Sulforaphane (SF), found in broccoli as its inactive precursor glucoraphanin (GRP), is considered to be responsible for the reduction of cancer risk that is associated with broccoli consumption. Upon crushing or chewing of fresh broccoli or broccoli sprouts, GRP is hydrolysed to SF by the plant thiohydrolase myrosinase. In instances of myrosinase inactivation, such as overcooking of broccoli, GRP can be hydrolysed to SF by microflora present in the lower gut(Reference Shapiro, Fahey and Wade1, Reference Lai, Miller and Jeffery2). However, GRP hydrolysis by microflora of the lower gut is far less efficient than hydrolysis by endogenous broccoli myrosinase(Reference Conaway, Getahun and Liebes3–Reference Cramer and Jeffery6).
SF protects against the incidence and progression of cancer via several mechanisms including inhibiting phase I cytochrome P450 enzymes, inducing cell-cycle arrest and apoptosis, reducing inflammation, and perhaps most well-characterised, modulating the nuclear factor-erythroid-2-related factor 2/Kelch-like ECH-associated protein 1 pathway(Reference Herr and Buchler7). In the body, SF is metabolised by the mercapturic acid pathway and excreted in the urine, mostly as SF-N-acetylcysteine (SF-NAC)(Reference Al Janobi, Mithen and Gasper8, Reference Egner, Chen and Wang9). The fate of non-hydrolysed GRP is less well understood. A recent study has reported that low amounts of intact GRP were recovered in the urine of human subjects after consumption of a GRP-rich beverage, but not after consumption of an SF-rich beverage(Reference Egner, Chen and Wang9). Another study has reported that low amounts of intact GRP were recovered in the urine, but not in the faeces of rats that were fed purified GRP(Reference Bheemreddy and Jeffery10).
Several small clinical studies have examined the absorption and excretion of SF in human subjects. When urinary excretion of isothiocyanates (ITC) was measured following ingestion of fully cooked or fresh/lightly cooked broccoli, urinary ITC metabolites were approximately three times greater from fresh/lightly cooked broccoli than from fully cooked broccoli where myrosinase had been heat-inactivated(Reference Conaway, Getahun and Liebes3, Reference Rungapamestry, Duncan and Fuller4). Similar studies have evaluated the appearance of total ITC in the urine following ingestion of broccoli sprouts that had been either completely hydrolysed to ITC using exogenous myrosinase or contained only GRP where the myrosinase had been destroyed by boiling(Reference Shapiro, Fahey and Wade5, Reference Egner, Chen and Wang9). It has been found that ITC excretion was much greater after consumption of the preformed ITC compared with the GRP preparations(Reference Shapiro, Fahey and Wade5, Reference Egner, Chen and Wang9). The results of these studies suggest that the conversion of GRP to SF and subsequent ITC bioavailability is dependent on active myrosinase.
In a previous study, a commercially available GRP powder devoid of myrosinase, typical of many dietary GRP supplements on the market, was examined for its potential to deliver bioactive SF to human subjects alone or in combination with the air-dried broccoli sprout powder, which served as an exogenous food source of myrosinase(Reference Cramer and Jeffery6). The results showed that the combination improved the absorption of SF, and thus opened the door to the potential for enhanced cancer risk reduction not only from GRP supplements, but also from specifically designed foods or food combinations(Reference Cramer and Jeffery6).
Due to commercial availability and consumer preferences, intact fresh broccoli sprouts are more likely to be ingested by humans than the air-dried broccoli sprout powder used in our previous study. However, fresh broccoli sprouts may present additional variables such as matrix effects or product variability that were not present when examining the air-dried broccoli sprout powder. Therefore, the present study examined the same commercially available GRP powder used in our previous study, but here, intact fresh broccoli sprouts were used as the exogenous food source of myrosinase. The present study also expanded the number of blood samples collected to better capture the differences in SF appearance in plasma from the test meals. The study sought to determine whether the fresh broccoli sprouts would enhance GRP conversion and ITC absorption from the GRP powder.
Methods
Fresh broccoli sprouts were donated by Tiny Greens Organic Farm (Urbana, IL, USA). Broccoli powder was a gift from Caudill Seed, Inc. (Louisville, KY, USA).
Human subject study population
A total of four healthy men, aged 18–30 years, were recruited by fliers at the University of Illinois at Urbana-Champaign. Before participating in the study, each subject completed baseline questionnaires regarding dietary supplement, tobacco and other drug use. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Illinois Institutional Review Board. Written informed consent was obtained from all subjects. The study took place between 31 January and 25 February 2009.
Study design, meal administration and sample collection
Subjects were randomly assigned to a four-by-four cross-over design. They were given a list of foods known to contain glucosinolates and asked to avoid these foods for 3 d before and throughout the entire duration of the study. Subjects were also requested to avoid the use of dietary supplements and to limit alcohol consumption to no more than two alcoholic beverages per d during the study. Experimental meals were given each Tuesday for 4 weeks resulting in a 6 d washout period between meals; the half-life for SF has been reported as approximately 2 h(Reference Ye, Dinkova-Kostova and Wade11, Reference Vermeulen, Klopping-Ketelaars and van den Berg12). On the morning of each trial, subjects were instructed to ingest a meal according to the cross-over design. In the case of sprout-containing meals, subjects were also instructed to chew the sprouts thoroughly. Meals included 5-d-old intact fresh broccoli sprouts of the calabrese variety (approximately 42 g) or GRP powder (2 g) in an amount that produced 70 or 120 μmol SF, respectively, determined by bench hydrolysis. The combination meal contained both intact fresh broccoli sprouts (approximately 42 g) and GRP powder (2 g). The GRP powder was a proprietary dry, defatted broccoli seed powder preparation that did not contain myrosinase. Experimental meals were accompanied by one cup (53 g) of dry cereal (Go Lean Crunch; Kashi Company, La Jolla, CA, USA) and half cup (113·5 g) of French vanilla fat-free yogurt (Stonyfield Farm, Londonderry, NH, USA) to serve as a vehicle and control meal. Thus, the control meal included cereal and yogurt only. Blood (8 ml) was drawn into EDTA vacutainer tubes (Becton, Dickinson & Company, Franklin Lakes, NJ, USA) immediately before ingestion of each meal (0 h), and at 0·5, 1·0, 1·5, 3·0 and 24 h following the meal. Plasma was immediately prepared by centrifugation and stored at − 80°C until analysed. Urine samples were collected at baseline (0 h), 0–6, 6–12 and 12–24 h after meal consumption. All urine voided during these time intervals was collected. The volumes were recorded and used to calculate total μmol of SF-NAC excreted. Baseline urine samples were kept on ice and ascorbic acid (Fisher Scientific, Pittsburgh, PA, USA) was added to the urine samples at 1 g/l urine no later than 1 h following collection. All other urine samples were collected into 500 ml bottles containing 0·5 g ascorbic acid. Subjects were instructed to store urine samples in a provided cooler and to return them the following morning when the 24 h blood samples were collected. Urine samples were then stored at − 80°C until analysed.
Sulforaphane analysis
In triplicate, GRP powder (50 mg) was added to 1·6 ml distilled water containing 0·8 U white mustard myrosinase (Sigma Chemical, St Louis, MO, USA), vortexed and left to hydrolyse in the dark for 24 h. (One unit of myrosinase produces 1·0 μmol glucose/min from sinigrin at pH 6·0 and 25 °C.) The mixture was then centrifuged for 5 min at 14 000 g and filtered through a 0·45 μm nylon filter. The supernatant was diluted 5-fold with distilled H2O and an internal standard of benzyl ITC (Sigma Chemical) was added. The analysis of the GRP powder was also conducted in the absence of added myrosinase to confirm the necessity of myrosinase in the conversion of GRP to SF. Fresh broccoli sprouts were obtained the day before each trial meal and analysed for SF production upon hydrolysis using a modification of a previously reported method(Reference Matusheski, Juvik and Jeffery13). In triplicate, 0·25 g fresh broccoli sprouts were heated at 90°C for 5 min in a glass vial containing 2 ml distilled water. Following heating, samples were cooled on ice, homogenised and 0·5 U white mustard myrosinase were added. Samples were vortexed and left to hydrolyse at room temperature for 2 h. The samples were then centrifuged for 5 min at 14 000 g. The supernatant was filtered through a 0·45 μm nylon filter and diluted 4-fold with distilled water. An internal standard of benzyl ITC was added. ITC were extracted into dichloromethane for analysis by GC, as described previously(Reference Cramer and Jeffery6).
Plasma total isothiocyanate analysis
Blood samples were collected in EDTA-coated tubes and centrifuged at 1000 g for 30 min. Plasma was collected and analysed as described previously(Reference Cramer and Jeffery6). This method provides a single total measurement for SF, other ITC and metabolites(Reference Zhang, Cho and Posner14, Reference Zhang, Wade and Prestera15).
Urinary sulforaphane-N-acetylcysteine analysis
Urine samples were analysed as described previously(Reference Cramer and Jeffery6). Briefly, the filtered urine (50 μl) was analysed by HPLC using a Hypersil C18 ODS column (10 μm, 250 × 4·6 mm; Phenomenex, Torrance, CA, USA) and detected at 254 nm using a Waters HPLC system. A gradient solvent system with a flow rate of 1 ml/min consisted of a starting solvent system of 5 % aqueous acetonitrile (Fisher Scientific) and 95 % water. Acetonitrile was increased linearly to 20 % over 3 min, held 4 min, then increased to 100 % over 2 min and held 13 min to rinse the column. Both solvents contained 1·0 % acetic acid (Fisher Scientific). A standard was generated in control urine using SF-NAC synthesised as described previously(Reference Keck, Qiao and Jeffery16).
Statistical analysis
Data were evaluated using the GLIMMIX procedure of SAS Statistical software (version 9.1; SAS Institute, Cary, NC, USA). Levels of SF metabolites in urine and blood were tested for interactions of treatment and time. Differences were separated using the SLICEDIFF option. Values were considered different at P < 0·05.
Results
Sulforaphane content of hydrolysed broccoli sprouts and glucoraphanin powder
Upon incubation in water at room temperature for 24 h with the addition of 0·8 U myrosinase, GRP powder produced 61·7 (se 2·1) μmol SF/g powder. No SF was produced in the absence of added myrosinase. Fresh broccoli sprouts produced 1·69 (se 0·12) μmol SF/g fresh weight.
Plasma total isothiocyanates
Plasma ITC were elevated compared with baseline at 0·5 h in both the sprout and combination meals (Table 1). The combination meal reached peak plasma concentration (2·86 (se 0·33) μmol/l) 1·5 h after ingestion. The sprout meal peaked at 3 h (1·53 (se 0·22) μmol/l), but this value was not different from the value at 1·5 h (1·43 (se 0·21) μmol/l). The GRP powder meal showed slightly elevated plasma concentration levels 3 h post-consumption (0·37 (se 0·25) μmol/l). However, values following the GRP powder meal were not different from the control meal or baseline measurements at any of the time points measured. All values returned to baseline values by 24 h.
Table 1 Total isothiocyanate (ITC) in plasma following test meal consumption
(Mean values with their standard errors for four subjects per group)
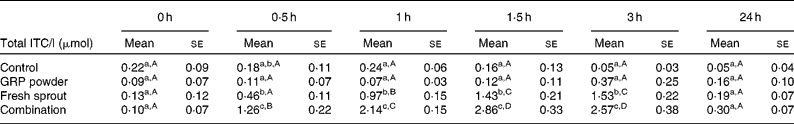
GRP, glucoraphanin.
a,b,c Mean values within a column (between-meal values) with unlike superscript letters were significantly different (P < 0·05).
A,B,C,D Mean values within a row (within-meal values) with unlike superscript letters were significantly different (P < 0·05).
Sulforaphane-N-acetylcysteine in the urine
The amount of SF-NAC excreted in the urine over 24 h following ingestion of each meal is shown in Fig. 1. After ingestion of fresh broccoli sprouts in combination with GRP powder, individuals excreted a mean of 123·8 (se 8·8) μmol SF-NAC over 24 h post-ingestion, 65 % of the dose ingested. After ingestion of broccoli sprouts alone, a mean of 42·0 (se 11·8) μmol SF-NAC was excreted, 60 % of the ingested dose. However, after ingesting GRP powder alone, a mean of only 29·2 (se 5·0) μmol SF-NAC was excreted, 24 % of the dose ingested.

Fig. 1 Urinary sulforaphane-N-acetylcysteine (SF-NAC) excretion after consumption of four different meals: control; glucoraphanin (GRP) powder; fresh broccoli sprout; combination. Baseline (■), 0–6 h (![]() ), 6–12 h (
), 6–12 h (![]() ) and 12–24 h (□) urine collection post-consumption. Values are means, with their standard errors represented by vertical bars for four subjects per group. a,b Mean values with unlike letters were significantly different between-meal (P < 0·05). A,B,C Mean values with unlike letters were significantly different within-meal (P < 0·05).
) and 12–24 h (□) urine collection post-consumption. Values are means, with their standard errors represented by vertical bars for four subjects per group. a,b Mean values with unlike letters were significantly different between-meal (P < 0·05). A,B,C Mean values with unlike letters were significantly different within-meal (P < 0·05).
Urine collection was separated into discrete intervals for evaluation of SF-NAC excretion: urine was collected for the first 6 h after meal ingestion, from 6 to 12 h and from 12 to 24 h post-ingestion. Significant differences were observed between the dietary groups. Considerable levels of SF-NAC were excreted during the first 6 h from individuals who received the combination meal or the broccoli sprout meal (61 and 62 % of the total SF-NAC that was excreted during the entire 24 h urine collection, respectively), but less than 22 % of the total 24 h SF-NAC was excreted during this first 6 h period from those receiving the GRP powder meal alone. In contrast, less than 10 % of the total 24 h SF-NAC recovered from the combination or sprout meal was excreted during the 12–24 h period, whereas 42 % of the total 24 h SF-NAC recovered following the GRP powder meal was excreted during this later time period.
Discussion
The main findings of the present study were that combining fresh broccoli sprouts with the GRP powder (1) increased the appearance of SF metabolites in plasma and urine and (2) removed the delay of metabolite appearance observed after the GRP powder, shifting the absorption/elimination pattern to the one similar to that seen after the consumption of fresh broccoli sprouts alone. This is the first study to determine whether combining two commercially available broccoli products, one containing and the other lacking myrosinase, would enhance SF availability from GRP. The present study could be extrapolated to hypothesise that combining fresh broccoli sprouts with well-cooked broccoli, where myrosinase is inactive, would also enhance SF availability. Additionally, it could be hypothesised that other sources of myrosinase, such as mustard, horseradish, cabbage, Brussels sprouts and watercress, would also enhance the conversion of GRP to SF.
The present study measured urinary SF-NAC excretion and plasma total ITC levels. The measurement of SF metabolites after consumption of broccoli, broccoli sprouts and other broccoli-related preparations has been a useful tool for assessing human exposure to SF, a compound associated with a reduced risk of cancer(Reference Shapiro, Fahey and Wade1, Reference Conaway, Getahun and Liebes3–Reference Cramer and Jeffery6, Reference Egner, Chen and Wang9, Reference Hanlon, Coldham and Gielbert17, Reference Shapiro, Fahey and Dinkova-Kostova18). SF metabolites in plasma reflect the amount of SF that tissues are being exposed to and are therefore important biomarkers of exposure to this cancer-preventive agent. SF metabolites in the urine reflect the absorption, metabolism and excretion of an ingested dose(Reference Shapiro, Fahey and Wade5). The major metabolite of SF appearing in the urine, SF-NAC, is often used as a marker of bioavailability, although it is not the only metabolite present in the urine(Reference Al Janobi, Mithen and Gasper8, Reference Egner, Chen and Wang9).
Only 24 % of the GRP dose from the GRP powder was recovered as SF-NAC in the urine, making it a poor source of dietary SF compared with fresh broccoli sprouts. This value is comparable with the reported recovery following ingestion of well-cooked sprouts or well-cooked mature broccoli, both of which also lacked myrosinase(Reference Conaway, Getahun and Liebes3, Reference Shapiro, Fahey and Wade5, Reference Shapiro, Fahey and Dinkova-Kostova18). Urinary values of SF-NAC after the GRP powder meal displayed a non-significant trend of increasing excretion over 24 h, suggestive of delayed absorption. The delayed absorption and low SF recovery was probably due to the lack of myrosinase in the powder and the resulting hydrolysis of GRP by microflora after the transit of GRP to the lower gut(Reference Shapiro, Fahey and Wade1, Reference Lai, Miller and Jeffery2, Reference Shapiro, Fahey and Dinkova-Kostova18, Reference Verhoeven, Verhagen and Goldbohm19). Plasma total ITC was not altered in response to the GRP powder, but there was a slight non-significant elevation at 3 h, also suggestive of delayed ITC absorption with low availability. It could be questioned whether the maximum plasma ITC level was further delayed rather than absent following ingestion of the GRP powder, and thus was not measured in the present study. Indeed, a slight, but non-significant peak 6 h post-consumption was observed in a study of well-cooked broccoli(Reference Conaway, Getahun and Liebes3). However, urinary recovery of SF-NAC was low not only for the first 6 h of the present study, but for the entire 24 h period following consumption of the GRP powder meal, confirming the absence of any significant elevation in plasma ITC levels. The low levels of SF metabolites detected in plasma and urine after consumption of the GRP powder may indicate lower anti-cancer potential for this product and other similar dietary supplements. For instance, it has been reported that similar GRP products lacking myrosinase induced detoxification enzymes in the colon, but not in the liver of rats, whereas unheated broccoli florets with functional myrosinase induced activity in both the colon and the liver(Reference Zhu, Soendergaard and Jeffery20).
Interestingly, data from the combination meal identified possible synergy among the fresh sprouts and GRP powder at early time points for SF and its metabolite appearance in plasma and urine. This indicates that GRP, not only from the broccoli sprouts, but also from the GRP powder, was hydrolysed by endogenous myrosinase from the broccoli sprouts. Additionally, excretion of SF-NAC following the combination meal was earlier than from the GRP powder alone and more similar to the excretion pattern following the consumption of broccoli sprouts alone, indicating that the fresh sprouts not only supported the hydrolysis of the GRP powder, but also caused it to occur earlier, resulting in earlier and more complete SF absorption. The trend for greater levels of SF-NAC to be excreted early (during the first 12 h following meal consumption) from the combination and sprout meals is consistent with metabolism occurring in the upper gastrointestinal tract in the presence of dietary myrosinase. A similar trend was observed in plasma where in both sprout and combination meals, plasma ITC levels were elevated by 0·5 h, and to a much higher level in the combination meal. Higher levels of SF metabolites in plasma and urine may indicate a greater reduction of cancer risk from consumption of this food combination.
Recovery of preformed ITC or ground, air-dried broccoli sprouts has been reported to be between 75 and 90 % of ingested doses(Reference Shapiro, Fahey and Wade5, Reference Cramer and Jeffery6, Reference Rouzaud, Young and Duncan21). This recovery decreased when a plant matrix was introduced, as is evidenced by several published papers, as well as the present paper where intact, but thoroughly chewed fresh sprouts resulted in a 60 % recovery of the dose(Reference Conaway, Getahun and Liebes3, Reference Shapiro, Fahey and Wade5, Reference Rouzaud, Young and Duncan21). Interestingly though, comparing an equimolar dose of SF from fresh sprouts (used here) with air-dried sprouts(Reference Cramer and Jeffery6) when combined with the GRP powder, an improved 24 h urinary recovery (65 v. 50 % of the ingested dose, respectively) and an elevated peak plasma ITC level (C max 2·9 v. 2·1 μmol total ITC/l, respectively) was observed in the combination using fresh intact broccoli sprouts. Based on this evidence, we conclude that fresh broccoli sprouts aided the conversion of GRP to SF from the GRP powder to a greater extent than air-dried broccoli sprouts. More research with larger study populations is needed.
One limitation of the present study is its small sample size. However, most human studies focusing on the bioavailability of SF have used similar small population sizes(Reference Conaway, Getahun and Liebes3, Reference Shapiro, Fahey and Wade5, Reference Cramer and Jeffery6, Reference Ye, Dinkova-Kostova and Wade11). The intent of the present study was to provide direction as a pilot study. Future large-scale work is needed.
Conclusion
GRP powder that lacked myrosinase was a poor dietary source of SF compared with broccoli sprouts. Fresh intact broccoli sprouts were able to synergistically enhance the hydrolysis of GRP from the GRP powder, perhaps more efficiently than ground, air-dried broccoli sprouts. Because efficacy is related to plasma levels, the elevation seen in plasma levels probably translates to a greater potential for cancer risk reduction. These findings provide important insights into the protective health benefit of broccoli products and preparations and can be used to develop foods with enhanced anti-cancer properties.
Acknowledgements
We thank Tiny Greens Organic Farm for supplying the fresh broccoli sprouts that were used in this study. We also thank John Jerrell for his help and expertise with HPLC analysis, as well as Heather Mangian and Melissa Sorrells for support in preparation for and during sample collection. J. M. C. was supported by a fellowship from Philip and Juanita Francis. This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that they have no conflicts of interest. The authors' contributions were as follows: J. M. C. participated in the study design, completed Institutional Review Board application, carried out meal administration and sample collection, completed all analyses and drafted the manuscript. M. T.-G. carried out phlebotomy for blood sample collection. E. H. J. participated in the study design and oversight of Institutional Review Board application. All authors participated in the manuscript discussion as well as read and approved the final manuscript.




