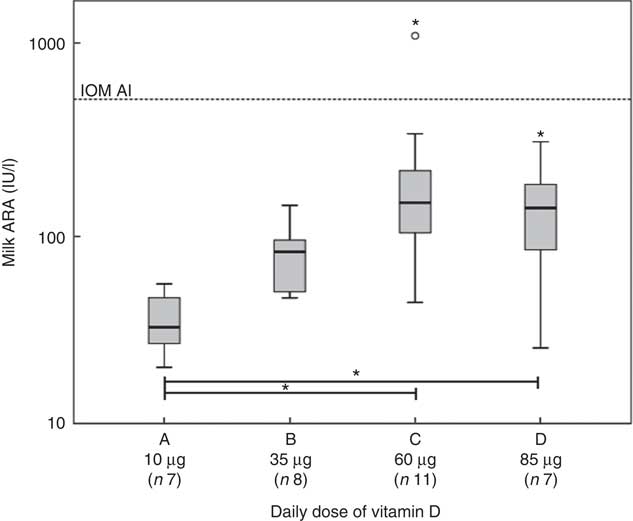
Fig. 6 Relations at 4 weeks postpartum (PP) between vitamin D dosages and milk antirachitic activity (ARA). The participants took 10 (group A), 35 (group B), 60 (group C) or 85 (group D) µg vitamin D3/d from 20 gestational weeks to 4 weeks PP. The linear relation between the vitamin D dosages and milk ARA was y=29·5+2·27x (x in µg and y in IU/l). The calculated vitamin D dosages needed to reach the target of 513 IU/l was 213 µg/d. The horizontal line indicates the infant adequate intake (AI) of the Institute of Medicine (IOM) at a milk ARA of 513 IU/l (i.e. 10 µg vitamin D/d at 780 ml milk/d). Except for one in group C, none of the mothers reached the IOM AI. * Significance (P<0·05). The increasing ARA with dose is mainly on account of increasing vitamin D concentrations.
Possible adverse effects
Online Supplementary Table S1 presents the data at the various visits of plasma Ca, plasma phosphate and the urine Ca:creatinine ratio. Plasma Ca and phosphate were below the upper limits of their reference ranges, as employed in our laboratory, while the urinary Ca:creatinine ratio was in agreement with those reported by Steegers et al. ( Reference Steegers, Thomas and de Boo 45 ) for pregnant and lactating women. None of the groups exhibited longitudinal changes in these parameters of potential vitamin D toxicity.
Discussion
As a primary aim of our ZOOG study, we were interested to see the maternal plasma 25(OH)D and parent vitamin D concentrations that would be reached by supplementing pregnant women with 10, 35, 60 and 85 µg vitamin D3/d from 20 GW up to 4 weeks PP, and notably the corresponding milk ARA that would be reached at 4 weeks PP. The investigated women had relatively high 25(OH)D at 20 GW (median 85; range 25–131 nmol/l). We found that, in general, the supplements increased both plasma parent vitamin D (Fig. 5) and 25(OH)D (Fig. 2) in a seemingly dose-dependent manner, from 20 GW to 36 GW, but not from 36 GW to 4 weeks PP. The dose-dependent increase in plasma 25(OH)D was confirmed by GEE analysis. Dosages of 35 µg vitamin D3/d or higher were needed to augment 25(OH)D to adequacy (80–249 nmol/l) in >97·5 % of the participants at 36 GW, while >85 µg/d was needed to reach the same criterion at 4 weeks PP (Fig. 3). The increments of 25(OH)D from 20 to 36 GW (Fig. 4(a)) and from 20 GW to 4 weeks PP (Fig. 4(b)) related inversely to the 25(OH)D concentration at 20 GW and diminished with dose. The supplements also caused a dose-dependent increase in the milk ARA at 4 weeks PP (Fig. 6). Except for one, none of the women reached a milk ARA of 513 IU/l, which corresponds with the AI of the IOM for 6 months old infants. Using a linear equation, it was estimated that this target would require a vitamin D3 supplemental dose of 213 µg/d.
Baseline vitamin D status and achievement of the 80 nmol/l 25-hydroxyvitamin D/l cut-off
The median 25(OH)D concentration of 85 (range 25–131) nmol/l at 20 GW (baseline) was higher than expected on forehand. Two studies, the ‘KOALA’( Reference Cremers, Thijs and Penders 46 ) and ‘Generation R’( Reference Vinkhuyzen, Eyles and Burne 12 ), both conducted with pregnant women in the Netherlands, found lower 25(OH)D of 44 (±18) and 65 (interquartile range: 43–87) nmol/l, respectively. In our study, only one woman (3 %) was classified as vitamin D deficient at enrolment, while only three (8 %) exhibited vitamin insufficiency. The ‘Generation R’ study revealed that among the women with European ethnic background, 7 % had vitamin D deficiency and 25 % had vitamin D insufficiency (25–49·9 nmol/l). The high vitamin D status of our study group is likely explained by the high socio-economic status of the recruited participants. They might have been more health conscious than their counterparts in the general population. Most of them (72 %) used a daily vitamin D supplement of 10 (range: 5–20) µg before the study start.
We found that all employed vitamin D3 dosages seemed effective in increasing the prevalence of vitamin D adequacy from 20 to 36 GW, but this was not the case for the comparison of 20 GW with 4 weeks PP. Obviously, it seems easier to reach vitamin D adequacy in pregnancy than in lactation. The discrepancy might be explained by vitamin D mobilisation from stores during pregnancy (see below). A dosage of 35 µg vitamin D3/d or higher was needed to reach vitamin D adequacy in >97·5 % of the participants at 36 GW, while >85 µg/d was needed to reach this criterion at 4 weeks PP. However, we believe one participant in the highest supplementation group to be non-compliant, on basis of her unresponsiveness on plasma 25(OH)D, parent vitamin D and fish oil results. When excluding this participant, we found that 85 µg/d was sufficient to reach vitamin D adequacy in >97·5 % of the participants at 4 weeks PP.
Courses of plasma 25-hydroxyvitamin D and parent vitamin D during the study
Supplementation with vitamin D3 dose dependently maintained or increased plasma 25(OH)D concentration at the pregnancy’s end (Fig. 2). This finding is in line with March et al. ( Reference March, Chen and Karakochuk 20 ) who demonstrated a dose-dependent 25(OH)D increase following administration of 10, 25 and 50 µg vitamin D/d from 13 to 24 GW until 36 GW. Upon continuing supplementation, their study subjects maintained or even increased their 25(OH)D concentration from 36 GW to 8 weeks PP. Unlike March et al., we found that the 25(OH)D concentration decreased, or tended to decrease, from 36 GW to 4 weeks PP (Fig. 2). A similar initial increase during pregnancy and a subsequent decrease during lactation was observed for the parent vitamin D (Fig. 5). An explanation could be non-compliance shortly after giving birth, however, the women in our study reported >75 % compliance to the supplements during the whole study. Although the GEE revealed that ‘month of the year’ followed a second-order polynomial, evaluation of the individual pregnancy periods suggested that it is unlikely that these courses are explained by seasonal cycling of vitamin D status, with highest 25(OH)D concentration in the Netherlands occurring around early August( Reference van Grootheest, Milaneschi and Lips 37 ). The women estimated that during the study they spent 1 h outside on a weekday and 2 h on a weekend day, depending on season and weather. In the Netherlands, during November to March, the required wavelength of sunlight exposure is insufficient to support vitamin D synthesis in skin. Furthermore, from April until October, vitamin D synthesis is highest/possible between 11.00 and 15.00 hours. The Dutch Health Council calculated that persons with skin type II, who spend 21 min/d outside between 11.00 and 15.00 hours, while wearing summer clothes, would produce between 6 and 7 µg vitamin D( 17 ). However, as many variables( Reference Wacker and Holick 47 ), such as clothing, sunscreen, air pollution and clock time, influence vitamin D synthesis in the skin, it is impossible to estimate the vitamin D contribution from sunlight exposure. Furthermore, pregnant women are advised to minimise sunbathing and to use a sunscreen. In all, thirty-four of the women reported to use a sunscreen upon going outside, with a median SPF of 30. If applied correctly, sunscreen protection factor 30 could reduce the vitamin D production in the skin by 95–98 %( Reference Holick 48 ). In conclusion, we consider it unlikely that sunlight exposure explains the observed courses of parent vitamin D and 25(OH)D in our study.
In a previous cross-sectional study of unsupplemented traditionally living Tanzanian women, we found higher 25(OH)D in pregnancy but similar concentrations at 3 d and 3 months PP, when compared with non-pregnant counterparts. These women are exposed to year-long abundant sunshine. We are aware of one other study in which 25(OH)D increased during pregnancy and fell after delivery( Reference Jones, Assar and Prentice 49 ). The higher 25(OH)D of unsupplemented mothers during pregnancy contrasts with the vast majority of literature data. Some authors showed higher concentrations( Reference Park, Brannon and West 50 – Reference Schleicher, Encisco and Chaudhary-Webb 52 ), but the most showed no change (Grant( Reference Grant, Stewart and Scragg 19 ) placebo group) or even declining concentrations( Reference Dent and Gupta 53 – Reference Ardawi, Nasrat and BA’Aqueel 56 ). The discrepancies may relate to different magnitudes of maternal vitamin D stores.
We previously suggested( Reference Luxwolda, Kuipers and Kema 38 ) that the higher 25(OH)D during pregnancy in Tanzania might be caused by the well-known higher circulating vitamin D binding protein (DBP) concentrations, which in turn may be driven by oestrogens( Reference Hadden and McLaughlin 57 , Reference Gomme and Bertolini 58 ). DBP has high affinity for 25(OH)D and to lesser extent for the parent vitamin D and the 1,25(OH)2D hormone( Reference Hollis and Wagner 59 ). While the mechanism underlying the 2- to 3-fold 1,25(OH)2D increases during pregnancy is unclear( Reference Wagner, Hollis and Kotsa 10 ), it is possible that induction of DBP extracts parent vitamin D from adipose tissue stores( Reference Luxwolda, Kuipers and Kema 38 ) and 25(OH)D from muscle( Reference Abboud, Rybchyn and Rizk 60 , Reference Heaney, Horst and Cullen 61 ) for subsequent transplacental transfer. Mobilisation of parent vitamin D from adipose tissue during pregnancy might be facilitated by the reducing insulin sensitivity in the second and third trimesters( Reference Hadden and McLaughlin 57 ), while it has been suggested that 25(OH)D is mobilised from muscle by physical activity( Reference Abboud, Rybchyn and Rizk 60 ). Uptake of DBP-bound 25(OH)D in the placenta may be facilitated by the megalin–cubilin system( Reference Hollis and Wagner 59 ), while parent vitamin D, because of its higher free fraction( Reference Hollis and Wagner 59 ), may more intensively cross by diffusion. Accumulation of the parent vitamin D may take place in the rapidly growing, vitamin D-naive, fetal adipose tissue compartment. This compartment amounts to about 0·35 kg at birth( Reference Carberry, Colditz and Lingwood 62 ), has been predominantly synthesised from maternal glucose and does not become mobilised during intrauterine life.
Dependence of plasma 25-hydroxyvitamin D increments on baseline status and dose
We found that the increments of plasma 25(OH)D from 20 GW to 36 GW (Fig. 4(a)) and from 20 GW to 4 weeks PP (Fig. 4(b)) relate inversely to the plasma 25(OH)D at 20 GW. In other words, the higher the 25(OH)D level at 20 GW, the lower the 25(OH)D response, irrespective of vitamin D3 supplemental dose. Such saturation effects of plasma 25(OH)D during vitamin D3 supplementation have been previously observed( Reference Dawodu and Akinbi 63 – Reference Vaes, Tieland and de Regt 65 ), either suggesting increasing storage, deactivation (e.g. 24-hydroxylation( Reference Schlingmann, Kaufmann and Weber 66 )) or both. It seems that at high baseline 25(OH)D concentration vitamin D3 supplementation might even cause a decrease, which is in line with previous findings( Reference Borst, Duk and Tromp 67 ). Especially at low supplemental dosages, such a decrease might, however, also be caused by supplement use before the study, incompliance, analytical variation, uncontrolled sunlight exposure, BMI, age and influence of stores. Altogether we suggest that next to vitamin D’s immune function( Reference Tamblyn, Hewison and Wagner 68 ), the deviant vitamin D physiology in pregnancy aims at the building of infant stores.
Milk antirachitic activity
The daily vitamin D3 dosages provided by us during pregnancy and lactation gave rise to a dose-dependent increase in the milk ARA, as measured at 4 weeks PP (Fig. 6). Except for one woman with a milk ARA of 1089 IU/l, none of the participants reached the 10 µg/d vitamin D output consistent with the IOM AI for 0–6 months old infants, and as translated to a milk ARA of 513 IU/l. We previously reported that vitamin D unsupplemented lactating women inhabiting various countries, including women with lifetime abundant sunlight exposure and high vitamin D status, had milk ARA ranging from 1 to 247 IU/l and did not reach this output either( Reference Stoutjesdijk, Schaafsma and Nhien 27 ).
Using linear extrapolation, we estimated that a daily supplemental dose of 213 µg would be needed to reach a milk ARA of 513 IU/l, which seems in reasonable agreement with the study of Wagner et al. ( Reference Wagner, Hulsey and Fanning 31 ). They supplemented with 160 µg vitamin D3/d for 6 months during lactation to find a milk ARA of 874 IU/l at the study end( Reference Wagner, Hulsey and Fanning 31 ).
Taken together, it seems that a daily dose of 50 µg vitamin D3/d( Reference Grant, Stewart and Scragg 19 , Reference Wall, Stewart and Camargo 30 ) and likely up to 85 µg/d as in the present study, provided during pregnancy and/or lactation, is unable to increase the milk ARA to the IOM AI for 0–6 months old infants.
Potentially adverse effects
None of the women reported adverse effects. Laboratory signs of vitamin D toxicity are hypercalciuria, hypercalcaemia and low serum PTH. Plasma 25(OH)D concentration did not exceed 250 nmol/l at any sampling point, while plasma Ca and phosphate remained below the upper limits of the reference ranges employed in our laboratory. The urinary Ca:creatinine ratios were in agreement with those reported by Steegers et al. ( Reference Steegers, Thomas and de Boo 45 ) for pregnant and lactating women. We did not analyse plasma PTH, but other studies did not detect abnormalities following supplementation with up to 160 µg vitamin D3/d( Reference Wagner, Hulsey and Fanning 31 , Reference Hollis, Wagner and Howard 32 ).
Limitations
The high plasma 25(OH)D concentrations in our study group at 20 GW, exhibiting almost no vitamin D deficiency or insufficiency, limits our findings to women with high vitamin D status at baseline. We nevertheless found that even these women needed vitamin D3 supplements to maintain a high vitamin D status. Other limitations include the small subject numbers per supplemental group, high socio-economic status and the mere European ethnic background of the women. We chose to use four different dosages as opposed to larger subgroups with less dose variation. Confounding factors that might have influenced inter-individual variation are BMI, age, season, clothing and behaviour with regard to sunlight exposure. We did find that month of year influenced the 25(OH)D increase. Another limitation is that we did not collect infant (cord) blood samples.
Conclusions
Both plasma parent vitamin D and 25(OH)D increased seemingly dose dependent from 20 to 36 GW and decreased subsequently from 36 GW to 4 weeks PP. Dosages of 35 µg vitamin D3/d or higher were needed to increase 25(OH)D to adequacy in >97·5 % of participants at 36 GW, while >85 µg/d was needed to reach this target in >97·5 % of participants at 4 weeks PP. The lower dose needed in pregnancy may relate to mobilisation of maternal vitamin D stores during pregnancy. The magnitude of the 25(OH)D increment from 20 to 36 GW and from 20 GW to 4 weeks PP diminished with dose and related inversely to 25(OH)D at 20 GW. Milk ARA at 4 weeks PP increased in a dose-dependent manner. However, except for one, none of the women reached a milk ARA of 513 IU/l. A 213 µg/d supplement may be needed to reach the infant AI.
Acknowledgements
The authors thank the participants, the participating obstetric practices, ‘Moeders voor Moeders’ and the UMCG Department of Obstetrics and Gynecology. The UMCG Laboratory for Special Chemistry is gratefully acknowledged for performing the analyses, Wim Calame for help with statistical analysis, Herman J. A. Velvis and master students Eline Hemelt and Wietske Hemminga for their participation in this study.
This work was supported by the Ministry of Economic Affairs, the Provinces of Groningen and Drenthe.
All authors designed the research; E. S. conducted the research; E. S. was involved in statistical analysis; E. S. D. A. J. D.-B. and F. A. J. M. wrote the paper; F. A. J. M. was involved with primary responsibility of the final content. All authors read and approved the final manuscript.
None of the authors has any conflict of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003598











