Glucagon-like peptide-1 (GLP-1) is a gut hormone that is produced and released by enteroendocrine L-cells. GLP-1 potentiates insulin secretion from pancreatic β-cells, attenuates gastric emptying rate and reduces appetite(Reference Drucker1). These physiological effects make GLP-1 a convincing target for treating type 2 diabetes. A serine protease dipeptidyl peptidase-4 (DPP-4) cleaves the N-terminal dipeptide (X-Pro, X-Ala; X is an amino acid) from peptides, including GLP-1(Reference Deacon2), which results in the loss of its bioactive properties as incretin. Thus, DPP-4 inhibitors help to preserve the bioactivity of GLP-1.
It has been well recognised that digestive products from macronutrients (glucose(Reference Kokrashvili, Mosinger and Margolskee3), fatty acids(Reference Tanaka, Yano and Adachi4), peptides(Reference Diakogiannaki, Pais and Tolhurst5) and amino acids(Reference Oya, Kitaguchi and Pais6)) enhance plasma GLP-1 levels. In addition, dietary proteins and peptides may be promising sources of ‘DPP-4 inhibitory peptides’. Several studies reported DPP-4 inhibitory action of peptides derived from milk protein(Reference Tulipano, Sibilia and Caroli7–Reference Nongonierma and FitzGerald9), rice protein(Reference Hatanaka, Inoue and Arima10), wheat protein(Reference Taga, Hayashida and Kusubata11) and Atlantic salmon protein(Reference Neves, Harnedy and O’Keeffe12), in vitro.
We previously demonstrated that rice protein and corn zein hydrolysate stimulated GLP-1 secretion in vitro and in vivo; moreover, these peptides decreased plasma DPP-4 activity in rats in situ studies(Reference Ishikawa, Hira and Inoue13–Reference Mochida, Hira and Hara15). Here, we hypothesised that protein ingestion increases plasma GLP-1 concentrations, larger than carbohydrate ingestion, possibly through both stimulating GLP-1 secretion by luminal peptides and potential inhibition of plasma DPP-4 activity by absorbed small peptides. Experimental data supporting the hypothesis were mainly obtained by in vitro experiments, but not by in vivo experiments.
In the current in vivo study, we measured plasma GLP-1 concentrations in rats after oral administration of dextrin and whey protein as a general dietary carbohydrate and protein, respectively. Whey protein is well known to have potent GLP-1 releasing activity(Reference Hutchison, Piscitelli and Horowitz16–Reference Giezenaar, Lange and Hausken18). To examine the involvement of DPP-4 activity in protein/carbohydrate-induced GLP-1 responses, we employed normal rats, treated with a DPP-4 inhibitor, and DPP-4-deficient F344/DuCrl/Crlj rats(Reference Erickson, Suzuki and Slmayer19). DPP-4 inhibitors or DPP-4-deficient models have been used to investigate the role of endogenous DPP-4, but there are few studies comparing GLP-1 responses to nutrients in the presence or absence of DPP-4, in order to test the possible role of exogenous DPP-4 inhibitory factors. Moreover, we examined the effect of another dietary protein and a lipid on GLP-1 responses in rats treated with a DPP-4 inhibitor, to clarify the specificity of whey protein and/or dietary proteins.
Materials and methods
Materials
Whey protein isolate (whey protein, WPI8855, Fonterra) was provided by Morinaga Milk Industry Co., Ltd., and dextrin (TK-16, the degree of polymerisation: 5·5) was provided by Matsutani Chemical Industry. The remaining reagents were purchased from Wako Pure Chemical Industries unless otherwise specified.
Animals and measurements of plasma glucagon-like peptide-1 concentration, dipeptidyl peptidase-4 activity and acetaminophen concentration
Male Sprague–Dawley (SD) rats (210–230 g), F344/DuCrl/Crlj rats (DPP-4 (–), 120–140 g) and F344/Jcl rats (DPP-4 (+), 140–170 g) aged 7 weeks were purchased from Japan SLC (Hamamatsu), Charles River Japan (Yokohama) and CLEA Japan, Inc., respectively. Animals were housed in individual cages, had free access to water and received an AIN-93G diet(Reference Reeves20) in a controlled room at 23 (sem 2)°C temperature, 60 (sem 5) % humidity and a 12 h light/dark cycle (light period, 08.00–20.00 hours). Animal experiments were performed after an acclimation period of 1 week. Rats were fasted overnight (approximately 16 h) before the experiments. This study was approved by the Hokkaido University Animal Committee, and the animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals of Hokkaido University.
To measure plasma GLP-1 concentration, blood samples were immediately mixed with aprotinin (final concentration 500 kIU/ml), heparin (final concentration 50 IU/ml; Nacalai Tesque, Inc.) and a DPP-IV inhibitor (final concentration, 50 μm; DPP4-010; Millipore Co.) on ice. To measure plasma DPP-4 activity, blood samples were immediately mixed with heparin (final concentration 50 IU/ml) on ice. Plasma was separated from the blood samples by centrifugation at 2300 g for 10 min at 4°C and then frozen at –80°C until measurements were taken.
Active GLP-1 concentrations were measured using an ELISA kit (27700; Immuno-Biological Laboratories Co, Ltd). The plasma acetaminophen concentrations were measured using an acetaminophen detection kit (01601-96; Kanto Kagaku). The DPP-4 activity was determined by using a surrogate substrate (Gly-Pro-p-nitroaniline, Gly-Pro-pNA, Sigma), as previously described(Reference Ishikawa, Hira and Inoue13,Reference Flock, Baggio and Longuet21) . One unit of DPP-4 activity was defined as the liberation of 1 μmol of pNA in 1 min.
Glucagon-like peptide-1 responses to oral administration of whey protein, dextrin, casein and Intralipos in Sprague–Dawley rats treated with or without dipeptidyl peptidase-4 inhibitor
Sitagliptin (BioVision, Inc. or Sigma) was used as an orally effective DPP-4 inhibitor. Sitagliptin (50 mg/kg dissolved in deionised water) or deionised water (5 ml/kg) was orally administered at –120 min to SD rats using a feeding tube (6 Fr; Atom Medical). Then, whey protein, dextrin (both at 2·0 g/kg) or deionised water (control) was orally administered (10 ml/kg) at 0 min. These test solutions contained acetaminophen (100 mg/kg) to assess the gastric emptying rate(Reference Heading, Nimmo and Prescott22,Reference Maida, Lovshin and Baggio23) . Blood samples were collected from the tail vein from –120 to 120 min for GLP-1, DPP-4 activity and acetaminophen measurement, respectively. Furthermore, GLP-1 responses to casein Na (casein, 2 g/15 ml/kg) and a lipid (Intralipos at 0·89 g/10 ml/kg, soyabean oil, Otsuka Pharmaceutical Co., Ltd.) were examined under the same experimental procedure. Because plasma DPP-4 activity was suppressed from 60 to 240 min after the sitagliptin treatment in our preliminary study, nutrients were orally given 120 min after oral sitagliptin treatment.
Glucagon-like peptide-1 responses to oral administration of whey protein and dextrin in dipeptidyl peptidase-4-deficient (F344/DuCrl/Crlj) rats and dipeptidyl peptidase-4-positive (F344/Jcl) rats
After basal blood collection (0 min), whey protein, dextrin (both at 4·0 g/kg) or deionised water (control) was orally administered (10 ml/kg) to F344/DuCrl/Crlj rats and F344/Jcl rats. Test solutions contained acetaminophen (100 mg/kg), and blood samples were collected from the tail vein until 60 min after the oral administration to measure GLP-1 and acetaminophen concentrations.
Collection of intestinal tissue and blood from vena cava in F344 rats
After overnight fasting, blood samples were collected from the vena cava under sodium pentobarbital anaesthesia (50 mg/kg, Somnopentyl injection; Kyoritsu Seiyaku Corporation) to measure plasma DPP-4 activity. After the rats were euthanised by exsanguination, the duodenum, jejunum, ileum, caecum and colon were removed. Luminal contents of intestinal tissues were washed out with cold saline, and then, a 2 cm segment was respectively collected from middle regions of these tissues for GLP-1 content measurement. The intestinal segments were immediately frozen in N2 and stored at –80°C.
Measurement of glucagon-like peptide-1 content in intestinal tissues
Intestinal segments were homogenised in ethanol–acid solution (ethanol/water/12 M HCl, 74:25:1; 5 ml/g of tissue)(Reference Cani, Hoste and Guiot24) and extracted for 24 h at 4°C. After centrifugation (2000 g for 20 min), the supernatants were collected and diluted (250-fold) with saline to measure the total GLP-1 concentration by ELISA (EZGLP1T-36K; Millipore). Tissue protein contents were measured using Lowry’s method.
Determination of dipeptidyl peptidase-4 inhibitory activity of luminal contents
After overnight fasting, 2 g/kg whey protein or deionised water (control) was orally administered (15 ml/kg) to SD rats. In this study, dextrin was not included because it was not expected that dextrin digestive products (glucose and its oligomers) inhibit DPP-4. At 15 min later, rats were euthanised by exsanguination under sodium pentobarbital anaesthesia (50 mg/kg, Somnopentyl injection; Kyoritsu Seiyaku Corporation), and the small intestine was removed. Luminal contents of the small intestine were washed out with ice-cold deionised water (10 ml) and collected. The collected luminal contents were stored at –30°C.
The luminal contents were boiled for 20 min to inactivate existing enzymes including DPP-4. After centrifugation (3500 g for 10 min at 22°C), the supernatant was collected; deionised water (5 ml) was added to the precipitate and then centrifuged again (3500 g for 10 min at 22°C) to collect the supernatant. The supernatants were combined, freeze-dried and then dissolved in 500 µl of deionised water. The solution (450 µl) was mixed with ice-cold acetonitrile (900 µl) and left for 3 h at –30°C to precipitate insoluble materials, including proteins. Then, the mixture was centrifuged (15 000 g for 5 min at 4°C), and the collected supernatant was evaporated. The luminal extract was dissolved in 48·6 µl of assay buffer (0·25 m Tris-HCl buffer, pH 8·0). The volume (48·6 µl) was determined based on the rough estimation that the liquid volume of luminal content was approximately 2–3 ml in rats used in the present study.
To investigate the DPP-4 inhibitory activity of the extracts from luminal contents, 75 µl of assay buffer (0·25 M Tris-HCl buffer, pH 8·0) was added to each well of a flat-bottomed ninety-six-well plate, and then, 5 µl of human DPP-4 (20 mU/ml in the reaction, Sigma) was added. The luminal extract was further diluted (× 1, × 1/5, × 1/25) to cover a wide range of estimated luminal volume. The luminal extract (5 µl) with various dilutions, or Diprotin A (Ile-Pro-Ile as a positive control, final concentration in the reaction at 10 µm, Peptide Institute, Inc.) was added to the well. The reaction was started by adding 80 μl of 1·6 mm Gly-Pro-pNA. After incubation at 37°C for 60 min, 40 μl of 1 m acetate (pH 4·2) was added to stop the reaction, and the absorbance at 405 nm was measured (‘absorbance A’) using a microplate reader (Infinite 200; Tecan Group Ltd). As the negative control for each extract, the absorbance at 405 nm was measured (‘absorbance B’) when the human DPP-4 was added to the assay mixture containing the extract, substrate and acetate after the 60-min incubation. The activity of DPP-IV was calculated by using the absorbance subtracting ‘absorbance B’ from ‘absorbance A’.
Statistical analysis
The sample size was calculated based on the experimental design (two-way repeated-measures ANOVA) determining the GLP-1 responses to the nutrients as the primary outcome measure. We used G × Power software (version 3.1.9.4) for the power analysis with following variables: the power = 0·8, significant level = 0·05 and effect size = 0·37 in the experiment in Fig. 5 or effect size = 0·3 in the other experiments. The effect size was estimated based on the results from a preliminary study. Hence, the required sample size was five (Figs. 1 and 5) and seven (Fig. 2) rats per group.

Fig. 1. Plasma glucagon-like peptide-1 (GLP-1) responses and dipeptidyl peptidase-4 (DPP-4) activity after oral administration of dextrin or whey protein in rats with or without sitagliptin treatment. (A and C) Changes in plasma GLP-1 concentrations after oral administration of water (control, 10 ml/kg, ![]() ), 2 g/kg dextrin (
), 2 g/kg dextrin (![]() ), 2 g/kg whey protein (
), 2 g/kg whey protein (![]() ) with or without 50 mg/kg sitagliptin treatment. (B and D) The AUC of changes (ΔAUC) in GLP-1 concentrations from 0 min. (E) Changes in plasma DPP-4 activity after sitagliptin treatment in conscious rats. Blood samples were collected at –120, –60, 0, 15, 30, 60, 90 and 120 min. Values are means with their standard errors (n 5–6). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). a,b Plots that do not share the same letter differ significantly between the treatments (P < 0·05, Tukey–Kramer’s test). In (E), significant differences compared with –120 min values within each treatment were evaluated using Dunnett’s test (P < 0·01). The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel (A, C, E).
) with or without 50 mg/kg sitagliptin treatment. (B and D) The AUC of changes (ΔAUC) in GLP-1 concentrations from 0 min. (E) Changes in plasma DPP-4 activity after sitagliptin treatment in conscious rats. Blood samples were collected at –120, –60, 0, 15, 30, 60, 90 and 120 min. Values are means with their standard errors (n 5–6). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). a,b Plots that do not share the same letter differ significantly between the treatments (P < 0·05, Tukey–Kramer’s test). In (E), significant differences compared with –120 min values within each treatment were evaluated using Dunnett’s test (P < 0·01). The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel (A, C, E).

Fig. 2. Plasma glucagon-like peptide-1 (GLP-1) responses after oral administration of dextrin or whey protein and plasma dipeptidyl peptidase-4 (DPP-4) activity in DPP-4 (+) and DPP-4 (–) rats. (A and C) Changes in plasma GLP-1 concentrations after oral administration of water (control, ![]() ), 4 g/kg dextrin (
), 4 g/kg dextrin (![]() ), 4 g/kg whey protein (
), 4 g/kg whey protein (![]() ) in conscious DPP-4 (+) and DPP-4 (–) rats. (B and D) The AUC of changes (ΔAUC) in GLP-1 concentrations from 0 min. Blood samples were collected before (0 min) and after the oral administration of test solutions (10 ml/kg). Values are means with their standard errors (n 7–9). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). a,b Plots that do not share the same letter differ significantly between the treatments (P < 0·05, Tukey-Kramer’s test). (E) Plasma DPP-4 activity after overnight fasting in anesthetised DPP-4 (+) and DPP-4 (–) rats. Blood samples were collected from the vena cava. Values are means with their standard errors (n 12–13). N.D., not detected. The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel (A, C).
) in conscious DPP-4 (+) and DPP-4 (–) rats. (B and D) The AUC of changes (ΔAUC) in GLP-1 concentrations from 0 min. Blood samples were collected before (0 min) and after the oral administration of test solutions (10 ml/kg). Values are means with their standard errors (n 7–9). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). a,b Plots that do not share the same letter differ significantly between the treatments (P < 0·05, Tukey-Kramer’s test). (E) Plasma DPP-4 activity after overnight fasting in anesthetised DPP-4 (+) and DPP-4 (–) rats. Blood samples were collected from the vena cava. Values are means with their standard errors (n 12–13). N.D., not detected. The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel (A, C).
Results are expressed as mean values with their standard errors. The AUC was calculated by the trapezoidal rule. Statistical significance was determined using one-way or two-way ANOVA to assess the main effects (treatment and time), as well as the interaction effects (treatment × time), using JMP Pro software version 13 (SAS Institute). Statistical significances between mean values were evaluated using Tukey–Kramer’s test, Dunnett’s test or Student’s t test as appropriate.
Results
Whey protein increased plasma glucagon-like peptide-1 concentrations with or without sitagliptin treatment, but dextrin failed without sitagliptin treatment in Sprague–Dawley rats
In rats without sitagliptin treatment, plasma GLP-1 concentrations significantly increased 30 and 60 min after the administration of whey protein compared with the basal (0 min) value (approximately 1·5 pm), while the same dose of dextrin had only small effects on GLP-1 concentrations (Fig. 1(A)). The AUC (ΔAUC) for changes in GLP-1 levels in the whey protein group was apparently higher than the values in the control and dextrin groups (Fig. 1(B)).
When rats were pretreated with sitagliptin, plasma DPP-4 activity was largely and continuously decreased by 20–50 % throughout the experimental period (Fig. 1(E)). Accordingly, plasma GLP-1 levels were elevated (approximately 1 pM) before the nutrient challenge (Fig. 1(C)). Plasma GLP-1 concentrations were largely increased 15 and 30 min after the oral administration of whey protein (approximately 2·5 pm) and dextrin (approximately 2 pm), with significant differences compared with the basal levels (Fig. 1(C)). Oral administration of dextrin elevated plasma GLP-1 concentrations until 60 min, similar to that of whey protein. The GLP-1 concentrations in whey protein and dextrin groups at 15, 30 and 60 min were significantly higher than those in the control group. The elevated GLP-1 levels were maintained in the whey protein group, but were gradually decreased after 90 min towards the basal level in the dextrin group. These results were reflected in ΔAUC values where the dextrin group had an intermediate value between those in the control and whey protein groups (Fig. 1(D)). When the data were presented as the changes from the value at 0 min (ΔGLP-1, online Supplementary Fig. S1(A) and (B)), sitagliptin treatment largely enhanced GLP-1 response to dextrin (approximately 1·2–1·4 pm, >3-fold increment) at 15 and 30 min compared with the response without sitagliptin, but the treatment had a relatively small effect on enhancing GLP-1 response to whey protein (approximately 0·8 pm, 1·5-fold increment) at 30 min.
Whey protein increased plasma glucagon-like peptide-1 concentrations in F344 rats with or without endogenous dipeptidyl peptidase-4, but dextrin increased glucagon-like peptide-1 levels only in dipeptidyl peptidase-4-deficient rats
To examine whether DPP-4 was involved in the difference in GLP-1 responses to whey protein and dextrin, we employed F344/DuCrl/Crlj rats as a DPP-4 (–) model and F344/Jcl rats as a DPP-4 (+) control. The deficiency of plasma DPP-4 activity in F344/DuCrl/Crlj rats was confirmed as shown in Fig. 2(E).
Consistent with the result in untreated SD rats (Fig. 1(A)), in DPP-4 (+) rats, plasma GLP-1 concentrations only increased in the whey protein group and that was significantly higher compared with those in the control and dextrin groups, from 15 to 60 min (Fig. 2(A)). Dextrin did not increase, rather decreased GLP-1 concentrations. The ΔAUC in the whey protein group was significantly higher than that in the control and dextrin groups (Fig. 2(B)).
In DPP-4 (–) rats, plasma GLP-1 concentrations increased 15 min after whey protein and dextrin administration (Fig. 2(C)). The GLP-1 concentrations at 30 min and ΔAUC of GLP-1 (Fig. 2(D)) in these groups were significantly higher than that in the control group.
Effects on gastric emptying rates after whey protein and dextrin administration
The rate of gastric emptying determines nutrient delivery into the intestinal lumen and could affect gut hormone secretory responses. In the absence of sitagliptin, changes in plasma acetaminophen concentration were significantly lower at 15 and 30 min, in both the whey protein and dextrin groups, compared with the control group (Fig. 3(A)). Upon treatment with sitagliptin, although elevation of plasma acetaminophen concentrations was apparently smaller in the whey protein group, those in the dextrin group were partially lower than the control group (Fig. 3(B)). Overall, regardless of sitagliptin treatment, whey protein had relatively larger effects on reducing gastric emptying rate compared with dextrin.
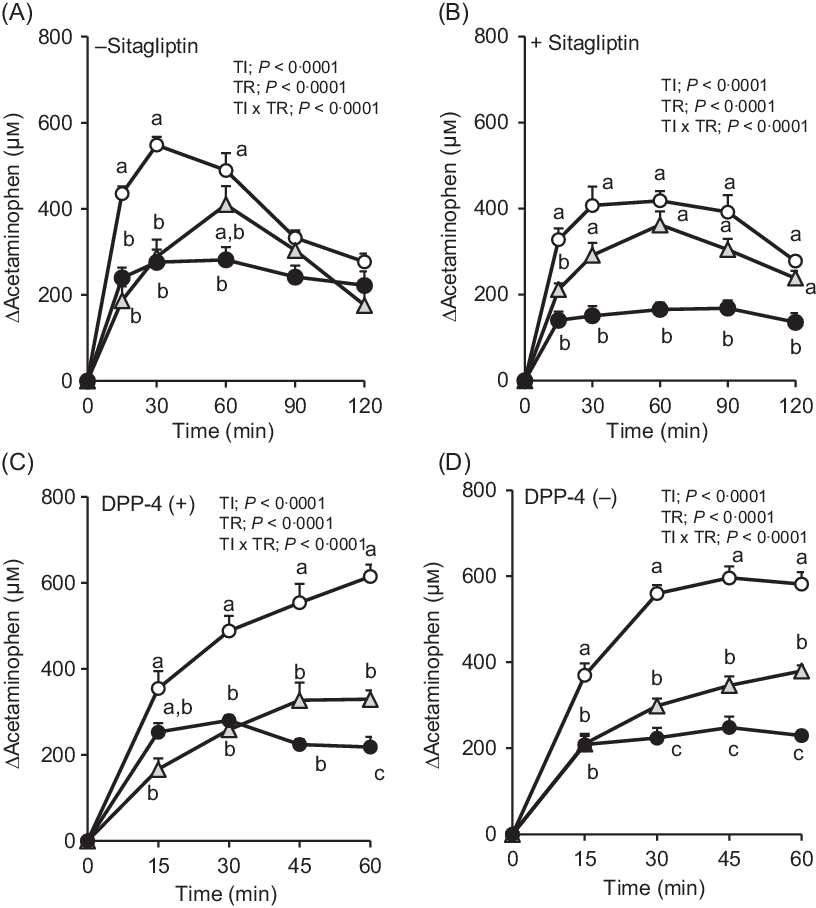
Fig. 3. Changes in plasma acetaminophen concentrations after oral administration of dextrin whey protein. Values are expressed as changes from basal (0 min) values and as mean values with their standard errors (n 5–6) (A, B). Values are expressed as changes from basal (0 min) values and as means with their standard errors (n 7–9) (C, D). a,b,c Plots that do not share the same letter differ significantly between the treatments (P < 0·05, Tukey–Kramer’s test). The P values of two-way repeated ANOVA for time (TI), treatment (TR), and interactions of time and treatment (TI × TR) are shown in each panel. ![]() , Control;
, Control; ![]() , dextrin;
, dextrin; ![]() , whey protein.
, whey protein.
In F344 rats with or without DPP-4, the elevation of plasma acetaminophen concentrations was similarly attenuated both in whey protein and dextrin groups compared with that in the control group (Fig. 3(C) and (D)). The whey protein group had significantly lower acetaminophen concentration than the dextrin group at 60 min in DPP-4 (+) rats, from 30 min to 60 min in DPP-4 (–) rats.
Intestinal tissue glucagon-like peptide-1 contents did not differ between dipeptidyl peptidase-4 (+) and dipeptidyl peptidase-4 (–) rats
Although both DPP-4 (+) and DPP-4 (–) rats originate from the F344 strain, there might be unexpected differences in the intestinal content or the distribution of GLP-1 between these strains. To address this issue, we measured GLP-1 contents in the duodenum, jejunum, ileum, caecum and colon of both strains. The GLP-1 content in the jejunum was tended to be higher in DPP-4 (–) rats than DPP-4 (+) rats (P = 0·068, Student’s t test), but significant differences were not observed in each part between the two strains (data not shown).
Luminal contents from the whey protein-treated rats decreased dipeptidyl peptidase-4 activity
The extracts from luminal contents in the rats gavaged water did not decrease DPP-4 activity, but extracts from whey protein-treated rats significantly decreased DPP-4 activity at ×1/5 and ×1 dilutions (Fig. 4).

Fig. 4. Dipeptidyl peptidase-4 (DPP-4) activity in the presence of luminal contents from rats treated with whey protein. Luminal contents were collected 15 min after oral administration of water or whey protein. DPP-4 activity was measured in the presence of extracts from the luminal contents and Diprotin A (final concentration in the reaction 10 µm, as a positive control). The luminal extracts (× 1) were diluted as indicated (× 1/5, × 1/25). The value of control treatment indicates DPP-4 activity without luminal extracts. Values are presented as means with their standard errors (control, Diprotin A: n 3, water, whey protein: n 4–5). Significant difference compared with the control group: * P < 0·05, ** P < 0·01 (Dunnett’s test).

Fig. 5. Plasma glucagon-like peptide-1 (GLP-1) responses after oral administration of casein or Intralipos in rats with or without sitagliptin treatment. (A and B) Changes in plasma GLP-1 concentrations from the basal (0 min) value. Rats were orally given casein (2 g/15 ml/kg; A), Intralipos (0·89 g/10 ml/kg; B) with (![]() ) or without (
) or without (![]() ) 50 mg/kg sitagliptin treatment. Blood samples were collected at 0, 15, 30, 60, 90 and 120 min. Values are means with their standard errors (n 5–6). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). Significant differences between the treatments: * P < 0·05, ** P < 0·01 (Student’s t test). The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel.
) 50 mg/kg sitagliptin treatment. Blood samples were collected at 0, 15, 30, 60, 90 and 120 min. Values are means with their standard errors (n 5–6). Significant difference compared with basal (0 min) values within each treatment: † P < 0·05, †† P < 0·01 (Dunnett’s test). Significant differences between the treatments: * P < 0·05, ** P < 0·01 (Student’s t test). The P values of two-way repeated ANOVA for time (TI), treatment (TR) and interactions of time and treatment (TI × TR) are shown in each panel.
Sitagliptin strongly enhanced glucagon-like peptide-1 responses to another dietary protein (casein) and a lipid (Intralipos)
Casein increased plasma GLP-1 levels in SD rats with or without sitagliptin treatment, which peaked at 15–30 min after the oral administration (Fig. 5(A)). However, the response to casein was much higher (approximately 1·7 pm at 30 min) with sitagliptin treatment than without the treatment (P = 0·059 at 30 min, Student’s t test). Similarly to casein administration, increased plasma GLP-1 levels after Intralipos administration were significantly enhanced (approximately 1·0 pm at 15 and 30 min, 2·4-fold increment) by sitagliptin treatment (Fig. 5(B)).
Discussion
In the present study, we demonstrated that oral administration of whey protein increased plasma GLP-1 concentrations, but dextrin failed in normal model rats. However, in the absence of DPP-4 activity (pretreatment with a DPP-4 inhibitor and in DPP-4 (–)-deficient model), both nutrients clearly increased plasma GLP-1 concentrations. The gastric emptying rate was unlikely associated with the difference in GLP-1 responses to whey protein and dextrin. The luminal contents from rats gavaged with whey protein decreased DPP-4 activity. Sitagliptin treatment strongly enhanced GLP-1 responses to casein and Intralipos, but the treatment has a relatively small effect on enhancing GLP-1 response to whey protein. These results suggest that whey protein induces higher GLP-1 responses than dextrin, Intralipos, and casein, possibly through both stimulating GLP-1 release potently and protecting released GLP-1 from degradation by DPP-4.
The ingestion of whey protein increased plasma GLP-1 levels dose dependently in healthy men(Reference Hutchison, Piscitelli and Horowitz16). In the present study, oral administration of whey protein potently increased plasma GLP-1 concentrations in normal model rats (SD and F344/Jcl rats, Fig. 1(A) and (B)). Dietary peptides(Reference Hira, Mochida and Miyashita14) and amino acids (glutamine(Reference Tolhurst, Zheng and Parker25), ornithine(Reference Oya, Kitaguchi and Pais6) and phenylalanine(Reference Alamshah, Spreckley and Norton26)) have been reported to stimulate GLP-1 secretion in vivo and in vitro. Amino acids and peptide fragments produced by digestion of whey protein likely induce GLP-1 secretion through direct stimulation on GLP-1-producing enteroendocrine cells.
Oral administration of dextrin, which liberates glucose in the lumen, failed to increase GLP-1 concentrations in normal model rats in the present study (Fig. 1(A) and (B)). The majority of human studies and some of the animal studies demonstrated glucose-induced GLP-1 secretion; however, a previous human study demonstrated a failure of oral glucose (75 g) to increase GLP-1 levels in non-diabetic subjects(Reference Yanagimachi, Fujita and Takeda27). Furthermore, an increase in GLP-1 after glucose ingestion without DPP-4 inhibitor treatment was remarkably lower than that with DPP-4 inhibitor treatment(Reference Tsuchimochi, Ueno and Yamashita28). These reports suggest that GLP-1 released into the blood circulation by luminal glucose is rapidly degraded by DPP-4.
Whey protein had a greater potency of increasing plasma GLP-1 levels than dextrin at the same dose, in normal rats (Fig. 1(A) and (B), and online Supplementary Fig. S1(A)). This is supported by a previous finding that a high protein diet induced higher GLP-1 response than a low protein diet(Reference Lejeune, Westerterp and Adam29). Although the experiments were done separately, whey protein (2 g/kg = 33·5 kJ (8 kcal)/kg) appeared to enhance plasma GLP-1 concentrations (online Supplementary Fig. S1(A)) higher than Intralipos (0·89 g/kg = 33·5 kJ (8 kcal)/kg, Fig. 5(B)) with or without sitagliptin treatment, which implies that the whey protein has potent ability to stimulate GLP-1 release than a lipid emulsion. Sitagliptin treatment largely enhanced GLP-1 response to Intralipos (Fig. 5(B)). These results indicate that GLP-1 response does not simply depend on the energy value of nutrients ingested and suggest that GLP-1 released by Intralipos is rapidly degraded by DPP-4.
The effects of dextrin on delaying gastric emptying rate were relatively smaller (Fig. 3(A), (B) and (D)) or almost similar (Fig. 3(C)), compared with that of whey protein, suggesting relatively faster delivery of dextrin into the small intestine than that of whey protein. Also, the GLP-1 contents in the respective intestinal tissues were almost similar between DPP-4 (+) and DPP-4 (–) rats (data not shown). These results suggest that higher GLP-1 responses by whey protein than dextrin were attributed neither to the faster delivery of whey protein into the intestinal lumen compared with that of dextrin nor to differences in intestinal GLP-1 content between these strains. The major difference in these two strains is that SD rats are outbred strain and F344 rats are inbred strain. Body weight at the same age was relatively smaller in F344 rats than in SD rats. However, it is difficult to specify the reason for the different magnitude of GLP-1 response to the same ingredient (whey protein or dextrin) between two strains.
It is not very surprising that luminal extract from whey-administered rats had an inhibitory effect on DPP-4 activity in vitro (Fig. 4). However, the result indicates that peptides which inhibit DPP-4 activity(Reference Silveira, Martínez-Maqueda and Recio30,Reference Lacroix and Li-Chan31) were liberated by luminal digestion of whey protein, and they existed in the lumen for 15 min after the oral administration of whey protein. We estimated that the liquid volume of luminal content was approximately 2–3 ml in rats here and that the concentration of extract (× 1 dilution) in the experiment was equivalent to that of luminal contents. Even if our estimated liquid volume had been smaller than the actual volume, it would be probably not more than 10 ml and thus the concentration of extract (× 1/5 dilution) is considered to be still lower than or equivalent to that of actual luminal contents.
Low-molecular-weight peptides (di- and tripeptides; Leu-Ala, Leu-Leu, Ile-Pro-Ala) derived from whey protein reportedly decreased DPP-4 activity in vitro; moreover, Ile-Pro-Ala was considered as a competitive inhibitor of DPP-4(Reference Tulipano, Sibilia and Caroli7). Oligopeptides containing Ile-Pro-Ala or Leu-Leu can be generated by trypsin digestion of whey protein(Reference Silveira, Martínez-Maqueda and Recio30). Furthermore, whey protein ingestion decreased DPP-4 activity in the proximal small intestine of mice(Reference Gunnarsson, Winzell and Deacon32). These findings suggest that the digestion of whey protein generates low-molecular-weight peptides, which act as inhibitors of DPP-4.
The intestinal absorption rate or bioavailability of bioactive peptides is generally considered to be low(Reference Foltz, van der Pijl and Duchateau33–Reference Miner-Williams, Stevens and Moughan36). Studies that demonstrate the appearance of intact food peptides in plasma after oral administration of dietary peptides are limited(Reference Foltz, Meynen and Bianco37–Reference Sánchez-Rivera, Ares and Miralles40). The intestinal transport (absorption) of DPP-4 inhibitory peptides has not been clarified yet, either in animal or in human studies. An in vitro study using Caco-2 cells demonstrated only a small percentage (<1 %) of the peptides added to the apical side were transported intact across the cell monolayers(Reference Lacroix, Chen and Kitts41). Because we have not yet succeeded in detecting such peptides in plasma, we only speculate that di- or tri-peptides might have been absorbed into the circulation through the intestinal epithelium and inhibited (masked) DPP-4 activity in the mesenteric vein in our rat study. Accordingly, the degradation of GLP-1 might be reduced in the whey protein group, which resulted in preserving higher GLP-1 concentrations than that in the dextrin group.
Previous human studies demonstrated increased GLP-1 secretion induced by the ingestion of whey protein(Reference Hutchison, Piscitelli and Horowitz16–Reference Giezenaar, Lange and Hausken18). However, there is no human study directly demonstrating that whey protein ingestion attenuates DPP-4 activity. Ohlsson et al. conducted a clinical experiment(Reference Ohlsson, Alsalim and Carr42) investigating GLP-1 responses to dietary protein (a protein mixture containing milk and egg protein) under inhibition of DPP-4 activity. They focused on the clinical efficacy of sitagliptin but not on the distinct effect of whey protein in the study. The result showed that sitagliptin treatment enhanced GLP-1 response to glucose and lipid ingestion (2–3-fold), and the treatment enhanced GLP-1 response to protein ingestion less than 2-fold. The relatively lower efficacy of sitagliptin in enhancing protein-induced GLP-1 response compared with glucose/lipid-induced GLP-1 response partly supports our hypothesis, and our findings could have translational potential for human applications.
DPP-4 activity was reduced by various dietary peptides in previous in vitro (Reference Hatanaka, Inoue and Arima10) and in situ studies(Reference Ishikawa, Hira and Inoue13), in addition to whey peptides. These findings and the present study, using whey protein, suggest that various kinds of dietary proteins(Reference Taga, Hayashida and Kusubata11,Reference Ishikawa, Hira and Inoue13,Reference Geraedts, Troost and Fischer43) might commonly increase plasma GLP-1 concentrations through the dual action similar to the whey protein. However, this was not the case because sitagliptin treatment largely enhanced GLP-1 response to casein (Fig. 5(A)). Although some peptides derived from casein also have DPP-4 inhibitory activity, such as Ile-Pro-Ile(Reference Nongonierma and FitzGerald9), such difference between whey and casein might be attributed to different properties of these proteins including amino acid sequences, digestibility and absorption rates. The absorption rates of casein have been considered lower than whey protein because the acidic conditions in the stomach coagulate casein and thus delay its emptying from the stomach(Reference Dangin, Boirie and Garcia-Rodenas44,Reference Mahé, Messing and Thuillier45) .
We have attempted to demonstrate the decrease in plasma DPP-4 activity and to identify possible DPP-4 inhibitory peptides in plasma after whey protein administration; however, further studies are needed to clarify these issues. The results of this study showed an association between high GLP-1 responses to whey protein and DPP-4 activity, but did not directly show the mechanism of action. These are the limitations of the present study. On the other hand, there are also some strengths of this study. The previous in vitro (Reference Hatanaka, Inoue and Arima10) and in situ (Reference Ishikawa, Hira and Inoue13) studies provided the possibility that peptides derived from dietary proteins not only stimulates GLP-1 secretion but also protects GLP-1 from degradation through inhibiting DPP-4 activity. Few reports are proposing the relationship between dietary protein and DPP-4 activity in vivo (Reference Gunnarsson, Winzell and Deacon32). In the present study, we demonstrated a clear association between GLP-1 responses to nutrients and plasma DPP-4 activity, by conducting separated experiments in rats having no- or reduced-DPP-4 activity. Such an experiment employing DPP-4-deficient animals is hardly replaced by a clinical trial.
In summary, oral administration of whey protein increased plasma GLP-1 concentrations with or without DPP-4 activity, while oral dextrin increased GLP-1 concentrations only in the absence of DPP-4 activity. These findings demonstrate that different GLP-1 responses between whey protein and dextrin are attributed to DPP-4 activity. Our findings do not directly prove, but support our hypothesis that although it is not universal, peptides generated by dietary protein (such as whey protein) digestion have two functions: stimulating GLP-1 secretion in the lumen and inhibiting DPP-4 activity in the local blood. In light of our findings, high GLP-1 responses to dietary protein could hold the key to the prevention and dietary therapy of glucose intolerance.
Acknowledgements
The authors thank Morinaga Milk Industry Co., Ltd. for providing whey protein isolate.
This work was supported by JSPS KAKENHI grant number 18K19158 and The Tojuro Iijima Foundation for Food Science and Technology.
Y. S., T. H. and H. H. designed the research; Y. S. conducted the research, analysed the data and performed statistical analysis; Y. S. and T. H. wrote the paper; T. H. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520002834








