Garlic (Allium sativum) is a medicinal plant that has historically been used for the treatment and prevention of some diseases and has recently been shown to have antimicrobial, antitumour, antioxidant and immunostimulatory properties(Reference Imai, Ide and Nagae1–Reference Tsao and Yin4). The pharmacological effects of garlic on cardiovascular disease (CVD) are mediated through the alteration of cholesterol and triglyceride (TAG) levels in the bloodstream(Reference Kyo, Uda and Kasuga5). The medicinal effects of garlic are derived from its flavonoid and organosulphur components. Studies in mammalian systems have indicated that these active pharmacological components act on multiple intracellular signalling pathways. In chickens, garlic compounds have also been shown to lower cholesterol and exert immunomodulating and antimicrobial activities(Reference Choi, Park and Kim6–Reference Kim and Kim9). However, compared with human studies, there is relatively limited information on the use of phytonutrients in general, and garlic in particular, in poultry veterinary medicine.
In addition to its effects on the cardiovascular system, garlic has been suggested for the treatment of parasitoses and other intestinal diseases(Reference Iciek, Kwiecien and Wlodek10). For example, crude extracts of garlic reduced or eliminated Hymenolepis, Aspiculuris, Histomonas and Eimeria parasites in animal models of infection(Reference Ayaz, Turel and Gul11–Reference Toulah and Al-Rawi15). Of these parasites, coccidia protozoa comprise a subclass of single-celled eukaryotic micro-organisms belonging to the phylum Apicomplexa and class Conoidasida. Coccidia of the genus Eimeria infect the intestinal epithelia of chickens, turkeys and some mammalian hosts.
The intestinal intraepithelial lymphocytes (IEL), the primary immune effector cells of the gut-associated lymphoid tissues, recognise and destroy pathogens that breach the intestinal epithelium(Reference Neutra, Mantis and Kraehenbuhl16). Chicken intestinal IEL are composed of two phenotypically and functionally distinct subpopulations, natural-killer (NK) cells and T-lymphocytes(Reference Gobel, Kaspers and Stangassinger17). In our previous studies, we have shown that IEL are the major effectors against coccidia parasites, Eimeria (Reference Lillehoj and Trout18, Reference Hong, Lillehoj and Lillehoj19). In chickens, disruption of the gut epithelium during avian coccidiosis severely restricts the feed conversion efficiency during commercial poultry production. While in-feed anticoccidial drugs and antibiotic growth promoters can be used to mitigate some of the effects of avian coccidiosis, the emergence of drug-resistant parasites and legislative restrictions on the use of in-feed antibiotic growth promoters are driving the development of alternative disease control strategies for poultry production(Reference Lillehoj, Kim and Bravo20, Reference Lillehoj and Lillehoj21). Because garlic has been shown to exert anticoccidial effects in mouse and rabbit infection models(Reference Dkhil, Abdel-Baki and Wunderlich14), in the present study we evaluated the effects of a compound including garlic-derived secondary metabolites, propyl thiosulphinate (PTS) and propyl thiosulphinate oxide (PTSO), on intestinal immunity to E. acervulina.
Materials and methods
Parasite cytotoxicity assay
Freshly sporulated E. acervulina oocysts were disrupted with 0·5 mm glass beads for 5–7 s and the freed sporocysts were incubated for 45 min at 41°C in PBS containing 0·014 m-taurodeoxycholic acid (Sigma) and 0·25 % trypsin (Sigma) to release infective sporozoites. Sporozoites were separated from debris by filtration, resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen), and isolated by centrifugation at 2100 g for 10 min at 4°C. Isolated sporozoites (1·0 × 106/ml) were incubated with PBS (negative control), 10 μg/ml of Garlicon40® or 5·0 μg/ml of chicken purified recombinant NK-lysin (positive control)(Reference Hong, Lillehoj and Siragusa22) for 2 or 4 h at 4°C, and viability was measured by trypan blue exclusion by counting a minimum of 100 sporozoites.
Garlicon40®, a product containing 40 % of a compound made of 67 % of propyl thiosulphinate oxide (PTSO) and 33 % of PTS, was provided by Pancosma S.A. The biosynthesis of PTS is made from propiin (S-propyl-L-cysteine sulphoxide), an amino acid derived from l-cysteine found in Allium species. The first step of the biosynthesis (of thiosulphinates) is the formation of the sulphenic acid associated (in this case propyl-1-sulphenic acid; CH3CH2CH2SOH) plus pyruvate plus ammonia. However, these compounds (sulphenic acids) are highly reactive, so they immediately produce thiosulphinates by a condensation reaction. In the last step, heating PTS induces its dismutation in PTSO and propyldisulphide. Propyldisulphide can be oxidised and transformed to PTSO and the oxidation of PTS to PTSO is never totally completed. Therefore, the two molecules are generally present in commercial samples. Garlicon40® was notated as PTSO/PTS in the present study.
Spleen lymphocyte proliferation
All experiments were approved by the Agricultural Research Service Institutional Animal Care and Use Committee. For the purpose of the experiment, 3-week-old Ross/Ross broiler chickens (Longenecker's Hatchery) were euthanised by cervical dislocation, and the spleens were removed and placed in Petri dishes with 10 ml of Hank's balanced salt solution (HBSS) supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin (Sigma). Cell suspensions were prepared by gently flushing through a cell strainer and lymphocytes were purified by density gradient centrifugation through Histopaque-1077 (Sigma). The cells were adjusted to 1·0 × 107 cells/ml in RPMI-1640 medium without phenol red (Sigma) and supplemented with 10 % fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Then, 100 μl/well of the sample were added to ninety-six-well flat-bottomed plates containing 100 μl/well of PTSO/PTS (10, 5, 2·5, 1·25, 0·6 or 0·3 μg/ml) from Pancosma S.A., 5 μg/ml of concanavalin A (Sigma) as a positive control, or medium alone as a negative control. The cells were incubated at 41°C in a humidified incubator (Forma) with 5 % CO2 for 48 h, and the cell numbers were measured using WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium, monosodium salt) (Dojindo Molecular Technologies) at 450 nm using a microplate spectrophotometer (Bio-Rad).
Experimental animals and diets
Broiler chickens (Ross/Ross, Longenecker's Hatchery) were randomly housed in Petersime starter brooder units and fed, from hatch, a standard diet alone (control) or a diet supplemented with 10·0 parts per million of PTSO/PTS. The composition of the standard diet was prepared as recommended by the National Research Council(23). The PTSO/PTS dose was chosen based on preliminary dose–response experiments.
Eimeria acervulina infection of chickens
At 10 d post-hatch, the chickens were transferred to large hanging cages (two birds/cage) and were orally infected with 1·0 × 104 sporulated oocysts of E. acervulina. The remaining chickens were housed in neighbouring cages, but were uninfected. Body weights (twenty birds/group) were measured at 0 and 10 d post-infection. Faecal samples (twenty birds/group) were collected between 6 and 9 d post-infection, and oocyst numbers were determined using a McMaster chamber (HK Inc.) according to the formula(Reference Lee, Lillehoj and Park24): total oocysts/bird = oocyst count × dilution factor × (faecal sample volume/counting chamber volume)/2.
Anti-profilin serum antibody assay
Peripheral blood (four birds/group) was collected from uninfected and infected chickens at 10 d post-infection, and sera were prepared by centrifugation and analysed for anti-profilin antibody levels by ELISA. For this, ninety-six-well plates were coated overnight with 10 μg/well of E. acervulina purified recombinant profilin protein as described(Reference Jang, Lillehoj and Lee25). The plates were washed with PBS containing 0·05 % Tween and blocked with PBS containing 1·0 % bovine serum albumin (Sigma). Diluted sera at 1:50 were added (100 μl/well), incubated with agitation for 1 h at room temperature and washed with PBS containing 0·05 % Tween. Bound antibody was detected with horseradish peroxidase-conjugated rabbit anti-chicken IgG (Sigma) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma) by measuring the optical density at 450 nm. All samples were analysed in quadruplicate.
Microarray hybridisation
Chicken intestinal IEL were isolated from the non-infected chickens at 14 d post-hatch as described(Reference Min, Lillehoj and Ashwell26) and gene expression analysis was performed by microarray hybridisation. The intestinal jejunum was removed, cut longitudinally and washed three times with ice-cold HBSS. Then, the samples were incubated in HBSS containing 0·5 mm-EDTA and 5 % fetal calf serum for 20 min at 37°C with constant swirling. Cells released into the supernatant were passed through nylon wool (Robbins Scientific), and washed twice with HBSS containing 5 % fetal calf serum. The IEL were purified by Percoll density gradient centrifugation and washed three times with HBSS containing 5 % fetal calf serum.
In preliminary experiments, we determined that the percentage of CD45-positive IEL ranges from 45 to 70 % depending upon the genetics, age and infection status of chickens used (data not shown). Total RNA (six birds/group) was isolated using Trizol (Invitrogen) and pooled in two samples with equal amount from three birds each. RNA were amplified using the Two-Color Quick Amp Labeling Kit (Agilent Technologies) with cyanine 3 (Cy3)- or Cy5-labelled CTP. The RNA probes from the control and treatment groups labelled with two different colours were hybridised with one Chicken Gene Expression Microarray (Agilent Technologies) containing 43 803 elements. Then, two biological replicates were conducted with alternation of Cy3- and Cy5-labelled RNA to prevent data distortion from sample labelling(Reference McShane, Shih and Michalowska27). Microarray images were scanned, and data extraction and analysis were performed using Feature Extraction software version 10.7.3.1 (Agilent Technologies).
Microarray data analysis
GeneSpring GX10 software (Silicon Genetics) was used to qualify and normalise image analysis data and to determine the fold changes in gene expression. Average signal intensities were corrected for background signals and normalised by the locally weighted regression and smoothing scatter plots method. Flag information was applied to strain the spots with 100 % valid values from each sample and an asymptotic t test analysis was performed to analyse the significance between PTSO/PTS-fed and non-supplemented control groups. To generate signal ratios, signal channel values from PTSO/PTS-fed birds were divided by values from negative controls. Modulated elements were defined as RNA with >2·0-fold increased or decreased levels with P < 0·05, as determined by the Student's t test. The differentially expressed genes were filtered using the Volcano Plot method(Reference Jin, Mao and Eshoo28) built by the t test. All microarray information and data were deposited in the Gene Expression Omnibus database (series record no. GSE36302).
Quantitative RT-PCR
Gene expression changes observed by microarray analysis were confirmed by quantitative RT-PCR as described(Reference Hong, Lillehoj and Lee29). Equivalent amounts of the same RNA samples used for microarray hybridisations were reverse-transcribed using the StrataScript First Strand Synthesis System (Stratagene). Oligonucleotide primers are listed in Table 1. Amplification and detection were carried out using the Mx3000P system and Brilliant SYBR Green quantitative RT-PCR master mix (SABioscience, Stratagene). Standard curves were generated using log10 diluted standard RNA and the levels of individual transcripts were normalised to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by the Q-gene program(Reference Muller, Janovjak and Miserez30). For the calculation of fold changes between treatment groups, the cycle threshold (C t) value of the target gene was normalised to GAPDH and calibrated to the relevant control value. Each analysis was performed in triplicate.
Table 1 Primers used for quantitative RT-PCR

* Calculated by the Q-gene program. Efficiency = 10( − 1/regressionslope).
Bioinformatics data analysis
Differentially expressed genes between the PTSO/PTS-fed and control chickens were analysed using Ingenuity Pathway Analysis software (IPA) (Ingenuity Systems). Each identifier was mapped to its corresponding gene in IPA (Ingenuity Systems). Both 2·0-fold increased and 2·0-fold decreased identifiers were defined as value parameters for the analysis. These genes, termed ‘focus genes’, were superimposed onto the global molecular networks contained within IPA (Ingenuity Systems). Functional gene analysis was performed to identify the biological functions and canonical pathways of genes from the mapped data sets. The Fisher's exact test was used to calculate P values to assess the probability of each biological function and pathway assigned to that data set. The pathways of focus genes were algorithmically generated based on their connectivity.
Statistical analysis
Data from parasite cytotoxicity assays, body weight gains, oocyst shedding, spleen lymphoproliferation and profilin antibody levels were expressed as the mean and standard deviation values. Comparisons of the mean values were performed by one-way ANOVA followed by Duncan's multiple-range test or Student's t test using SPSS software (SPSS 15.0 for Windows); differences between groups were considered statistically significant at P < 0·05.
Results
Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate on in vitro sporozoite viability and spleen cell proliferation
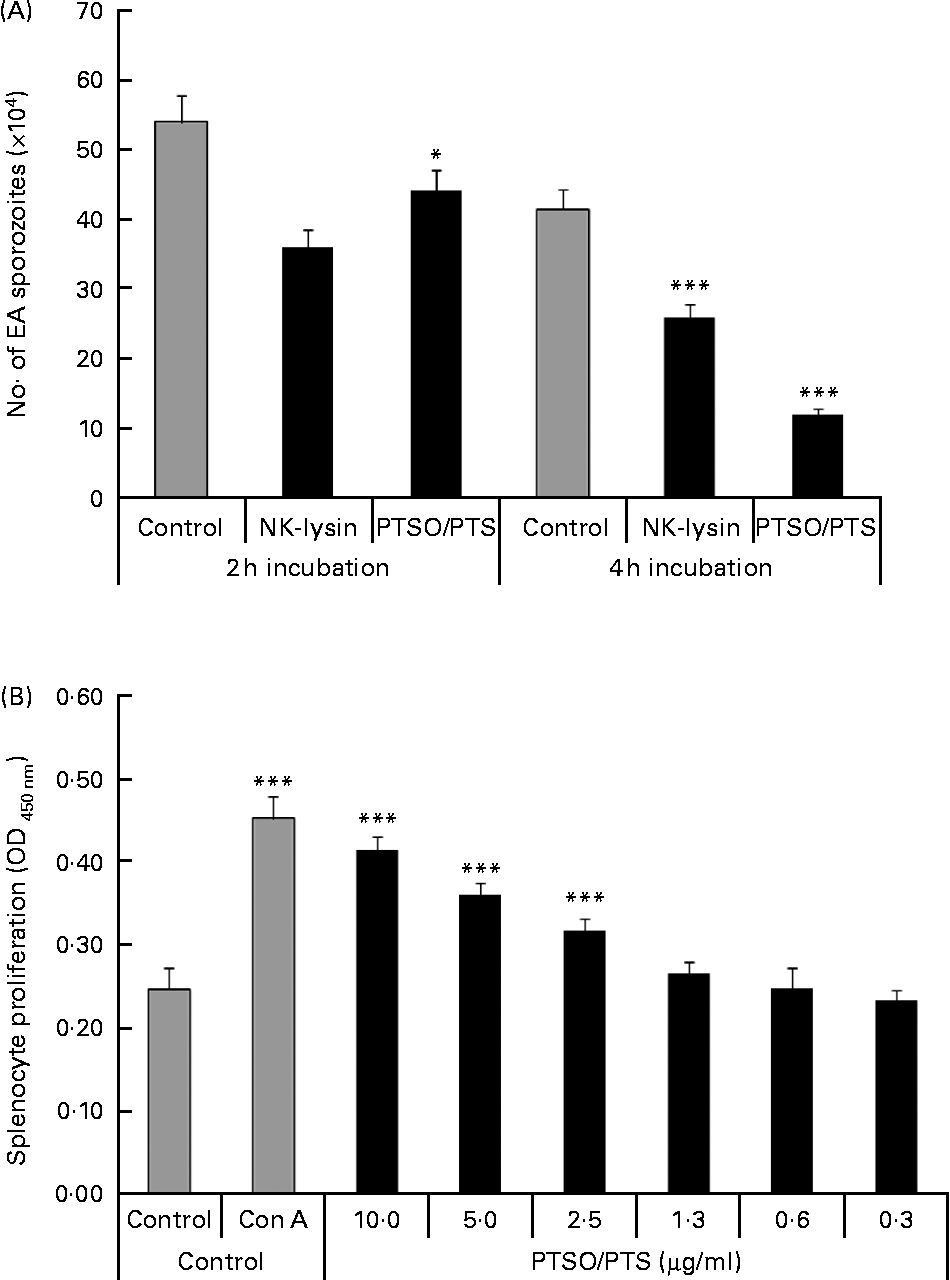
Treatment of freshly prepared E. acervulina sporozoites with 10 μg/ml of PTSO/PTS for 2 or 4 h significantly decreased parasite viability by 18 and 71 %, respectively (Fig. 1A). The reduced sporozoite viability was comparable with that produced by 5·0 μg/ml of chicken purified recombinant NK lysin, previously shown to be cytotoxic for E. acervulina and E. maxima parasites(Reference Hong, Lillehoj and Siragusa22).

Fig. 1 Effect of propyl thiosulphinate oxide/propyl thiosulphinate (PTSO/PTS) in vitro. (A) Eimeria acervulina (EA) sporozoites (1·0 × 106/ml) were incubated with PBS (control), 10 μg/ml of PTSO/PTS or 5·0 μg/ml of chicken recombinant natural killer (NK) lysin, for 2 or 4 h at 4°C, and viability was measured by trypan blue exclusion by counting a minimum of 100 sporozoites. (B) Spleen cells were treated with the indicated concentrations of PTSO/PTS, concanavalin A (Con A; 5 μg/ml) or medium (control) for 48 h and viable cell numbers were measured using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium. Values are means, with standard deviations represented by vertical bars (n 3). Mean values were significantly different from those of PTSO/PTS-treated with control groups according to the Student's t test: * P < 0·05; *** P < 0·001. OD, optical density.
PTSO/PTS significantly increased splenocyte proliferation at 2·5 μg/ml or higher concentrations tested compared with the medium control at P < 0·001 (Fig. 1B).
Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate on body weight gain and faecal oocyst excretion following experimental Eimeria acervulina infection
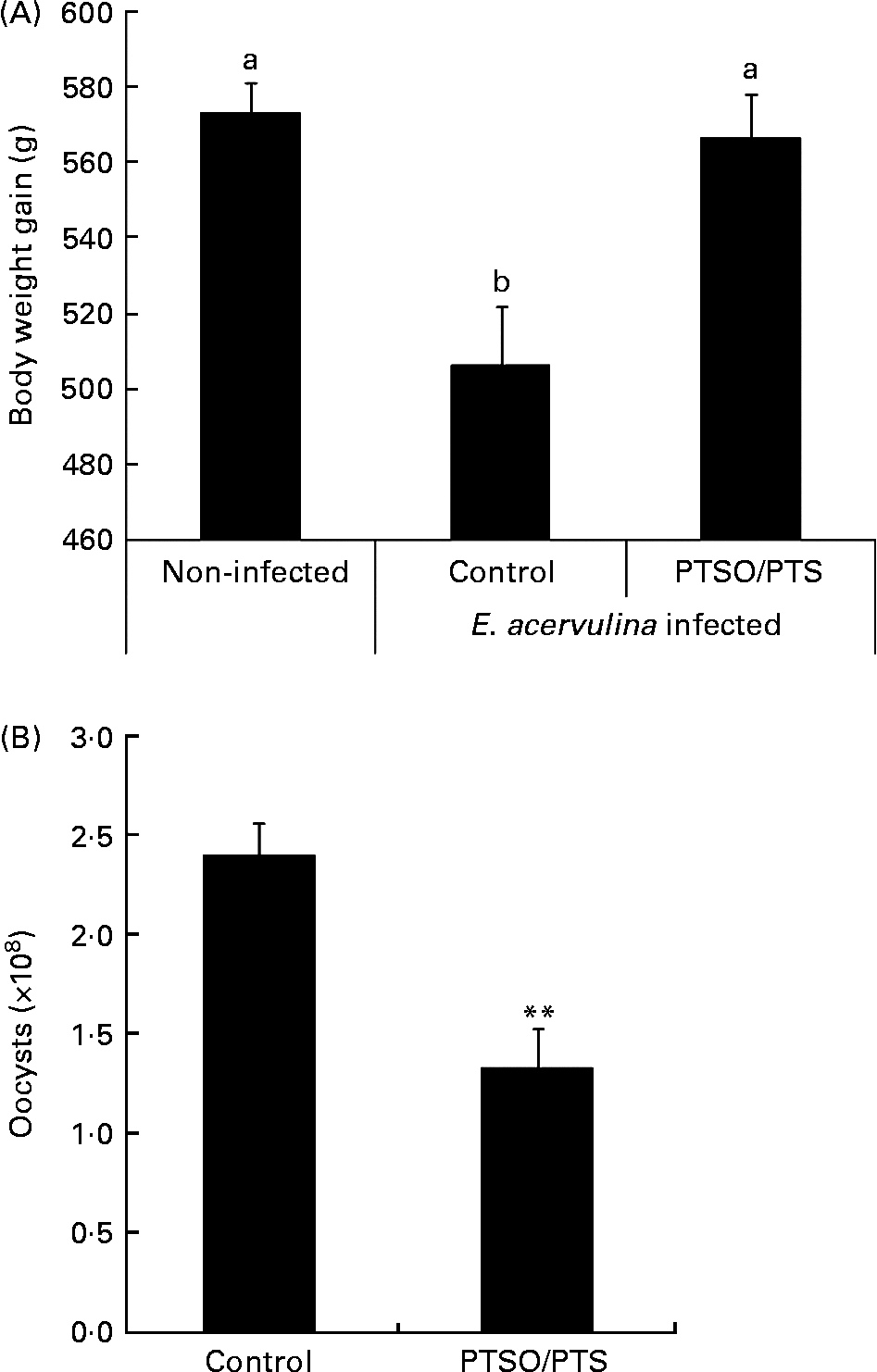
Dietary supplementation with PTSO/PTS significantly increased body weight gain in E. acervulina-infected chickens compared with infected birds given a non-supplemented control diet (Fig. 2A). In addition, chickens fed a diet supplemented with PTSO/PTS showed decreased faecal oocyst excretion compared with non-supplemented controls (Fig. 2B).

Fig. 2 Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate (PTSO/PTS) on body weight gain and faecal oocyst excretion following experimental Eimeria acervulina infection. Chickens were fed from hatch with non-supplemented or PTSO/PTS-supplemented diets and either uninfected or orally infected with 1·0 × 104 oocysts of E. acervulina at 10 d post-hatch. (A) Body weights (twenty birds/group) were measured in non-infected and infected chickens on the non-supplemented diet (control), and in infected chickens on the PTSO/PTS-supplemented diet at 0 and 10 d post-infection. Values are means, with standard deviations represented by vertical bars. a,b Mean values with unlike letters were significantly different according to Duncan's multiple-range test (P < 0·05). (B) Faecal samples (twenty birds/group) were collected from chickens on the non-supplemented (control) and PTSO/PTS-supplemented diets between 6 and 9 d post-infection and total oocyst numbers were determined using a McMaster chamber. Values are means, with standard deviations represented by vertical bars. ** Mean value was significantly different from that of the control group (P < 0·01; Students t test).
Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate on serum anti-profilin antibody levels
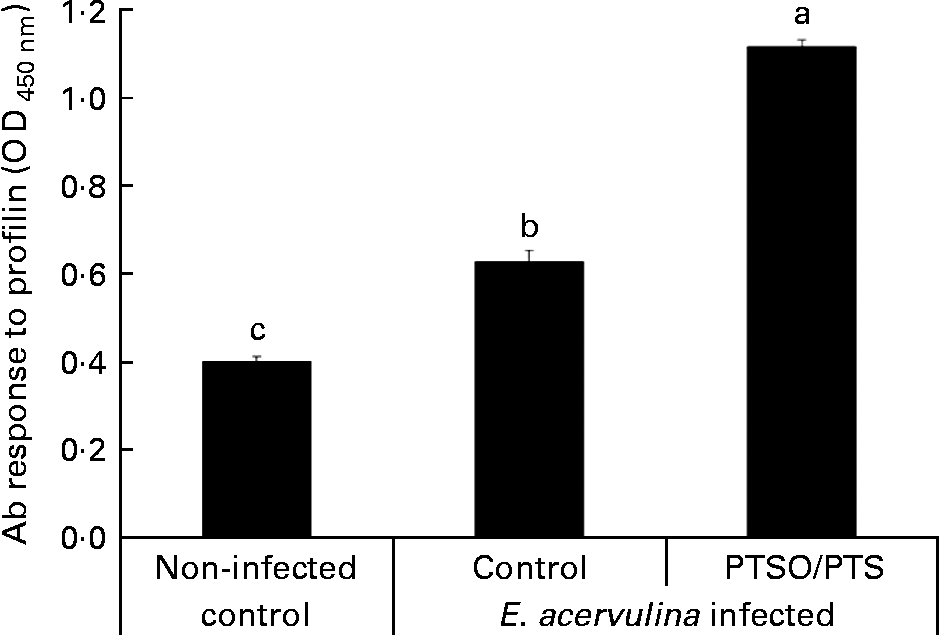
Serum antibody levels against E. acervulina profilin were measured as a parameter of humoral immunity in PTSO/PTS-supplemented and non-supplemented chickens. The profilin antibody levels were higher in Eimeria-infected chickens than in the uninfected control at 10 d post-infection, and dietary PTSO/PTS significantly (P < 0·05) increased serum profilin antibody levels (Fig. 3) compared with non-supplemented controls.

Fig. 3 Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate (PTSO/PTS) on profilin antibody (Ab) levels. Chickens were fed from hatch with non-supplemented (control) or PTSO/PTS-supplemented diets and orally infected with 1·0 × 104 oocysts of Eimeria acervulina at 10 d post-hatch. Peripheral blood (four birds/group) was collected at 10 d post-infection and sera were analysed for anti-profilin Ab levels by ELISA. Values are means, with standard deviations represented by vertical bars (n 4). a,b,c Mean values with unlike letters are significantly different according to Duncan's multiple-range test (P < 0·05). OD, optical density.
Effect of dietary propyl thiosulphinate oxide/propyl thiosulphinate on differential gene expression
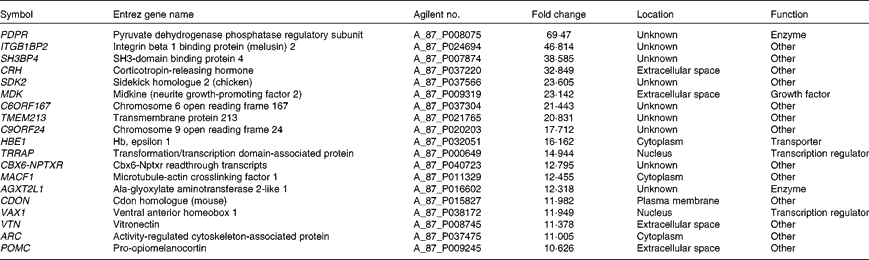
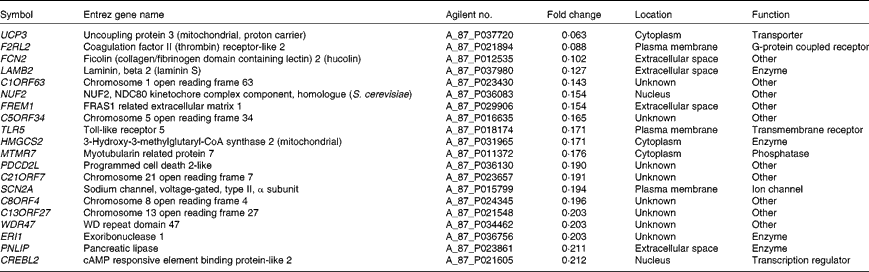
Microarray hybridisation analysis using Agilent Technologies' Chicken Gene Expression Microarray which contains 43 803 elements identified 1227 mRNA whose levels were altered >2·0-fold in intestinal IEL of PTSO/PTS-treated chickens compared with non-supplemented controls. Of these, 552 were increased and 675 were decreased. This data set was mapped to the corresponding genes of the human, mouse and rat genome using Ingenuity Knowledge Base software (Ingenuity Systems), and 288 chicken genes were identified and annotated. Tables 2 and 3 list the genes corresponding to the twenty most up- or down-regulated transcripts, respectively.
Table 2 Genes corresponding to up-regulated intestinal intraepithelial lymphocyte transcripts in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet

Table 3 Genes corresponding to down-regulated intestinal intraepithelial lymphocyte transcripts in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet

Quantitative RT-PCR validation
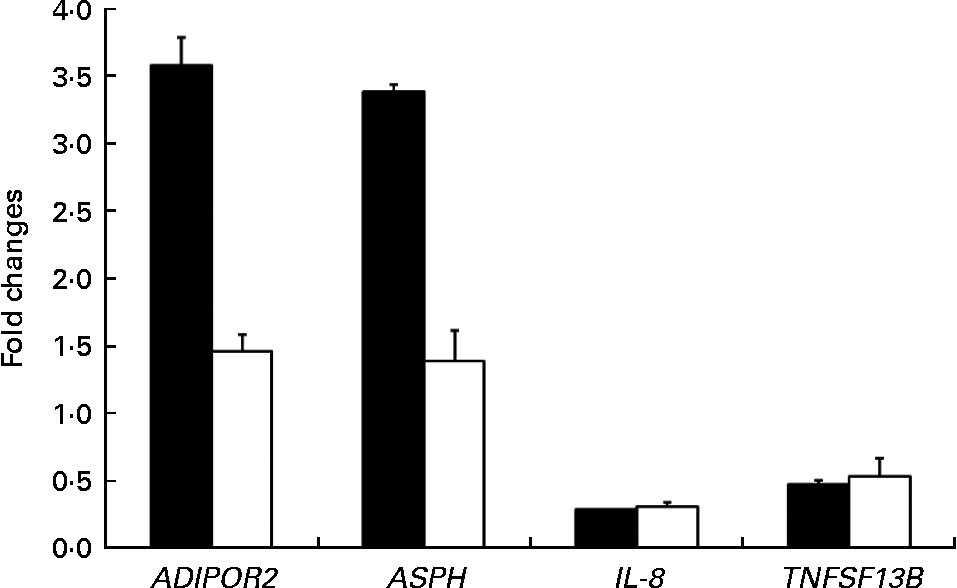
The expression patterns observed by microarray analysis were validated by quantitative RT-PCR with four selected transcripts whose levels were significantly modulated between PTSO/PTS-fed and non-treated chickens. These genes were adiponectin receptor 2, aspartate β-hydroxylase, IL-8 and TNF (ligand) superfamily member 13b (TNFSF13B). The levels of these transcripts that were up- or down-regulated by microarray hybridisation were also correspondingly altered when analysed by quantitative RT-PCR (Fig. 4). As previously discussed, the differences in the magnitude between the changes observed, particularly with adiponectin receptor 2 and aspartate β-hydroxylase, might be due to differences in the normalisation methods and/or the different fluorescent dyes used in the two techniques(Reference Lee, Sladek and Greenwood31).

Fig. 4 Comparison between microarray (■) analysis and quantitative RT-PCR (□) for the levels of mRNA corresponding to selected genes. Each bar represents fold changes of mRNA levels in propyl thiosulphinate oxide/propyl thiosulphinate-fed chickens compared with non-supplemented controls. ADIPOR2, adiponectin receptor 2; ASPH, aspartate β-hydroxylase; TNFSF13B, TNF (ligand) superfamily, member 13b.
Biological function and pathway analyses
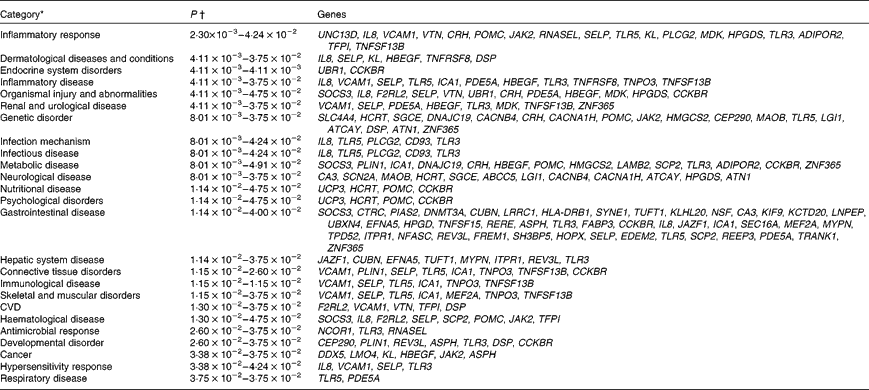
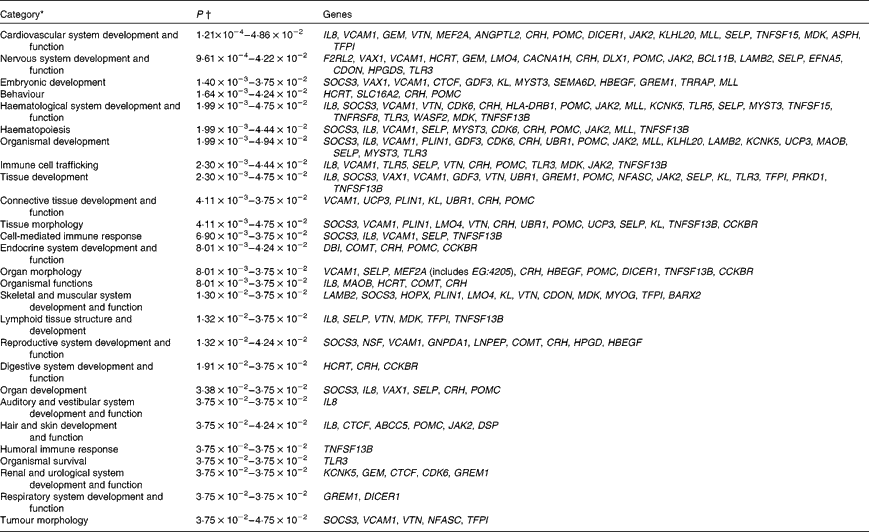
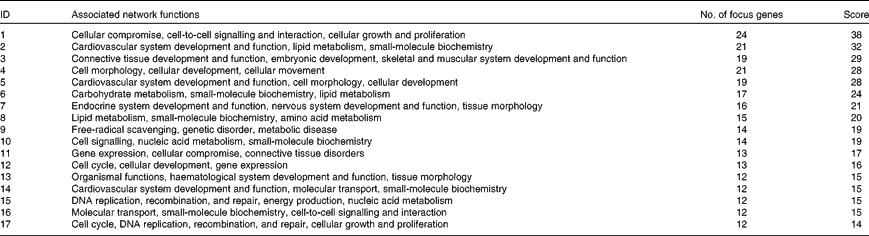
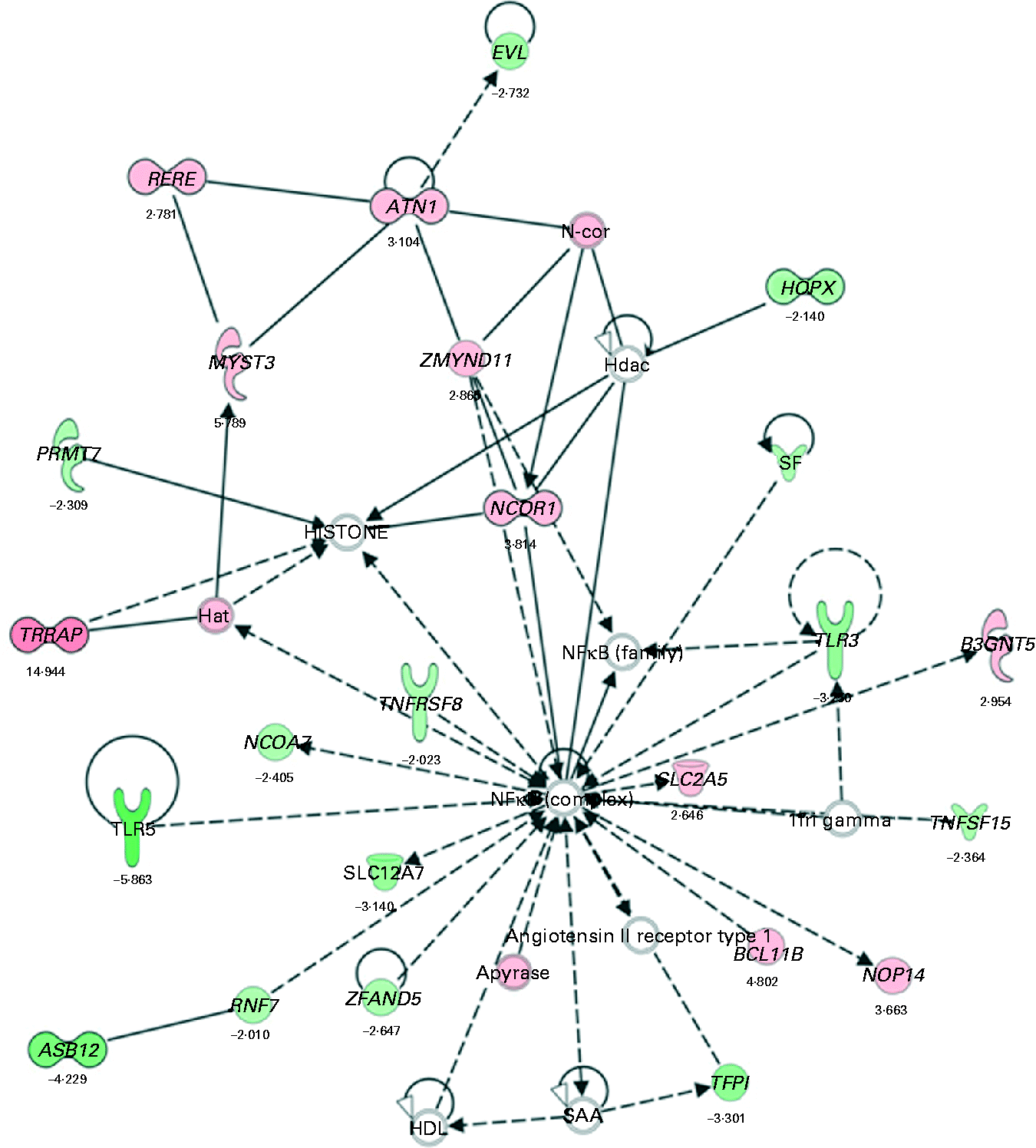
Biological function analysis using the IPA (Ingenuity Systems) database was performed for the 288 genes corresponding to the mRNA that were differently altered following dietary PTSO/PTS treatment compared with untreated controls. The P value associated with a particular function in this analysis is a statistical measure of the likelihood that genes from the data set under investigation participate in that function. In this manner, these genes were classified under the categories ‘Disease and Disorders’ and ‘Physiological System Development and Function’. In the category ‘Disease and Disorders’, the most significant function that was identified was ‘Inflammatory Response’ (Table 4). In the category ‘Physiological System Development and Function’, the most significant function that was identified was ‘Cardiovascular System Development and Function’ (Table 5). Canonical Pathway analysis classified the genes whose expressions were significantly modified by PTSO/PTS dietary supplementation to eight selected biological functions (Table 6). Of these, the pathway of ‘Communication Between Innate and Adaptive Immune Cells’ was identified as the most significant pathway and included five genes. Overall, seventeen groups of pathway networks, classified according to the number of focus genes, are listed in Table 7. Fig. 5 illustrates the most significant network converging on the NF-κB transcription factor with twenty-four focus genes identified.
Table 4 Significant functions in the category ‘Disease and Disorders’ in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet

* Data sets were analysed by BioFunction analysis using Ingenuity Pathway Analysis software (Ingenuity Systems).
† P-value ranges of the biological functions included in the category. P values were calculated using the right-tailed Fisher's exact test.
Table 5 Significant functions in the category ‘Physiological System Development and Function’ in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet

* Data sets were analysed by BioFunction analysis using Ingenuity Pathway Analysis software (Ingenuity Systems).
† P-value ranges of the biological functions included in the category. P values were calculated using the right-tailed Fisher's exact test.
Table 6 Significant canonical pathways in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet

TREM1, triggering receptor expressed on myeloid cells 1.
* Data sets were analysed by Bio Function analysis using Ingenuity Pathway Analysis software (Ingenuity Systems).
† P values were calculated using the right-tailed Fisher's exact test.
‡ The ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway.
Table 7 Significant networks in chickens given a propyl thiosulphinate oxide/propyl thiosulphinate-supplemented diet compared with a non-supplemented diet


Fig. 5 The most significant network with the indicated genes corresponding to mRNA exhibiting >2·0-fold up- and down-regulated levels following dietary supplementation of propyl thiosulphinate oxide/propyl thiosulphinate compared with non-supplemented controls. Genes corresponding to increased and decreased mRNA are indicated by red and green colours, respectively, with the colour intensity indicating the relative expression levels. Overlaid numbers are the fold changes associated with each gene. ![]() , Complex;
, Complex; ![]() , cytokine/growth factor;
, cytokine/growth factor; ![]() , enzyme;
, enzyme; ![]() , group/complex/other;
, group/complex/other; ![]() , transcription regulator;
, transcription regulator; ![]() , transmembrane receptor;
, transmembrane receptor; ![]() , transporter;
, transporter; ![]() , unknown;
, unknown; ![]() , direct relationship;
, direct relationship; ![]() , indirect relationship.
, indirect relationship.
Discussion
The present study was conducted to investigate the immunologic and genomic alterations following dietary supplementation with PTSO/PTS, the secondary metabolites of garlic, during E. acervulina infection of chickens.
PTSO/PTS enhanced the in vitro and in vivo parameters of immunity. Treatment of chicken spleen cells with PTSO/PTS increased their proliferation and treatment of E. acervulina sporozoites with PTSO/PTS decreased cell viability. Chickens given a PTSO/PTS-supplemented diet and infected with E. acervulina had greater body weight gain, reduced faecal oocyst excretion and increased profilin antibody responses compared with chickens fed a non-supplemented diet. Some of these responses may have been due to a direct cytotoxic effect of PTSO/PTS on parasite sporozoites. Microarray hybridisation identified 1227 transcripts whose levels were significantly altered in the intestinal IEL of PTSO/PTS-fed birds compared with non-supplemented controls. Biological pathway analysis identified the altered transcripts as belonging to the categories ‘Disease and Disorder’ and ‘Physiological System Development and Function’.
Garlic has been traditionally used as a folk remedy to treat a variety of human diseases, the health-promoting effects of which are attributed to the presence of multiple sulphur-containing compounds, such as diallyl sulphide, diallyl disulphide, dipropyl sulphide and allicin. These compounds, and others, have demonstrated pharmacological activity in vitro and in vivo (Reference Murugavel and Pari32–Reference Son, Mo and Rhee35). Allicin is generally considered to be one of the primary active compounds that gives garlic its characteristic odour and many of its healing benefits. Thiacremonone, a novel sulphur compound isolated from garlic, possesses anti-inflammatory properties through the inhibition of NF-κB activation in rats and mice(Reference Ban, Oh and Kim36). NF-κB is a major transcription factor that plays a key role in regulating the immune response to infection following the binding of pathogenic micro-organisms to a variety of cell surface receptors, such as Toll-like receptors (TLR)(Reference Takeda and Akira37). Our bioinformatic analysis identified NF-κB as a central molecule in the most significant network that was activated in PTSO/PTS-treated chickens. This network also included TLR3 and TLR5 as down-regulated components. Further evidence for the modulation of TLR and NF-κB in response to garlic comes from the study of Youn et al. (Reference Youn, Lim and Lee38) showing that the ethyl acetate fraction of the plant bulb inhibited lipopolysaccharide-induced dimerisation of TLR4 and blocked NF-κB activation.
Interestingly, however, our functional studies indicated that dietary PTSO/PTS increased the resistance of chickens to experimental E. acervulina infection, as evidenced by greater weight gain and reduced parasite excretion, and augmented parameters of adaptive immunity, including a higher profilin antibody response and greater spleen cell proliferation, compared with chickens fed a non-supplemented diet. The ability of PTSO/PTS to directly kill sporozoites and stimulate spleen cell proliferation in vitro suggests that a similar mechanism may operate in vivo, thus contributing to increased body weight and decreased parasite fecundity.
The capability of the PTSO/PTS preparation to stimulate spleen cell mitogenesis (an indicator of adaptive cellular immunity), while simultaneously down-regulating TLR and NF-κB (indicators of innate immunity), suggests that the dietary supplement may have more than one active component. In this regard, Clement et al. (Reference Clement, Pramod and Venkatesh39) identified several immunomodulatory proteins from garlic with mitogenic activity towards human peripheral blood lymphocytes and murine splenocytes. Further studies to characterise the active components of garlic and their effects on innate and adaptive immunity will help to resolve these questions.
In the biological functional analysis, eighteen genes were related with the most reliable ‘Inflammatory Response’ category (Table 4). Among those genes, TLR are a major component of the pattern recognition receptor repertoire that detect invading micro-organisms and direct the vertebrate immune system to eliminate infection. TLR5 plays a role in restricting the entry of flagellated Salmonella into systemic sites(Reference Iqbal, Philbin and Withanage40) and TLR3 which recognises double-stranded RNA analogue, induces interferon-β through the recognition of viral double-stranded RNA in chicken(Reference Karpala, Lowenthal and Bean41). In the present study, TLR3 and TLR5 expression was down-regulated in the bioinfomatical analysis and this may explain the reduced inflammatory response seen at the later phase of coccidiosis infection. Although the underlying mechanisms are not yet known, it was found that garlic increases NK cell activity and T cell proliferation in mice(Reference Hassan, Yaraee and Zare42). Aged garlic extract treatment enhanced NK cell activity(Reference Morioka, Sze and Morton43, Reference Kyo, Uda and Ushijima44) and phagocytosis(Reference Kyo, Uda and Kasuga5). The balance of T helper 1/T helper 2-type cytokines, such as the secretion of interferon-γ and IL-2 v. IL-4 and IL-10, was regulated by the consumption of garlic products in a dose-dependent manner(Reference Liu, Su and Lii45–Reference Ghazanfari, Hassan and Ebtekar47). In chickens, IEL contains two major phenotypically and functionally distinct populations, NK and T cells, and these cells play important roles in avian coccidiosis(Reference Gobel, Kaspers and Stangassinger17). Therefore, it is possible that dietary garlic enhanced disease resistance against coccidiosis by activating these effector cells and enhancing the secretion of immunomodulating cytokines in the gut. The TNF family is an important regulator of inflammation, immune responses and tissue homeostasis(Reference Locksley, Killeen and Lenardo48). TNFSF13B, also known as B-cell activating factor (B-cell activating factor of the TNF family), plays a major role in B-cell survival, proliferation and differentiation. TNFSF13B induced the selective expansion of B cells in the spleen and caecal tonsils when administered to young chicks(Reference Schneider, Kothlow and Schneider49). The level of TNFSF13B was shown to increase during various diseases that may reflect the severity of disease in malaria, virus infection and multiple sclerosis(Reference Ittah, Miceli-Richard and Lebon50–Reference Ragheb, Li and Simon52). However, the overexpression of TNFSF13B is also involved in some autoimmune conditions(Reference Yamanishi, Kumagi and Yokota53, Reference Janda, Rensing-Ehl and Eibel54). In the present study, dietary feeding of normal chickens with PTSO/PTS enhanced serum antibody response to E. acervulina infection in Eimeria-infected chickens when antibodies were measured at 10 d after infection. However, for gene expression analysis, we used uninfected gut tissues from chickens treated with PTSO/PTS, and we saw the decreased TNFSF13B transcript compared to the chickens that were not fed with PTSO/PTS. Therefore, we speculate that the expression of TNFSF13B may have increased upon coccidiosis infection and this may have caused high antibody response. Chicken IL-8 (IL-8/CXCLi2) was the first chicken CXC chemokine identified and significantly triggered by the exposure of the gut to feed and bacteria in newly hatched chicks(Reference Bar-Shira and Friedman55). IL-8 has both chemotactic and angiogenic functions in the chicken(Reference Poh, Pease and Young56); so this gene was also identified in the ‘Cardiovascular System Development and Function’ category (Table 5). At low concentrations, IL-8 is chemotactic for monocyte/macrophages and lymphocytes. However, at high concentrations, IL-8 stimulates sprouting and growth of blood vessels(Reference Martins-Green and Feugate57).
In addition to effects on inflammation and immunity, dietary garlic dramatically affects the cardiovascular system(Reference Rahman58). For example, garlic consumption by humans may diminish the progression of CVD by decreasing the levels of LDL and total cholesterol, while concomitantly raising HDL levels. Consumption of garlic is also associated with the inhibition of platelet aggregation and reduced systolic and diastolic blood pressure. Recently, garlic was found to decrease two other markers of CVD, homocysteine and C-reactive protein. Interestingly, our present results identified ‘Cardiovascular System Development and Function’ as the most significant function in the category ‘Physiological System Development and Function’ of PTSO/PTS-fed chickens. This finding may extend the effects of garlic metabolites to poultry. However, the underlying pathways associated with the cardiovascular system in poultry still needs to be studied.
In conclusion, in vivo feeding of young broiler chickens with PTSO/PTS compound, a garlic-derived extract, improved resistance to experimental E. acervulina infection and induced significant alterations of the transcriptome in chicken intestinal lymphocytes involving immune- and cardiovascular-related gene pathways and networks. These results suggest that dietary immunomodulation by garlic-derived compounds may represent a possible alternative to current drug-based strategies for commercial poultry production.
Acknowledgements
The authors state that there are no conflicts of interest. D. K. K. conceived and performed the experiments, analysed the data and wrote the manuscript; H. S. L. conceived and designed the experiments; S. H. L. designed and performed the experiments; E. P. L. contributed to write the manuscript; D. B. conceived the experiments. The authors thank Marjorie Nichols and Stacy Torreyson for their technical assistance. This project was supported by a Trust agreement between Agricultural Research Service–United States Department of Agriculture and Pancosma.