Environmental conditions in early life are integral drivers in long-term health and susceptibility to disease. Postnatal nutritional conditions can alter the development of immune, metabolic and nervous systems(Reference Galic, Spencer and Mouihate1–Reference Sominsky, Ziko and Nguyen3). Breast milk is the first, and ideally sole, source of nutrients for newborns during a critical window of growth and development. Exclusive breast-feeding results in healthier outcomes for newborns in both infancy and adulthood, ranging from fewer infections to a lower risk of obesity(Reference Horta, Loret de Mola and Victora4,Reference Stuebe and Schwarz5) . Our understanding of the mechanisms mediating the protective effects of breast milk continues to evolve but is likely in part via bioactive factors transferred from mother to infant during lactation. Alongside the provision of macro- and micronutrients, breast milk contains a multitude of bioactive compounds, including immune factors, hormones and short RNA (i.e. microRNAs) that can directly alter cellular signalling pathways and subsequent health outcomes(Reference Lonnerdal6–Reference Melnik and Schmitz8). However, breast milk composition is highly variable according to time of collection, phase or duration of lactation and maternal characteristics including genetics, body weight and diet(Reference Andreas, Kampmann and Mehring Le-Doare7,Reference Demmelmair and Koletzko9) .
In addition to nutrition, the protein component of breast milk supplies the infant with bioactive proteins including lactoferrin, α-lactalbumin, osteopontin and milk fat globule proteins that protect from infant infections and aid in the absorption of minerals and digestion of fats(Reference Lonnerdal6). Maternal diet and nutritional status during lactation can affect the abundance of certain proteins in milk and potentially mediate offspring health outcomes(Reference Hue-Beauvais, Miranda and Aujean10,Reference Kucia, Langhammer and Gors11) . Hormones transferred from mother to infant via breast milk, specifically insulin and leptin, are associated with offspring body weight and adiposity levels, possibly through disturbances in the development of the hypothalamic feeding circuitry(Reference Vogt, Paeger and Hess12–Reference Logan, Siziba and Koenig15). Maternal diet, metabolic status and weight have been associated with leptin levels in breast milk(Reference Yu, Rong and Sun16,Reference Zamanillo, Sanchez and Serra17) . Differences in hormonal exposure through breast milk and bioactive proteins may influence the development of metabolic pathways and appetite regulation.
MicroRNA (miRNA) are short RNA averaging twenty-two nucleotides in length that target corresponding mRNA and inhibit their translation(Reference Gebert and MacRae18). miRNA are abundant in milk, and the majority are transported by exosomes and protected from degradation by the lipid bilayer(Reference Melnik and Schmitz8,Reference Zhou, Li and Wang19) . Studies have demonstrated the ability of milk-derived miRNA to enter offspring circulation and alter gene expression in target tissues(Reference Manca, Upadhyaya and Mutai20,Reference Lin, Chen and Xie21) . Therefore, milk-derived miRNA may serve as a key epigenetic regulator in developmental programming. Indeed, miRNA levels in milk are affected by maternal diet and correlate with maternal overweight/obesity status and with infant weight and adiposity(Reference Zamanillo, Sanchez and Serra17,Reference Pomar, Castro and Pico22–Reference Munch, Harris and Mohammad24) . In this study, we chose to examine seven miRNA (miR-222, miR-203a, miR-200a; miR-26a, miR-27a, miR-103 and miR-148a) based on their respective relationships with obesity and/or an obesogenic diet, regulation of adipogenesis and/or abundance in rodent and human breast milk(Reference Melnik and Schmitz8,Reference Zamanillo, Sanchez and Serra17,Reference Munch, Harris and Mohammad24–Reference Fu, Dong and Tian27) .
There is now abundant evidence that offspring metabolic health is profoundly influenced by maternal health, particularly through maternal diet and weight status(Reference Ainge, Thompson and Ozanne28–Reference Pomar, van Nes and Sánchez31). In particular, maternal obesogenic diets and maternal obesity have been shown to have adverse effects on offspring metabolism and obesity risk(Reference Ainge, Thompson and Ozanne28–Reference Pomar, van Nes and Sánchez31). Several dietary compounds have been investigated to mitigate the programmed obesity risk conferred to offspring via maternal obesity, including prebiotics. Prebiotics, substrates that are selectively utilised by host micro-organisms conferring a health benefit(Reference Gibson, Hutkins and Sanders32), have been shown in multiple rodent studies to mitigate the deleterious effects of obesogenic diets on both maternal and offspring adiposity and metabolism(Reference Paul, Bomhof and Vogel30,Reference Paul, Collins and Nicolucci33,Reference Paul, Collins and Bomhof34) . We previously showed in rats that supplementing a maternal high-fat/sucrose (HFS) diet during gestation and lactation with the prebiotic oligofructose (OFS) improved insulin sensitivity, gut microbiota profiles and serum inflammatory profiles and reduced fatty liver in the offspring(Reference Paul, Collins and Nicolucci33). The mechanism underlying the protective effects of maternal prebiotic intake on the offspring is not entirely understood but may be related to dietary-induced changes in maternal milk composition(Reference Hallam, Barile and Meyrand35). Based on samples collected in the aforementioned study, the goal of this study was to examine the effects of prebiotic supplementation of a maternal high-fat/high sucrose diet on milk composition (leptin, protein, miRNA expression) and the association between milk components and offspring outcomes.
Materials and methods
Animals and diets
The study protocol was approved by the University of Calgary Life and Environmental Sciences Animal Care Committee (No. AC15-0079) and conformed to the Guide to Care and Use of Experimental Animals (Canadian Council on Animal Care). The present study used milk samples from an experimental design previously described in detail(Reference Paul, Collins and Nicolucci33). Briefly, 6-week-old virgin female Sprague–Dawley rats were fed a HFS diet (Diet 102412; Dyets) ad libitum for 10 weeks to induce obesity. Of the rats with the greatest weight gain, forty-five were allocated to one of three experimental dietary groups during gestation and lactation (n 15/group): (1) HFS ad libitum (control HFS group); (2) HFS + 10 % (w/w) OFS (Orafti P95; Beneo) and (3) body weight-matched OFS group with limited HFS diet provision (WM). The females were bred at 16 weeks of age, and the diets started immediately upon identification of a copulation plug. The amount of HFS diet provided to WM dams was based on a combination of repeated body weight measurements of both the WM and OFS dams and energetic intake measurements of the OFS dams. A fourth lean reference group (n 15) was maintained on control AIN-93 diet throughout the study (Dyets, Bethlehem, PA, USA). Diet composition is described in Table 1. Female and male Sprague–Dawley rats were bred in wire-bottomed cages. The day a copulation plug was found was designated gestation day 0, and females were then housed individually and provided their respective experimental diet. The day after birth, litters were culled to ten pups with equal numbers of males and females; extra pups were cross-fostered to dams with similar-aged pups when needed to increase litter size. Pups were weaned at day 21. On the day of weaning, two males and two females from each litter were lightly anaesthetised with isoflurane and their body composition was measured via dual-energy X-ray absorptiometry scan with software for small animals and cardiac serum was collected. From the remaining offspring, two males and two females from each litter were weaned onto the HFS diet and consumed this until 24 weeks of age. Offspring body weight was measured weekly, and food intake was measured for five consecutive days each month. At 24 weeks of age, body composition was analysed via dual-energy X-ray absorptiometry. Faecal samples were collected at 3, 11 and 24 weeks of age. Given the small sample volumes at 3 weeks of age, faecal samples from littermates were combined. At 11 and 24 weeks of age, male and female microbiota was analysed separately. The relative abundance of a predetermined set of microbial groups was determined using real-time quantitative PCR as described previously(Reference Paul, Collins and Nicolucci33). Animals were killed by overanaesthetisation and aortic cut, and liver tissue was excised for later triglyceride (TAG) determination using a TAG (GPO) reagent set (Point Scientific Inc.).
Table 1. Experimental diet composition*

HFS, high fat/sucrose; HFS + OFS, high fat/sucrose supplemented with oligofructose.
* Oligofructose (Orafti P95; Beneo). Energy density of the diets in kcal/g is 3·76, 3·6, 4·6 and 4·3, respectively, for AIN-93G, AIN-93M, HFS and HFS + OFS.
Milk sample collection and protein and hormone analysis
Milk samples were collected from dams on the day that pups were weaned (day 21) according to our previously published protocol(Reference Paul, Hallam and Reimer36). Dams were anaesthetised with isoflurane, and milk letdown was stimulated with 2 IU of intraperitoneal oxytocin. After 5–15 min, milk was collected into capillary tubes via manual expression of the milk. Milk was stored at −80°C until further analysis. The following day, dams were lightly anaesthetised with isoflurane and their body composition was measured using dual-energy X-ray absorptiometry. They were then euthanised via overanaesthetisation and aortic cut.
The total milk protein concentration was determined using the Bradford Protein Assay (BioRad Inc.). Leptin concentration in milk samples was determined using ELISA (Millipore).
Milk microRNA isolation and quantification
Total RNA and miRNA were isolated using the miRNeasy Serum/Plasma kit (Qiagen) according to manufacturer’s instructions. The quantity and quality of RNA were measured using a Quant-iT RiboGreen RNA Assay kit (Thermo Fisher Scientific Life Sciences). Reverse transcription and quantitative PCR was performed on specific miRNA (miR-222, miR-203a, miR-200a, miR-26a, miR-27a, miR-103 and miR-148a) in milk according to manufacturer’s instructions for the miScript II RT Kit and the miScript Primer Assays (Qiagen). The real-time PCR protocol was: initial activation step of HotStarTaq DNA polymerase (95°C, 15 min); forty cycles of denaturation (15 s at 94°C), annealing (30 s at 55°C) and extension (34 s at 70°C); and melting curve analysis. SNORD68 and SNORD96A were determined to be appropriate reference genes based on their relatively stable expression levels across tissues and cell types and were used in the 2−ΔCT calculation of miRNA levels. Primer sequences are provided in Table 2.
Table 2. Primer sequences for real-time quantitative PCR

miRNA, microRNA.
Statistical analyses
Results are reported as mean values with their standard errors. Data were tested for normality using Shapiro–Wilk test (α = 0·05). Independent samples Kruskal–Wallis test with pairwise comparisons of treatment was used to determine differences between groups for non-parametric data (milk miRNA levels). For all other data with a normal distribution, differences between groups were evaluated using a one-way ANOVA with Tukey’s post hoc tests as appropriate. For correlation analysis, Pearson’s correlation was used. A P < 0·05 was considered significant. Data are available from the corresponding author upon reasonable request. Statistical analyses were performed using SPSS version 26.0 software (SPSS, Inc.).
Results
Maternal oligofructose supplementation attenuates postpartum weight retention
The body composition of dams was assessed when pups were weaned and showed that HFS dams had higher body weight, fat mass and body fat percentage compared with LEAN and HFS + OFS dams (Table 3). The HFS-WM group was weight-matched through controlled feeding to the HFS + OFS group and did not differ significantly from them in body weight, fat mass or body fat percentage. Prebiotic supplementation during gestation and lactation (HFS + OFS) attenuated the body weight, fat mass and body fat percentage gain seen in the HFS dams and resulted in values for the HFS + OFS group that were similar to the LEAN control dams. Lean mass, bone mineral content and bone mineral density did not differ according to diet.
Table 3. Maternal body composition (Mean values with their standard errors)

HFS, high fat/sucrose; HFS + OFS, high fat/sucrose supplemented with oligofructose; HFS-WM, high fat/sucrose fed weight-matched to HFS + OFS.
a,bTreatments with different superscript letters are significantly different at P < 0·05 (i.e. a is different from b, but ab does not differ from a or b).
Maternal diet and weight status influence maternal milk composition
Maternal milk was collected at weaning and assessed for protein and leptin concentrations and miRNA levels. Milk produced by HFS dams contained less protein than milk from LEAN controls (Fig. 1a). OFS supplementation normalised the milk protein content to LEAN, while the HFS-WM milk contained intermediary levels of protein. Leptin content did not differ between groups (Fig. 1b).
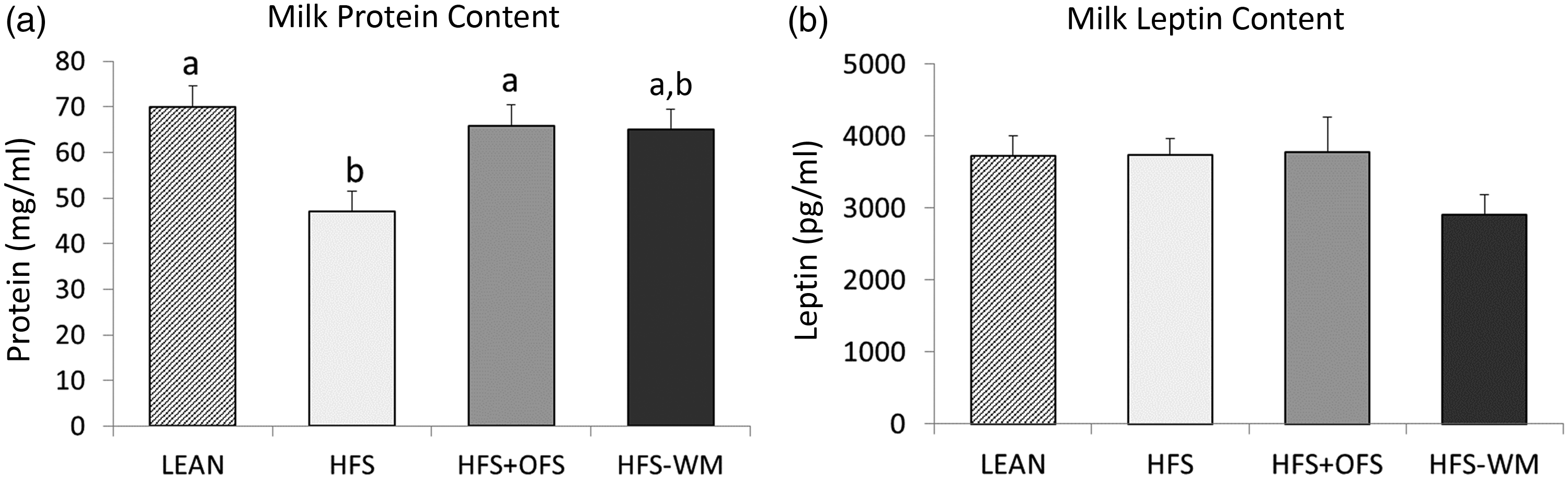
Fig. 1. Maternal diet influences maternal milk (A) protein content and (B) and leptin concentrations at weaning (day 21). Values are mean with their standard errors with n 4–9 per group. Treatments with different superscript letters are significantly different at P < 0·05 (i.e. ‘a’ is different from ‘b’, but ‘ab’ does not differ from ‘a’ or ‘b’). HFS, high fat/sucrose; HFS + OFS, high fat/sucrose supplemented with oligofructose; HFS-WM, high fat/sucrose fed weight-matched to HFS + OFS.
Of the seven miRNA analysed (miR-222, miR-203a, miR-200a, miR-26a, miR-27a, miR-103 and miR-148a), six were differentially expressed according to maternal diet (Fig. 2). HFS + OFS dams had greatly reduced miR-222 levels which were significantly lower than HFS (P = 0·033) and HFS-WM (P = 0·0001) dams (Fig. 2a). HFS + OFS and HFS-WM dams had increased miR-203a levels which were significantly greater than LEAN (P = 0·007; P = 0·019) dams (Fig. 2b). HFS + OFS dams had significantly reduced miR-200a levels compared with HFS-WM (P = 0·004) dams (Fig. 2c). HFS + OFS dams had greatly reduced miR-26a and miR27a levels which were significantly lower than HFS (P = 0·001; P = 0·012), HFS-WM (P = 0·002; P = 0·01) and LEAN (P = 0·001; P = 0·024) dams (Fig. 2d and e). HFS + OFS dams had significantly reduced miR-103 levels compared with HFS-WM (P = 0·012) and LEAN (P = 0·014) dams (Fig. 2f). Last, miR-222 and miR-200a are strongly correlated (R = 0·894, P < 0·001).

Fig. 2. Relative expression levels of seven microRNA in maternal milk at weaning (day 21). Values are mean with their standard errors with n 7–10 per group. Treatments with different superscript letters are significantly different at P < 0·05 (i.e. ‘a’ is different from ‘b’, but ‘ab’ does not differ from ‘a’ or ‘b’). HFS, high fat/sucrose; HFS + OFS, high fat/sucrose supplemented with oligofructose; HFS-WM, high fat/sucrose fed weight-matched to HFS + OFS.
Maternal milk composition correlates with maternal body composition
We used correlation analysis to investigate the relationship between maternal body composition at weaning and maternal milk content (Table 4). Milk leptin content was negatively correlated with maternal lean mass (P = 0·047) and positively correlated with maternal body fat percentage (P = 0·043). Milk protein content was inversely associated with maternal fat mass (P = 0·001), body fat percentage (P = 0·000) and body weight (P = 0·041). Of the miRNA analysed, miR-200a and miR-222 were positively associated with maternal fat mass (P = 0·001; P = 0·011), body fat percentage (P = 0·008; P = 0·031) and body weight (P = 0·001; P = 0·003). In terms of milk composition, milk leptin content and milk protein content were inversely correlated (P = 0·022).
Table 4. Correlations between maternal milk components and maternal body composition

Maternal milk composition correlates with offspring body composition by sex
We investigated the relationship between milk components and adult offspring body composition following their consumption of a HFS diet until 24 weeks of age. Data on the offspring outcomes have been previously published(Reference Paul, Collins and Nicolucci33) and used in the present analysis. Correlation analysis revealed associations between maternal milk content and body composition that differed by sex (Table 5). In female offspring, milk protein content was negatively associated with adult fat mass (P = 0·035), body fat percentage (P = 0·043) and body weight (P = 0·032). Expression levels of miR-222 in milk were positively correlated with female liver TAG (P = 0·046), and expression levels of miR-200a showed a similar trend (P = 0·067). In male offspring, expression levels of miR-27a in milk were positively correlated with fat mass (P = 0·004) and body fat percentage (P = 0·004); miR-26a was positively correlated with body fat percentage (P = 0·031); and miR-148a was positively associated with fat mass (P = 0·022). Similar trends were identified between the expression levels of miR-27a, miR-26a and miR-148a and male offspring body composition, including negative trends with lean mass and positive trends with body weight.
Table 5. Correlations between maternal milk components and offspring outcomes at 24 weeks of age

Maternal milk composition correlates with offspring microbiota measures at 3, 11 and 24 weeks of age
We used correlation analyses to investigate the relationship between milk components and previously published offspring faecal microbiota relative abundance of select bacterial groups at 3, 11 and 24 weeks of age(Reference Paul, Collins and Nicolucci33). Given the exploratory nature of the correlation analysis, we present all of the correlations with a P < 0·05 in Table 6, but have focused the description here on those with a P < 0·002. Certain bacteria were more likely to be highly significantly associated with milk miRNA. Enterobacteriaceae showed numerous correlations. At 3 weeks of age, it was positively associated with miR-203a (P = 0·0001); at 24 weeks of age in female offspring, it was associated with miR-200a and miR-148a (P < 0·001) and at 24 weeks of age in male offspring, it was associated with milk leptin (P = 0·001). Bifidobacterium spp. at 11 weeks of age in female offspring was positively associated with miR-200a and miR-148a (P = 0·0001). In male offspring at 24 weeks of age, Akkermansia muciniphila was associated with miR-203a (P = 0·0001) and Roseburia spp. was associated with miR-27a (P = 0·002) and miR-148a (P = 0·0001).
Table 6. Correlations between maternal milk components and offspring microbiota measured using quantitative PCR at 3, 11, and 24 weeks of age*

* Gut microbiota was previously reported as percentage relative abundance from faecal samples collected at 3, 11 and 24 weeks of age(Reference Paul, Collins and Nicolucci33). Faecal samples at 3 weeks were extremely small, and male and female samples were therefore pooled within litters.
Discussion
Breast milk, as the first and potentially sole source of neonatal nutrition, plays a key role in the development of various physiological systems. Despite definitive evidence indicating better health outcomes for exclusively breastfed infants(Reference Horta, Loret de Mola and Victora4,Reference Stuebe and Schwarz5) , the mechanisms underlying the health benefits remain incompletely understood. In this study, we show that breast milk composition, particularly miRNA levels, can be altered according to maternal diet. Specifically, our results demonstrate that protein content and miRNA levels in breast milk differ between dams consuming obesogenic diets alone or the same diet supplemented with a prebiotic OFS. Furthermore, these differences in milk composition correlate with both maternal and offspring body weight, fat mass and body fat percentage as well as offspring gut microbiota at 3, 11 and 24 weeks of age, indicating a potential link between maternal diet, milk composition and offspring phenotype.
The protein content in breast milk contains a variety of bioactive compounds that may contribute to the overall health benefits of breast-feeding(Reference Lonnerdal6). In our study, the milk of obese dams consuming a HFS diet had significantly lower protein content than lean dams and obese dams supplemented with OFS. Overall, the protein content in milk was negatively associated with maternal body weight, fat mass and body fat percentage. Although these correlations align with previous studies(Reference Pomar, van Nes and Sánchez31,Reference Bachour, Yafawi and Jaber37) , other studies have reported positive associations between maternal adiposity/BMI and milk protein composition(Reference Leghi, Netting and Middleton38). The discrepancy in results may be due to variations in the timing of milk collection, as protein concentration decreases over time(Reference Pomar, van Nes and Sánchez31,Reference Bzikowska-Jura, Czerwonogrodzka-Senczyna and Olędzka39) , and varies with diet during lactation, which may not have been uniformly accounted for in a number of studies(Reference Leghi, Netting and Middleton38).
The decrease in protein content in milk from obese HFS dams could plausibly reflect a reduction in bioactive proteins that have immunomodulatory and antibacterial properties. Indeed, a recent study demonstrated that cafeteria diet-fed dams produced milk with reduced bioactive protein concentrations compared with control rats’ milk(Reference Pomar, Sánchez and Palou40). Maternal secretory IgA is transferred via the enteromammary pathway into breast milk and provides protection against infection until the neonate can produce sufficient antibodies on their own(Reference Brandtzaeg41). The lack of protein seen in HFS dam milk may reflect a decrease in secretory IgA transferred to the offspring, although we did not directly measure this in our study. Lactoferrin, a bioactive protein highly abundant in breast milk, synergistically functions with breast milk-derived lysozyme to sequester Fe from pathogens. Clinical trials indicate that supplementation of infant formula with lactoferrin and lysozyme results in fewer infections and quicker diarrhoea relief due to both bactericidal action and immunomodulatory mechanisms(Reference King, Cummings and Guo42–Reference Zavaleta, Figueroa and Rivera45). Furthermore, the immunomodulatory effects and the antibacterial properties of bioactive proteins may serve to help shape the neonatal gut microbiota towards a more beneficial community and thereby enable typical development of the immune system and intestinal barrier(Reference Brandtzaeg41,Reference van den Elsen, Garssen and Burcelin46) . Future studies that analyse offspring gut microbiota in parallel with immunomodulatory effects would provide valuable insight into how this potential gut microbiota–breast milk–immune linkage is affected by maternal obesity and prebiotic supplementation.
We used a weight-matching protocol (HFS-WM) to investigate the effect of OFS independent of the commonly seen effects it has on reducing body weight and adiposity. The rescue of milk protein composition in prebiotic-fed dams is likely a result of OFS supplementation rather than weight loss because the HFS-WM milk protein content was not significantly different from the HFS dams. Although OFS supplementation rescued the protein content in milk, further studies are needed to determine the underlying mechanism behind OFS’ ability to alter breast milk protein content, as well as the specific protein fraction altered.
Milk leptin did not differ according to dietary group, but it was negatively correlated with milk protein content and maternal lean mass and positively correlated with maternal body weight. Milk leptin was not correlated with offspring body composition but was associated with certain faecal bacteria. Most notably, Enterobacteriaceae in males at 24 weeks of age was positively associated with milk leptin (r = 0·597; P = 0·001). If additional relationships with significance closer to P < 0·05 are considered, Lactobacillus spp. in males at 11 weeks of age (r = –0·397; P = 0·044) and Roseburia spp. abundance in females at 24 weeks of age (r = –0·396; P = 0·03) were also related to milk leptin. Although 11 and 24 weeks of age are far removed from the offspring’s exposure to maternal milk leptin, it is interesting that Enterobacteriaceae which is considered an inflammatory bacteria(Reference Zeng, Inohara and Nuñez47) and Roseburia spp. which is considered a beneficial butyrate producer(Reference Rivière, Selak and Lantin48) would be positively and negatively correlated, respectively, with milk leptin. Previous studies have reported strong correlations between breast milk leptin and maternal BMI and adiposity(Reference Andreas, Hyde and Gale49,Reference Uysal, Onal and Aral50) , although no definitive relationship between milk leptin content and infant body composition has been established(Reference Mazzocchi, Giannì and Morniroli51).
In addition to bioactive proteins, milk also contains bioactive components in the form of miRNA that may serve as epigenetic regulators and alter offspring development and metabolism. We demonstrated that miRNA levels differ according to maternal diet (miR-222, miR-203a, miR-200a, miR-26a, miR-27a and miR-103) and correlate with maternal (miR-200a and miR-222) and offspring (miR-222, miR-200a, miR-27a, miR-26a and miR-148a) body composition. Certain bacteria in the faecal matter of offspring at 3, 11 and 24 weeks of age were also correlated with milk miRNA levels that could provide insight into some of the phenotypic differences across groups. Although no studies currently link milk miRNA expression and offspring gut microbiota, Liu et al. (Reference Liu, da Cunha and Rezende52) demonstrated that miRNA can affect growth and performance of gut microbiota and experiments performed by Zhou et al. (Reference Zhou, Paz and Sadri53) indicate that RNA packaged in milk exosomes can shape overall gut microbiota communities.
Milk miR-222 and miR-200a levels were increased in the HFS-WM groups and decreased in the OFS-supplemented group. Furthermore, miR-222 and miR-200a were positively associated with maternal body weight, fat mass and body fat percentage and female offspring liver TAG. In addition, miR-200a was positively associated with the abundance of proinflammatory Enterobacteriaceae in female offspring at 24 weeks of age when the strongest metabolically disturbed phenotype was evident. miR-222 is up-regulated in adipose tissue and blood in patients with obesity and type 2 diabetes(Reference Xie, Lim and Lodish54,Reference Ortega, Mercader and Moreno-Navarrete55) , in milk of overweight/obese mothers(Reference Zamanillo, Sanchez and Serra17) and in the adipose tissue of diabetic rats(Reference Herrera, Lockstone and Taylor56). In vitro studies indicate that miR-222 may be part of the initial cellular response to hyperglycaemia(Reference Herrera, Lockstone and Taylor56) and may be associated with inflammation in adipose tissue(Reference Xie, Lim and Lodish54). We previously showed through serum metabolomics analysis that the HFS-WM dams were under stress and this may have contributed to an inflammatory profile that increased proinflammatory cytokines and subsequently increased miR-222 expression(Reference Paul, Bomhof and Vogel30). In addition, Ono et al.(Reference Ono, Igata and Kondo57) identified miR-222 as a negative regulator of insulin resistance via the down-regulation of insulin receptor substrate-1 in the liver. An up-regulation of miR-200a is associated with non-alcoholic fatty liver disease(Reference Alisi, Da Sacco and Bruscalupi58–Reference Ezaz, Trivedi and Connelly60), and in vitro studies show an increase in miR-200a expression in hepatocytes exposed to NEFA and proinflammatory factors(Reference Feng, Xu and Ji61). Similar to miR-222, miR-200a may have been up-regulated in HFS-WM dams due to stress and the positive correlation between female offspring liver TAG and miR-222 and miR-200a may be explained through these inflammatory mechanisms. In line with these observations, OFS supplementation has been associated with an anti-inflammatory profile(Reference Kumar, Ward and Brown62,Reference Dehghan, Pourghassem Gargari and Asghari Jafar-abadi63) ; therefore, the decrease in miR-222 and miR-200a in milk from HFS-OFS dams may be reflective of the inflammation-reducing effects of OFS supplementation.
Our results revealed a decrease in milk levels of miR-27a, miR-103 and miR-26a from OFS-supplemented dams compared with milk from lean, HFS-WM and HFS (except miR-103) dams and positive correlations between these miRNA and male offspring adiposity. Although our results do not align with previous studies that indicate miR-26a overexpression is beneficial, miR-27a and miR-103 are up-regulated in obesity, the metabolic syndrome and type 2 diabetes(Reference Pomar, Castro and Pico22,Reference Ji and Guo64,Reference Yu, Du and Wei65) . miR-27a, chiefly secreted by adipocytes, targets PPARγ and facilitates M1-macrophage polarisation(Reference Yu, Du and Wei65). miR-103 is involved in insulin signalling, increased in breast milk of mothers with overweight/obesity and anti-sense regulation rescues insulin resistance in diet-induced obesity animal models(Reference Zamanillo, Sanchez and Serra17,Reference Trajkovski, Hausser and Soutschek66) . The reduction in miR-27a and miR-103 concentration in milk from OFS-supplemented dams may reflect the lower levels of circulating miRNA in dams, rather than represent miRNA levels produced by the lactocytes specifically for the milk transfer. Although recent studies suggest that the miRNA levels in breast milk primarily originate from the mammary glands, there is evidence that there could be a small contribution of maternal circulation to miRNA in breast milk(Reference Alsaweed, Lai and Hartmann67).
To the best of our knowledge, this is the first paper to investigate the effects of OFS supplementation in an obese HFS model on the composition of breast milk and subsequent association with offspring metabolic markers. Furthermore, the weight-matching group in the study allowed us to separate the effects of OFS per se from the effects of OFS-induced weight loss. Although we measured differences in milk miRNA levels between groups, we did not measure the concentration in offspring plasma or target tissues to confirm the miRNA can reach the target tissue and produce relevant changes in gene expression. Second, we only collected milk at one time point, although studies suggest that protein, hormone and miRNA concentrations vary according to the period of lactation. Last, our dietary experiment lasted from gestation through lactation, so the results cannot be attributed exclusively to either period.
In conclusion, we show that breast milk composition varies in protein and miRNA levels according to maternal diet and obesity status and correlates with maternal and offspring body composition and offspring faecal microbiota. Specifically, obesogenic diets can alter the protein and miRNA content of breast milk, and OFS can partially rescue these alterations back towards lean dams. Future studies are needed to examine a broader range of offspring outcomes and other characteristics of milk derived from prebiotic-supplemented dams. Although much remains to be learned about breast milk miRNA and their impact on offspring health, this preclinical study suggests that they may be influenced by maternal diet.
Acknowledgements
The authors thank Kristine Lee for technical assistance with milk analysis.
The study was funded by the Canadian Institutes of Health Research (PJT-159626).
H. A. P. and R. A. R. were responsible for planning the original study the samples were derived from. D. E. L. and R. A. R. were responsible for the analysis. D. E. L. prepared the first draft of the manuscript which was revised by H. A. P. and R. A. R. All authors read and approved the final manuscript.
D. E. L. and H. A. P. declare no conflicts of interest. R. A. R. has received honoraria from Beneo GmbH for work distinct from the current study.










