Since the identification of vitamin C during the 1920s(Reference Carpenter1), the vitamin has become characterised as a molecule that neither animals nor plants can live without(Reference Grollman and Lehninger2). Arising from a glucose molecule, vitamin C is chemically one of the simplest vitamins, due to its water-soluble nature and low molecular weight. The most common bioavailable form that contributes multiple key biological functions is vitamin C’s reduced form known as ascorbic acid(Reference Kocot, Luchowska-Kocot and Kiełczykowska3).
Many of the vitamin’s functions arise as a result of ascorbate acting as a precursor for multiple enzymes. Examples include the biosynthesis of collagen (the body’s major building peptide), production of carnitine for mitochondrial support(Reference Rebouche4) and histamine. Additional cofactor roles include Fe and Cu absorption and mobilisation and the conversion of dopamine into norepinephrine(Reference Milne and Omaye5,Reference Turley, West and Horton6) . Immune support is actioned through vitamin C’s involvement in enhancing differentiation and proliferation of B and T cells(Reference Carr and Maggini7), and the enhancement of natural killer cell activity(Reference Heuser and Vojdani8). Ascorbate has also been identified as a major direct free radical scavenger and can further regenerate other important scavengers such as urate, glutathione, β-carotene and α-tocopherol(Reference Harrison, Allard and Bixler9). Furthermore, vitamin C supports the central nervous system(Reference Travica, Ried and Sali10), cardiovascular(Reference Kónya and Ferdinandy11), immunological(Reference Carr and Maggini7) and endocrine systems(Reference Marik12).
The importance of vitamin C is further exemplified by the physiological compromises that arise during states of plasma ascorbate deficiency (inadequate/deficient: ≤28 µmol/l, adequate: >28 µmol/l)(Reference Ravindran, Wiltshire and Das13,Reference Hampl, Taylor and Johnston14) . Low plasma levels are linked to deficiencies in tissues, as muscle vitamin C content depletes after plasma concentrations(Reference Spoelstra-de Man, Elbers and Oudemans-van Straaten15). Some of the common early symptoms that present during deficiency include fatigue and depression, loss of appetite, and painful joints and muscles(Reference Delanghe, Langlois and De Buyzere16). Further symptoms include swollen and bleeding gums, loss of teeth, haemorrhaging under the skin and slowed healing of wounds(Reference Gariballa17,Reference Lipner18) .
Due to its water-soluble properties and relatively short half-life, plasma ascorbate concentrations are transient, with a number of proposed factors contributing to its bioavailability and consequential deficiency. Habitual behaviours such as alcohol consumption(Reference Lim, Sharma and Thompson19), smoking(Reference Brubacher, Moser and Jordan20) and lack of fruit and vegetable consumption(Reference Michels, Welch and Luben21) are common contributors to deficiency. Furthermore, an array of chronic illnesses and conditions has been linked to deficiency(Reference Travica, Ried and Sali10,Reference Carr, Shaw and Natarajan22–Reference Mayland, Bennett and Allan25) . A number of cohort studies have revealed a vulnerability for plasma vitamin C deficiency amongst hospitalised patients(Reference Ravindran, Wiltshire and Das13).
Surgical trauma is often viewed as a neglected part of global health in terms of patient numbers, mortality, morbidity and costs(Reference Dobson26). Multiple short-term and longer-lasting chronic postoperative co-morbidities are reported following surgical procedures. The initial stress response involves the activation of resident and non-resident immune cells which are the ‘first responders’ to danger(Reference Dobson26), activating a cascade of pro-inflammatory cytokines(Reference Dąbrowska and Słotwiński27). A series of cellular disruptions such as mitochondrial damage are commonly observed postoperatively(Reference Dobson26). Further clinical symptoms that may arise include systemic coagulopathy, ischemia, reperfusion(Reference Hollenberg and Mangano28), elevated blood sugar levels, myocardial and liver injury, renal dysfunction, gastrointestinal complications(Reference Giglio, Marucci and Testini29), postoperative cognitive dysfunction and organ failure(Reference Dobson26).
Unlike humans, most animals, which can synthesise their own vitamin C, will increase and maintain circulatory concentrations of the vitamin following severe physiological stress, while humans may experience significant depletions following the body’s increased demand for the vitamin(Reference Drouin, Godin and Pagé30). Surgery has been shown to increase energy utilisation as well as decrease nutritional reserves(Reference Salah31). Postoperative depletions in plasma vitamin C were reported as early as 1940(Reference Bartlett, Jones and Ryan32). One of the first trials conducted on 105 surgical patients revealed a significant depletion in plasma vitamin C concentrations immediately after a range of surgery types(Reference Crandon, Landau and Mikal33).
Recent reviews have demonstrated that ascorbate supplementation may potentially alleviate post-surgical pathophysiologies such as organ failure(Reference Hill, Wendt and Benstoem34), sepsis(Reference Carr, Shaw and Natarajan22) and pain(Reference Carr and McCall24). A narrative review concluded that there is an increased postoperative vitamin C requirement for surgical patients, with the need for additional investigations to determine the optimal postoperative vitamin C dose for clinical benefits(Reference Fukushima and Yamazaki35). However, this review only briefly examined the literature which focused on changes in postoperative plasma vitamin C concentrations. In addition, the absence of both, a systematic search strategy, and a risk of bias assessment for the reported studies made this review vulnerable to a possible risk of bias. A review is yet to systematically assess the body of literature investigating the potential impact of surgical exposure on plasma vitamin C concentrations and determine the magnitude and time frame of any potential postoperative change in plasma concentrations.
The aim of the present systematic review and meta-analysis was to compare pre- and postoperative plasma vitamin C concentrations in patients exposed to surgery and to potentially determine the magnitude and time frame of any change. An additional aim was to investigate whether there may be a change in concentration according to the type of surgery. Understanding of vitamin C’s physiological response to surgery (surgical trauma) could assist with preventative and supportive interventions, including vitamin C supplementation, parenteral nutrition and dietary intake.
Methods
Literature search
This review used the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) flow chart. We searched the PUBMED, SCOPUS, SciSearch and the Cochrane Library for publications from 1970 to April 2020. To locate unpublished literature, we searched clinical trial registries such as ClinicalTrials.gov for relevant trials. There was no restriction to language. When only the abstract was available, authors were contacted to provide the required data. Keywords used were vitamin C, ascorbate, ascorbic acid, antioxidant, plasma/serum, surgery, surgical trauma, elective, emergency, and pre- and postoperative. We subsequently searched reference lists of previously published systematic reviews and meta-analyses, and reference lists of relevant papers for additional primary studies. Table 1 outlines the criteria for the literature search. Articles were first screened for eligibility based on titles and abstracts by two investigators. If considered potentially eligible, the full-text publication was retrieved and independently assessed by two authors. Authors were contacted for missing data.
Table 1. Study inclusion and exclusion criteria

Study selection
Studies eligible for inclusion were randomised controlled trials and observational studies assessing plasma/serum vitamin C concentration in adult patients undergoing all forms of surgery.
Systematic review
In the systematic review, we included prospective, observational studies and only the placebo/control groups from randomised controlled trials. As long as trials assessed plasma/serum vitamin C concentrations pre- and postoperatively, the type of surgery was unrestricted and included both elective and emergency surgeries. Preoperative assessments were only considered if taken in close proximity to surgery commencement (within 1 week), while the timing of the postoperative assessment was not restricted.
Meta-analyses
Stricter inclusion criteria were applied for the meta-analyses. In the meta-analyses, we excluded studies which administered a control/placebo intervention consisting of a vitamin C containing source, or a source that could influence plasma vitamin C concentrations directly for the duration of the trial. These were excluded from analysis to ensure no interference from pre- or postoperative intervention on plasma concentrations and to assess the true impact of the surgery on plasma concentrations. Only studies reporting mean plasma vitamin C concentrations and standard deviations were eligible for meta-analysis.
Data extraction
The number of participants, mean plasma vitamin C concentrations and standard deviation pre- and post-surgery were collated from text, tables or figures. Additional data were extracted for study design, mean/median age, total study period, timing of outcome measures, mean plasma vitamin C concentrations, type of surgery and any additional outcomes measures/blood biomarkers. For studies that assessed vitamin C at multiple postoperative time points, only the mean concentration of the last time point (longest postoperative assessment) was included into the meta-analysis.
Quality assessment/risk of bias
Methodological quality was assessed independently by two investigators (N. T. and K. R. using guidelines by the Cochrane Collaboration)(Reference Park, Lee and Seo36). The Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) was used to assess trial bias(Reference Kim, Park and Lee37). This tool was selected due to the review primarily observing differences between pre- and postoperative vitamin C concentrations and concentrations being observed prospectively without the implementation of an intervention per se. Only elements of bias relating to the potential change in vitamin C concentration were considered when assessing bias, rather than the allocation of an intervention. Consequently, the included RoBANS domains that were assessed included the selection of participants, confounding variables, incomplete outcome data, selective outcome reporting and the addition of other bias. The domains which included measurement of intervention (exposure) and blinding of outcome assessment (blinding) were not used as they were not applicable to the included studies. The tool weighted risk of a possible bias under ‘low’, ‘unclear’ and ‘high’ measures and did not attempt numerical scores. Studies that reported a high risk of bias on at least one out of the six domains, such as substantial confounding factors or missing data points, were not considered for meta-analysis.
Statistical analysis
Mean plasma vitamin C concentrations before and after surgery, standard deviation and number of participants were entered into the meta-analysis using Review Manager version 5.3(38). Heterogeneity was assessed using the I 2 statistic on all models. Heterogeneity was considered substantial if the I 2 statistic was ≥50 %. If the pooled studies exhibited significant heterogeneity, a random-effects model, which considers both between study and within-study variation, and sampling variability between studies (I 2 statistic >50 %) were applied for each meta-analysis(Reference Riley, Higgins and Deeks39). To further explore heterogeneity, we repeated the analyses by sequentially omitting one trial at a time to investigate the influence of a single trial on the overall effect estimate.
Studies were separated into those that assessed plasma vitamin C concentrations within the first postoperative week (short-term assessment) and those that assessed plasma vitamin C beyond 1 week postoperatively (long-term assessments). The first postoperative week is representative of the early postoperative recovery phase and during which several of the most common postoperative complications appear(Reference Santonocito, De Loecker and Donadello40,Reference Bowyer and Royse41) , while concentrations assessed following 7 d are more indicative of the later postoperative recovery phase(Reference Bowyer and Royse41). A number of studies assessed multiple time points providing data for both short-term and long-term postoperative meta-analyses. Subgroup analyses were conducted by type of surgery of trials with short-term (within seven postoperative days) assessment of vitamin C concentrations, including cardiac, orthopaedic or gastrointestinal surgical subgroups.
The overall effect sizes were subjected to two-tailed z tests for examining the significance of differences between means. We considered a statistically significant finding with P < 0·05. We calculated differences between pre- and postoperative concentrations of mean plasma concentrations. All vitamin C concentrations were converted into micromoles per litre (µmol/l) for a standardised concentration comparison between trials. Additionally, standard error of the mean was converted into standard deviation values. For analyses consisting of ≥10 studies, publication bias was assessed by visual inspection of funnel plots(Reference Higgins, Thomas and Chandler42). In the presence of publication bias, we performed a sensitivity analysis in which smaller studies that reported more extreme effect sizes were excluded.
Results
Thirty-one studies met the inclusion criteria for the systematic review(Reference Behrend, Lengnick and Zdravkovic43–Reference Hill-Mündel, Schlegl and Biesalski73). Twenty-three of thirty-one studies that were included in our systematic review met the inclusion criteria for meta-analysis(Reference Behrend, Lengnick and Zdravkovic43–Reference Barker, Leonard and Trawick62,Reference Fukushima, Koide and Yamazaki64,Reference Hill, Borgs and Fitzner71–Reference Hill-Mündel, Schlegl and Biesalski73) . The selection of studies is summarised in Fig. 1.
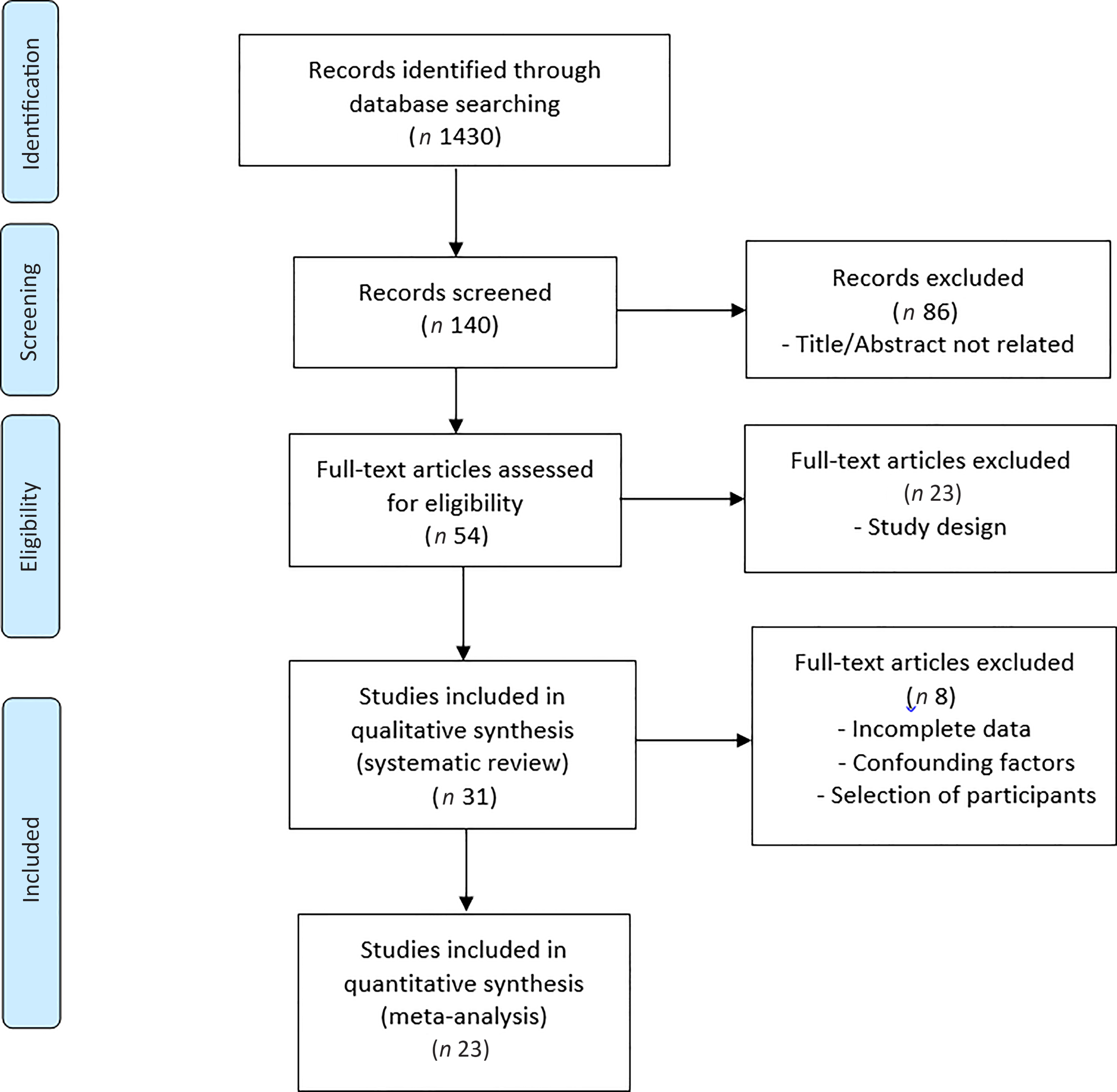
Fig. 1. PRISMA flow diagram of the literature search process.
Major characteristics of the studies included into the meta-analysis are presented in Table 2 and additional studies included into the systematic review in Table 3.
Table 2. Characteristics of studies included into both systematic review and meta-analysis
(Mean values and standard deviations)
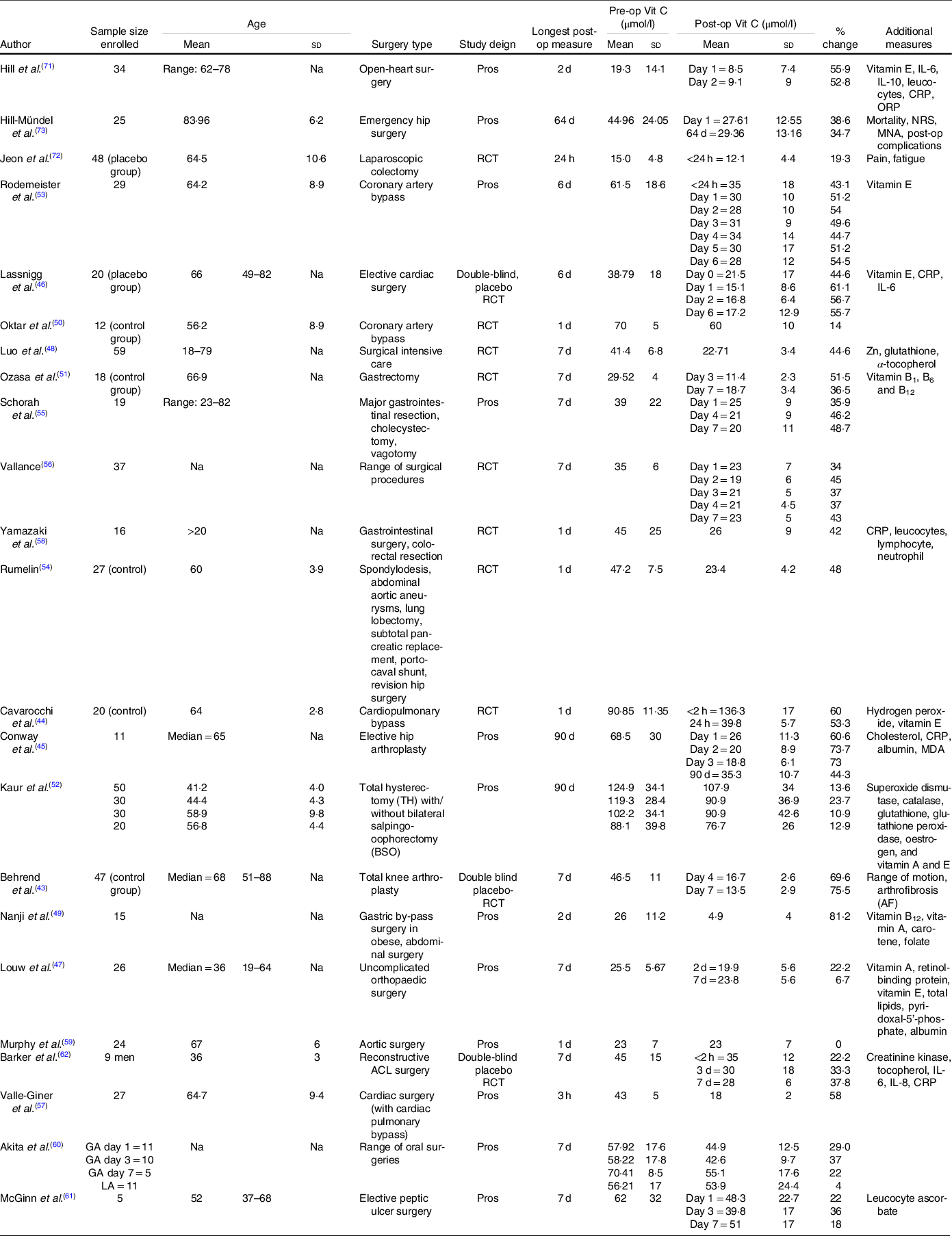
Post-op, postoperative; Pre-op, preoperative; Vit C, vitamin C; Na, not assessed; Pros, prospective; CRP, C-reactive protein; ORP, oxidation–reduction potential; NRS, nutritional risk screening; MNA, Mini Nutritional Assessment; RCT, randomised controlled trial; MDA, Malondialdehyde; ACL, anterior cruciate ligament.
Table 3. Characteristics of additional studies included into systematic review but excluded from meta-analysis
(Mean values and standard deviations)
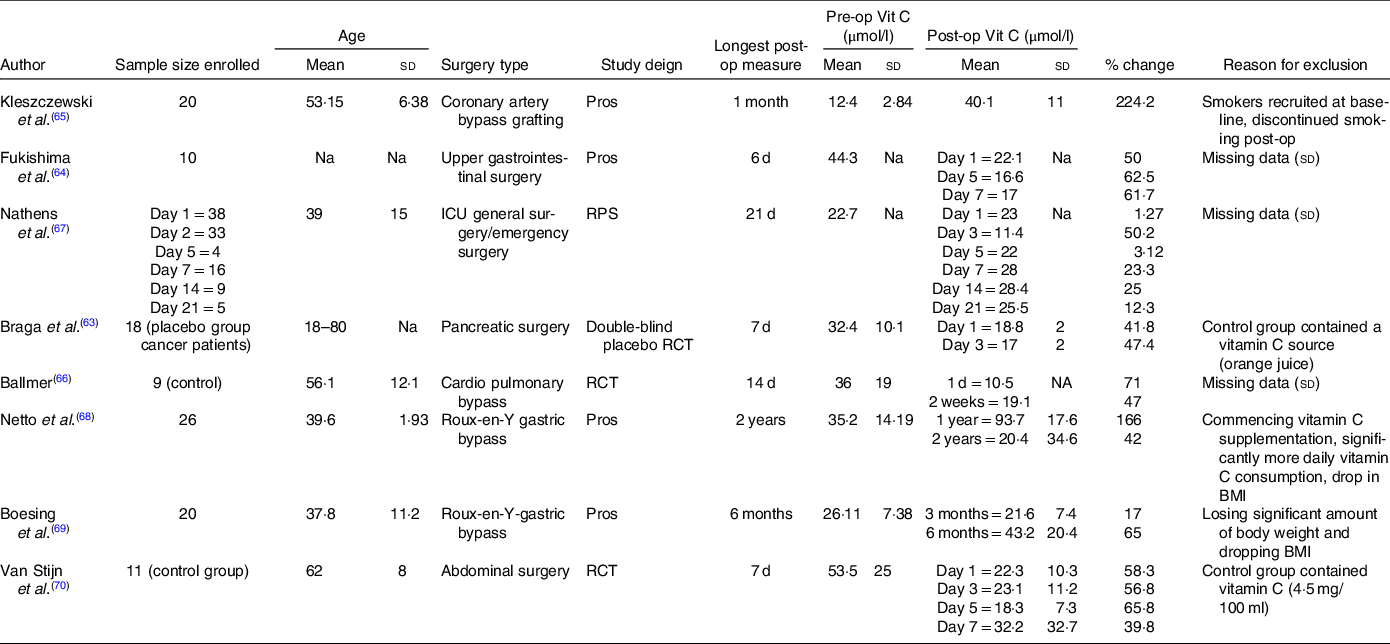
Post-op, postoperative; Pre-op, preoperative; Vit C, Vitamin C; Pros, prospective; Na, not assessed; RPS, randomised prospective trial; RCT, randomised controlled trial; ICU, intensive care unit.
Systematic review
The systematic review of 31 studies comprised of a total of 861 patients who had their plasma vitamin C concentrations assessed pre- and postoperatively. The ages ranged from 18 to 85 years, with studies predominantly including patients over the age of 60 years. All trials assessed preoperative vitamin C concentrations within 24 h of the surgery. A majority of studies assessed plasma concentrations using validated ascorbate biochemical analyses which included HPLC(Reference Chung, Chung and Szeto74) and electron paramagnetic resonance spectroscopy(Reference Rodemeister, Duquesne and Adolph53), while a number of studies failed to mention the biochemical analysis that was undertaken(Reference Behrend, Lengnick and Zdravkovic43,Reference Louw, Werbeck and Louw47,Reference Jeon, Park and Moon72) . Concentrations were predominantly assessed during the first postoperative week. Surgery type varied between studies and predominantly included elective surgeries. These included coronary artery bypass grafting, bariatric and gastrointestinal surgery, elective and emergency hip arthroplasty, elective limb surgery and subtotal pancreatic replacement. A number of studies including broader surgery categories (i.e. intensive care surgery, major surgery).
A common trend of included studies revealed a significant mean plasma vitamin C depletion at all postoperative assessments compared with preoperative assessments, with concentrations failing to return to preoperative levels for the duration of the studies. Depletion of vitamin C was most evident when plasma concentrations were assessed during the first seven postoperative days.
Quality of included studies
Out of thirty-one trials, eight of the studies included in the systematic review ranged from medium to high quality when weighed by the RoBANS (Fig. 3). Twenty-two out of the thirty-one studies had an unclear risk of bias for the confounding variable domain due to failing to report on variables which may influence plasma vitamin C concentrations such as medication intake, dietary intake (particularly vitamin C containing foods), supplementation and smoking/alcohol consumption, both prior to surgery and during the postoperative period (Figs. 2 and 3). In addition, an unclear risk of bias was applied to the confounding variable domain for studies which failed to report on the in vitro vitamin C probe handling, which is vital for preventing vitamin C oxidation prior to biochemical analysis. Similarly, measure of exposure was unclear in twenty-seven studies which failed to report on either the type or amount of anaesthetic, surgical details such as length of surgery, haemodilution and blood transfusion volume.

Fig. 2. Risk of bias assessment of studies included into meta-analysis and systematic review; yellow circle = unclear bias and green circle = low risk of bias.
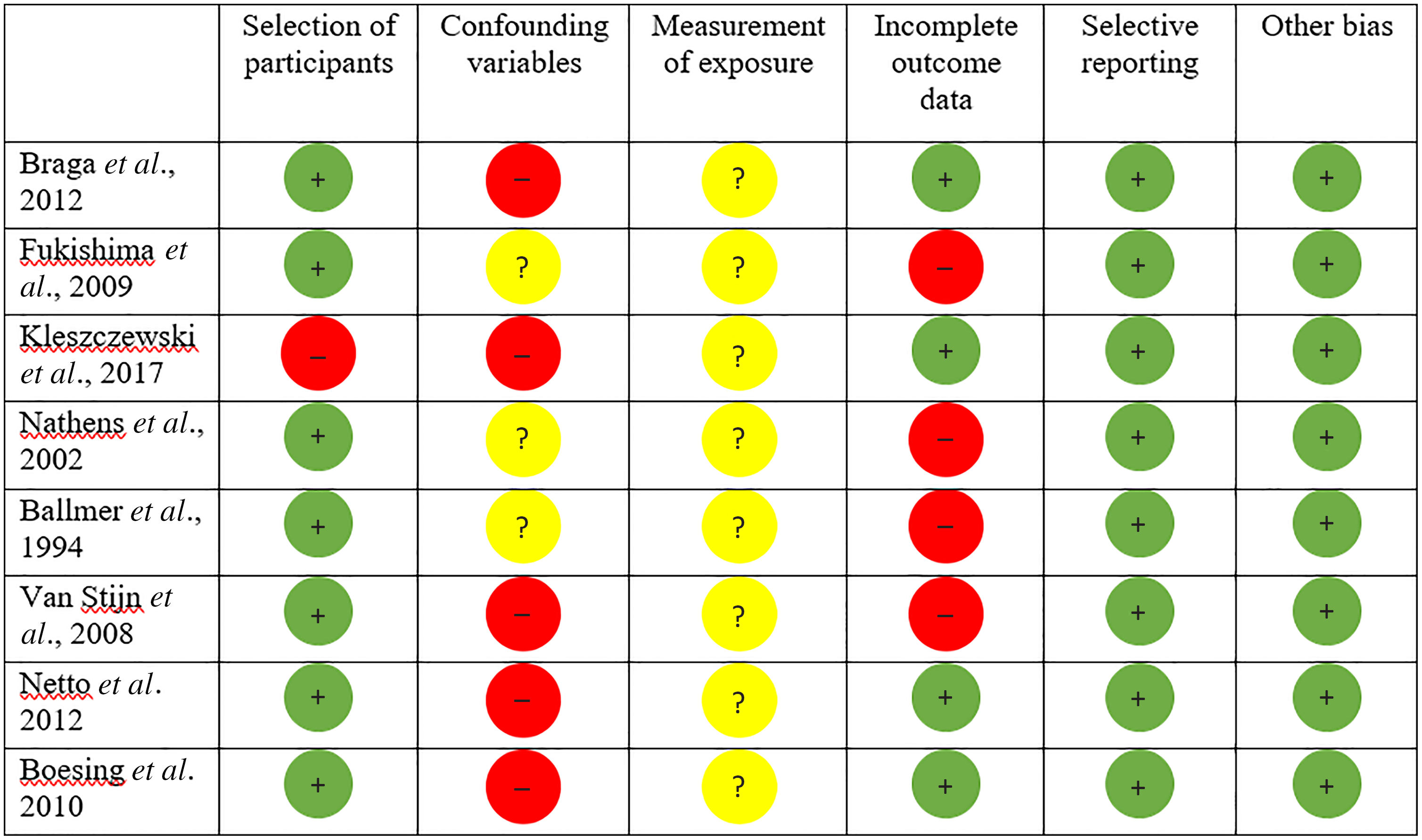
Fig. 3. Risk of bias assessment for studies included into systematic review but excluded from meta-analysis; red circle = high risk of bias, yellow circle = unclear bias and green circle = low risk of bias.
Only three studies displayed increases in postoperative plasma vitamin C concentrations(Reference Kleszczewski, Modzelewska and Lisowska65,Reference Nathens, Neff and Jurkovich67,Reference Netto, Moreira and Patiño68) . However, all of these studies had the potential to be significantly affected by confounding factors, presenting with a high risk of bias on at least one of the domains assessed by the RoBANS (Fig. 3). Additionally, four studies reported incomplete data for mean plasma vitamin C concentrations or standard deviation(Reference Fukushima, Koide and Yamazaki64,Reference Ballmer, Reinhart and Jordan66,Reference Nathens, Neff and Jurkovich67,Reference van Stijn, Ligthart-Melis and Boelens70) , another two consisted of control groups that contained a vitamin C source (4·5 mg/100 ml vitamin C(Reference van Stijn, Ligthart-Melis and Boelens70), orange juice concentrate(Reference Braga, Bissolati and Rocchetti63)) and one study recruited smokers at baseline who discontinued smoking postoperatively(Reference Kleszczewski, Modzelewska and Lisowska65). Additionally, two longitudinal studies assessing vitamin C concentrations following Roux-en-Y gastric bypass procedures presented with a high risk bias(Reference Netto, Moreira and Patiño68,Reference Boesing, Moreira and Wilhelm-Filho69) due to significant confounding factors such as participants losing a significant amount of body weight and dropping BMI postoperatively, and/or commencing vitamin C supplementation and the consumption of significantly more daily vitamin C on average in one study(Reference Netto, Moreira and Patiño68).
Meta-analysis
Twenty-three studies included into the meta-analysis involved 642 patients. Only studies presenting with a low to unclear risk of bias were included into the meta-analysis (Fig. 2), with those reporting a high risk of bias on at least one of the assessment domains being excluded from the meta-analysis (Fig. 3). Mean preoperative vitamin C concentrations varied between studies, with only three studies(Reference Nanji and Freeman49,Reference Murphy, Kolvenbach and Aleksis59,Reference Hill, Borgs and Fitzner71) reporting mean plasma vitamin C concentrations indicative of a inadequate/deficient concentration (<28 µmol/l)(Reference Travica, Ried and Sali75).
Twenty-one studies assessed plasma vitamin C concentrations within 7 d postoperatively. Mean postoperative plasma concentrations were used from various time points during the first postoperative week. One study separated mean pre- and postoperative plasma concentrations between sex groups(Reference Louw, Werbeck and Louw47), and these were averaged for the purpose of our analysis. One study assessed the effect of two types of anaesthetic agents (general v. local) on plasma vitamin C concentrations in two groups of patients(Reference Akita, Kawahara and Takeshita60). As a result, twenty-five trial arms were included into this meta-analysis.
Three studies assessed plasma vitamin C concentrations beyond 7 d postoperatively, with these postoperative assessments being conducted 2–3 months post-surgery. One study(Reference Kaur, Negi and Sarna52) recruited two different cohorts of participants (pre- and postmenopausal) undergoing two different types of surgeries (total hysterectomy with or without bilateral salpingo-oophorectomy). Consequently, these data were imputed as separate comparisons into the meta-analyses that assessed longer-term postoperative concentrations.
Concentrations ≤7 d postoperatively
The meta-analysis assessing mean plasma vitamin C concentrations within seven postoperative days (short term) is presented in Fig. 4. Surgery significantly decreased plasma vitamin C concentrations during the first postoperative week in patients. Mean difference (95 % CI) between preoperative and postoperative plasma vitamin C concentration was −17·99 µmol/l (CI −22·81, −13·17) (trial arms = 25, n 565, P < 0·001), a mean reduction of 39 % from a preoperative mean of 46·3 µmol/l. High heterogeneity was observed between these trials (I 2 97 %, P < 0·001).
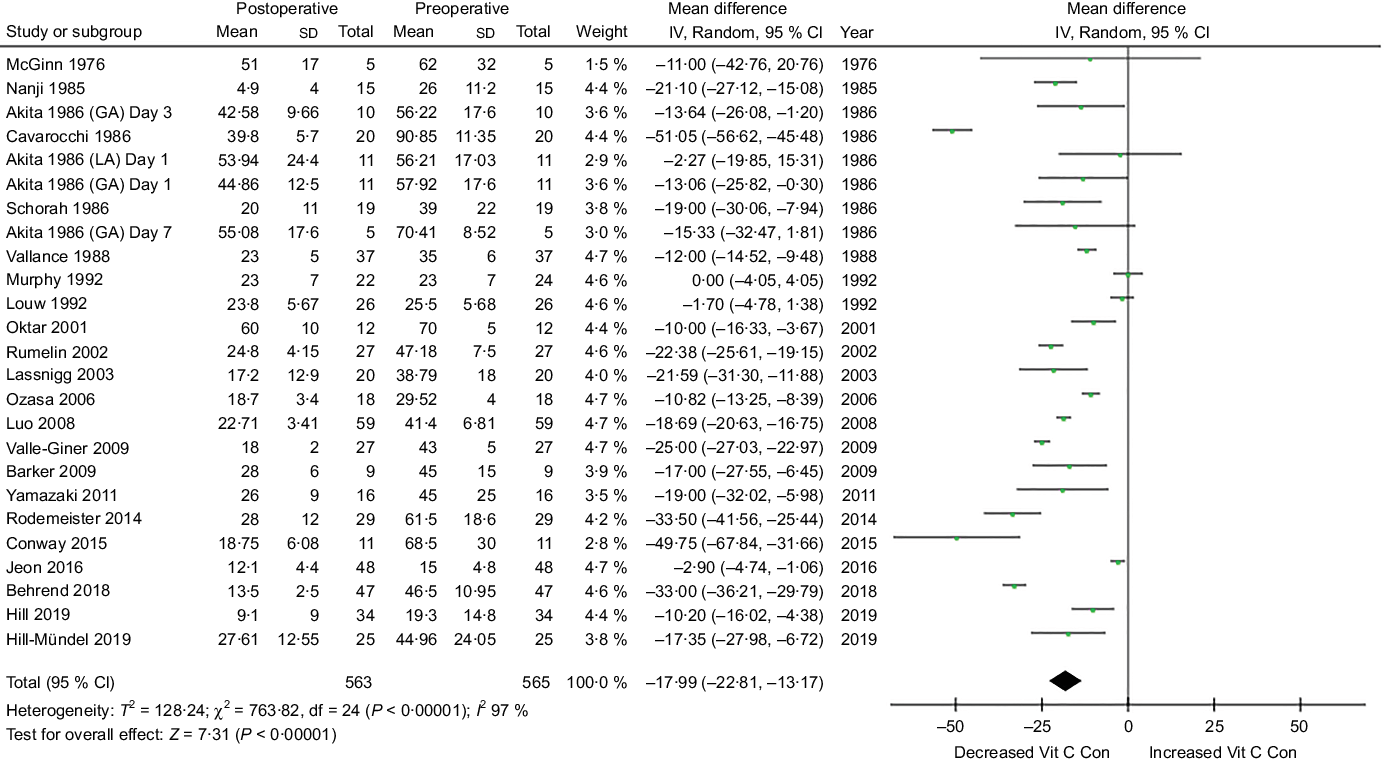
Fig. 4. Meta-analysis assessing mean plasma vitamin C concentrations within seven postoperative days. Diamond represents overall effect size of the meta-analysis. Boxes represent mean differences in concentrations and lines across the boxes represent respective 95 % CI. GA, general anaesthesia; LA, local anaesthesia; Vit, vitamin; Con, concentration.
Sensitivity analyses revealed that when specific studies were omitted, high heterogeneity was still present, suggesting that the heterogeneity was not related to a single trial. Inspection of the funnel plot suggested some evidence of publication bias. Additional analyses demonstrated that excluding small trials that reported extreme results had no noticeable affect on the pooled effect size.
Sixteen studies reported mean postoperative vitamin C concentrations representative of inadequate/deficient concentration (<28 µmol/l). Studies with higher mean preoperative concentrations (>55 µmol/l) seemed to display lower rates of postoperative deficiency, with the highest postoperative concentrations observed in two studies which displayed preoperative concentrations >70 µmol/l(Reference Cavarocchi, England and O’Brien44,Reference Oktar, Sinci and Kalaycioğlu50) .
Concentrations >7 d postoperatively
The meta-analysis assessing mean plasma vitamin C concentrations beyond seven postoperative days is presented in Fig. 5. The meta-analysis of six trial arms involving 166 patients assessed the effect of surgery on vitamin C levels 2–3 months post-surgery. The analysis revealed that surgery significantly decreased plasma vitamin C concentrations beyond the first postoperative week. The mean difference between preoperative and postoperative plasma vitamin C concentration was −18·80 µmol/l (CI −25·04, −12·56) (P < 0·001), a mean reduction of 21 % from a preoperative mean of 91·3 µmol/l. A relatively low heterogeneity was observed between these trials (I 2 0 %, P < 0·001).

Fig. 5. Meta-analysis assessing mean plasma vitamin C concentrations beyond seven postoperative days. Diamond represents overall effect size of the meta-analysis. Boxes represent mean differences in concentrations and lines across the boxes represent respective 95 % CI. TH, total hysterectomy; BSO, bilateral salpingo-oophorectomy; Vit, vitamin; Con, concentration.
Subgroup analyses (surgery type)
Additionally, a subgroup meta-analysis, categorising surgeries into surgical types, assessed changes in vitamin C concentrations within the first postoperative week (Fig. 6). A significant difference in mean plasma vitamin C concentrations was observed between pre- and post-cardiac surgery (43·5 % reduction) (subgroup 1·1·1), orthopaedic surgery (52 % reduction) (subgroup 1·1·2) and gastrointestinal surgery (34·6 % reduction) (subgroup 1·1·3). A high heterogeneity was observed between the trials of each subgroup analysis (I 2 > 90 %, P < 0·001).
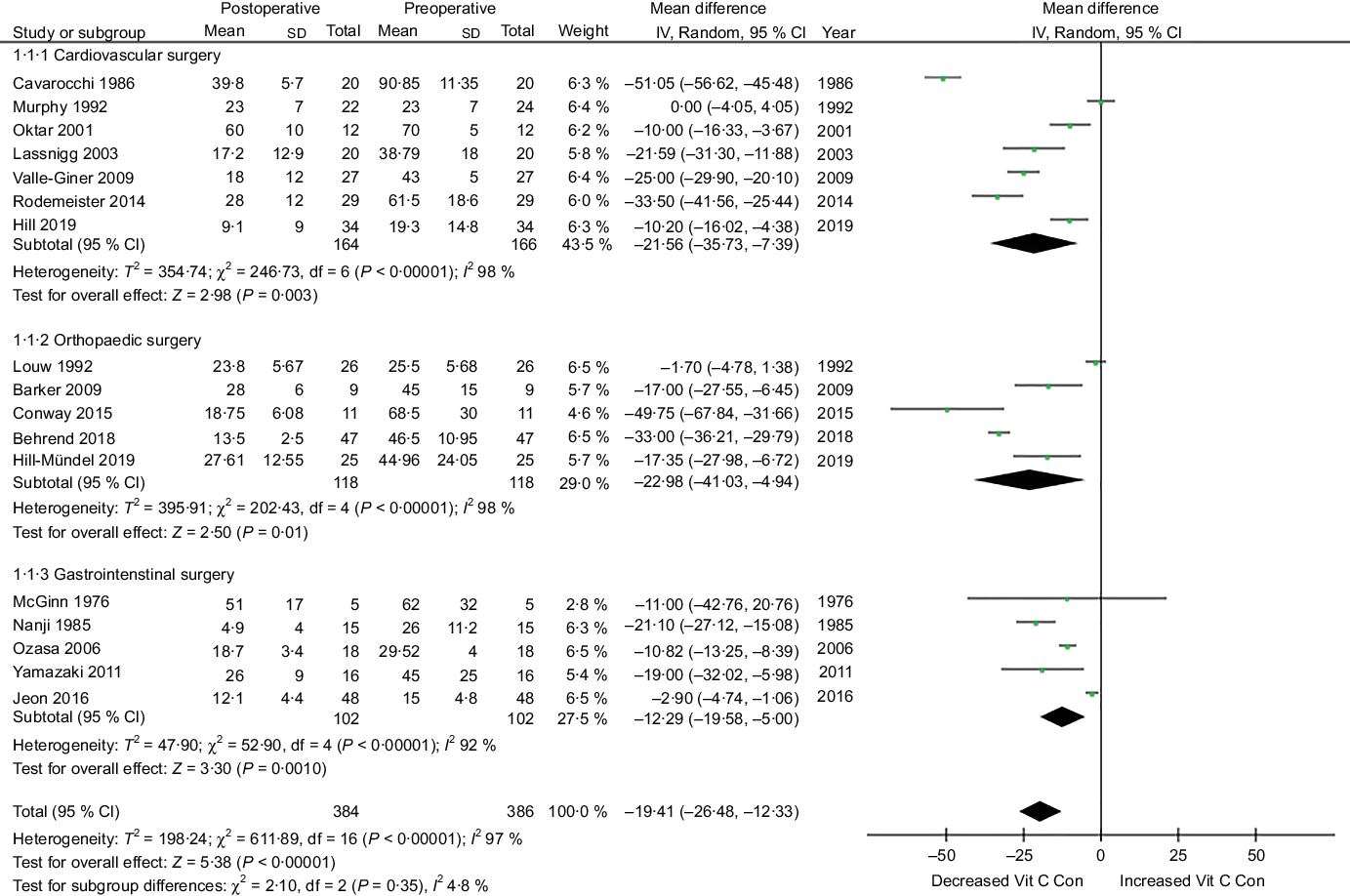
Fig. 6. Subgroup meta-analysis for different types of surgery (cardiovascular, orthopaedic and gastrointestinal) assessing vitamin C concentrations within the first postoperative week. Diamond represents overall effect size of the meta-analysis. Boxes represent mean differences in concentrations and lines across the boxes represent respective 95 % CI, Vit, vitamin; Con, concentration.
Discussion
This systematic review and meta-analysis were performed to summarise the effects of surgery on plasma vitamin C concentrations. The systematic review revealed consistent trends of significant postoperative depletions. This trend was observed in a number of high-quality trials that adhered to study designs with a low risk of bias. Pooled data from the meta-analysis, which excluded studies displaying a high risk of bias, revealed a mean plasma vitamin C concentration depletion of −17·99 µmol/l (39 %) (CI −22·81, −13·17) (P < 0·001) during the first postoperative week and −18·80 µmol/l (21 %) (CI −25·04, −12·56) (P < 0·001) 2–3 months postoperatively. Given the high heterogeneity between the aggregated studies, findings from the first postoperative week should be interpreted with caution. Additional subgroup analyses revealed significant mean vitamin C depletions following cardiac, orthopaedic and gastrointestinal surgery types within the first postoperative week. Nonetheless, this subgroup analysis failed to reduce the high heterogeneity between trials assessing changes in concentration during the first postoperative week.
A number of multi-factorial explanations may account for the observed results. Extensive research has demonstrated the prominence of oxidative stress (reactive oxygen species (ROS)) following surgical trauma(Reference Kotzampassi, Kolios and Manousou76). This uprise may result from postoperative biochemical alternations(Reference Kotzampassi, Kolios and Manousou76) alongside the stimulation of inflammatory responses(Reference Dobson26) and the hypothalamus pituitary axis(Reference Desborough77). Various postoperative conditions such as infections, sepsis and ischemia can further exacerbate the generation of ROS(Reference Dobson26). Due to ascorbate’s antioxidant properties, the vitamin is heavily utilised during periods of oxidative stress(Reference Sasazuki, Hayashi and Nakachi78) which can deplete concentrations(Reference Mezzetti, Lapenna and Romano79). Ascorbate is known as a ‘first-line’ responder due to preserving other antioxidants such as vitamin E(Reference Ballmer, Reinhart and Jordan66).
Ascorbate may be used for a number of additional postoperative physiological processes such as l-carnitine production, collagen, nitric oxide synthesis/preservation and immune system modulation, in an effort to return to postoperative physiological homoeostasis. Consequently, postoperative vitamin C depletions may interfere with a number of these functions. Preliminary studies have revealed that postoperative depletions in vitamin C may be potentially associated with impaired wound healing(Reference Bikker, Wielders and van Loo80), disrupted pain modulation (through impaired vitamin C histamine synthesis)(Reference Jeon, Park and Moon72), impaired cognition(Reference Travica, Ried and Pipingas81), increased risk of arthrofibrosis(Reference Behrend, Lengnick and Zdravkovic43) and prolonged hospital stay(Reference Sharma, Miller and Shahi82).
Another proposed mechanism involves the redistribution of vitamin C following surgical trauma. Studies have demonstrated both depletions and increases in ascorbate leucocyte concentrations during the first postoperative week(Reference Vallance56,Reference McGinn and Hamilton61) , despite a significant increase in leucocyte numbers. This may be due to ascorbate being compartmentalised from plasma into leucocytes(Reference Hornig83), given the high affinity for ascorbate displayed by leucocytes(Reference Bergsten, Amitai and Kehrl84). Further reports have suggested that haemodilution, characterised by decreased blood concentration of cells and solids in the blood resulting from the gain of fluid(Reference Avidan, Silvergleid and Hines85), may lead to reductions in postoperative vitamin C concentrations(Reference McGinn and Hamilton61).
The oxidised form of ascorbate, dehydroascorbic acid, is transported through glucose receptors (such as GLUT1 and GLUT3)(Reference Ben-Poorat and Moridani86,Reference Corpe, Eck and Wang87) . Research has postulated that glucose (which is commonly elevated postoperatively)(Reference Dobson26) may compete with dehydroascorbic acid for absorption through the GLUT receptors and impede the ability of intracellular ascorbate recycling.
Additionally, anaesthetic type may play a role in ascorbate concentrations, as exemplified in one of the included studies in which participants exposed to general anaesthesia experienced significant reductions in plasma vitamin C, whereas those exposed to local anaesthetic failed to demonstrate significant depletions(Reference Akita, Kawahara and Takeshita60). However, these results have not been validated by more recent studies with larger sample sizes. A number of commonly prescribed postoperative medications including aspirin have also demonstrated a capacity to reduce plasma vitamin C concentrations(Reference Basu88).
The smaller percentage reduction of mean vitamin C concentrations beyond seven postoperative days in comparison with those that assessed concentrations during the first postoperative week may be explained by a higher degree of oxidative stress and physiological processes requiring ascorbate, and a greater re-distribution and malabsorption of ascorbate during the short-term postoperative period. In addition, perioperative acute starvation and hospitalisation-associated malnutrition(Reference Kirkland and Shaughnessy89) may play a role, with disrupted eating patterns, suppressed appetite and suboptimal storage/preparation of vitamin C-rich foods during the inpatient phase(Reference Avelino-Silva and Jaluul90,Reference Armstrong, Jamieson and Porter91) .
A number of studies demonstrating mean preoperative concentrations of vitamin C (>70 µmol/l) demonstrated higher postoperative vitamin C concentrations, representative of adequate concentrations during the first postoperative week. This is consistent with a number of randomised controlled trials which administered a vitamin C intervention preoperatively as a means of achieving high plasma concentrations and observed non-deficient postoperative concentrations during the first postoperative week(Reference Barker, Leonard and Trawick62,Reference van Stijn, Ligthart-Melis and Boelens70) .
Conversely, the mean percentage of reduction in vitamin C concentrations during the first postoperative week appeared large in a number of studies that reported mean preoperative concentrations over 60 µmol/l(Reference Conway, Talwar and McMillan45,Reference Rodemeister, Duquesne and Adolph53) . This is in line with previous studies which demonstrated a more dramatic depletion in vitamin C concentrations amongst patients with higher preoperative levels(Reference Bartlett, Jones and Ryan32,Reference Crandon, Landau and Mikal33) . This may be explained by the pharmacokinetics of plasma ascorbate. At normal intake, ascorbate has an estimated half-life between 7 and 14 d in plasma(Reference Collie, Greaves and Jones92); however, if ingestion stops completely, or if at low levels, vitamin C’s half-life is significantly longer, between 35 and 40 d(Reference Kallner, Hartmann and Hornig93, Reference Noble, Healey and McDougal-Chukwumah94). Based on these results, more research is required to determine whether preoperative concentrations and perioperative supplementation make a difference to postoperative outcomes.
We acknowledge a number of limitations in the present meta-analysis. Despite studies with a high risk of bias being excluded from the meta-analysis, twenty out of the twenty-three studies presented with an unclear risk of bias for confounding factors. These trials failed to assess either dietary intake of vitamin C-containing foods, supplementation, smoking status, alcohol consumption or pre- and postoperative medications. This information would have been crucial considering a number of studies(Reference Conway, Talwar and McMillan45,Reference Kaur, Negi and Sarna52,Reference Akita, Kawahara and Takeshita60) demonstrated preoperative concentrations indicative of levels likely achievable through supplementation and/or high dietary intakes.
It is well known that exogenous factors such as heat, light and storage(Reference Pullar, Bayer and Carr95) can influence the rate of in vitro vitamin C oxidation, requiring appropriate vitamin C probe handling methods that ensure the stability and accurate measurement of absolute plasma vitamin C concentrations(Reference Pullar, Bayer and Carr95). Nineteen out of the twenty-three studies provided information that related to established vitamin C probe handling techniques prior to biochemical analysis; however, four studies failed to report this information(Reference Behrend, Lengnick and Zdravkovic43,Reference Oktar, Sinci and Kalaycioğlu50,Reference McGinn and Hamilton61,Reference Jeon, Park and Moon72) . In order to ensure accurate assessment, studies should adhere to the various established vitamin C probe handling techniques.
Additionally, surgical information such as the length of surgery, type of anaesthetic and surgical complications were only reported in four trials(Reference Akita, Kawahara and Takeshita60,Reference Barker, Leonard and Trawick62,Reference Hill, Borgs and Fitzner71,Reference Jeon, Park and Moon72) . Various studies did take into account a range of additional vital biomarkers, including vitamin E concentrations, haematocrit concentrations indicative of haemodilution(Reference Ballmer, Reinhart and Jordan66), biomarkers of oxidative stress(Reference Cavarocchi, England and O’Brien44,Reference Conway, Talwar and McMillan45) and the type of anaesthetic administered(Reference Akita, Kawahara and Takeshita60). Sex differences were only considered in one trial(Reference Louw, Werbeck and Louw47), which were averaged for the purpose of our analysis. It has recently been proposed that plasma and tissue vitamin C may be regulated diversely between sexes(Reference Travica, Ried and Hudson96), suggesting that sex may be a variable that should be considered when assessing perioperative vitamin C concentrations.
Moreover, pre-surgical co-morbidities needs to be considered, which could affect both plasma vitamin C concentrations and the body’s response to surgical trauma. Disease-related malnutrition, often associated with inflammation, may be exacerbated during the perioperative period, increasing malnutrition and potentially affecting perioperative vitamin C concentrations(Reference Norman, Pichard and Lochs97). One of the studies(Reference Jeon, Park and Moon72) included in our meta-analysis did consist of participants diagnosed with colon cancer; however, postoperative concentration was measured within 24 h of the procedure, minimising the potential confounding effects of cancer on plasma concentrations (i.e. malnutrition, treatments).
High heterogeneity was observed for a number of our meta-analyses, including the subgroup analyses. This is the first meta-analysis to pool data from the array of studies that have explored changes in postoperative plasma vitamin C concentrations. Subsequently, the observed heterogeneity may be attributed to varying surgery types, types of anaesthetic administered, differences in mean preoperative vitamin C concentrations, length of patient hospital stay and participant pre- and postoperative health status between trials. Moreover, although concentrations were split into the first postoperative week and those beyond, concentrations were derived from varying postoperative time points despite efforts to categorise them into the broader time point groups.
Additional analyses revealed a potential publication bias amongst the trials assessing changes in concentrations during the first postoperative week. However, a number of explanations may account for this, including clinical and methodological heterogeneity between trials and the observation of postoperative vitamin C depletion in all of the trials, irrespective of sample size. A paucity of published studies reporting increases or no effects of surgery on postoperative vitamin C concentrations could further explain this.
To date, an overwhelming number of studies assessing postoperative vitamin C concentrations are within time points during the first postoperative week (21/23 studies). Longer-term assessments, during which patients are outpatients, are necessary for both more conclusive results and a clinical significance to be established. Vital information revolving around the patient’s sex, pre-surgical co-morbidities, perioperative dietary intake, supplementation, smoking/alcohol intake, medications and surgical details will provide the opportunity for future investigations to subgroup the studies and compare them accordingly with less heterogeneity. Future studies should also consider preoperative levels and assess how varying preoperative concentrations respond to surgery.
Conclusion
This is the first meta-analysis to systematically assess the effects of surgery on mean plasma vitamin C concentrations. Based on the studies conducted to date, our meta-analysis has revealed a significant mean plasma vitamin C depletion following surgery both acutely (≤7 d postoperatively) and during longer-term postoperative time points (>7 d from surgery). Significant mean plasma vitamin C depletions were independent of surgery type such as cardiac, orthopaedic and gastrointestinal surgery within the first postoperative week. Despite the exclusion of studies presenting with a high risk of bias, the degree of heterogeneity weakens the inferences that can be made from the study results obtained from the first postoperative week. Large-scale, long-term trials are required to confirm these results and examine the types of postoperative pathophysiologies which may arise as a consequence of the vitamin C depletion, in an effort to guide potential approaches that could ameliorate this depletion.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
N. T. and A. S. formulated the meta-analysis, data analysis was conducted by N. T., K. R. and I. H., N. T. and K. R. interpreted the results, N. T., K. R., I. H., A. Sc., A. P. and A. S. contributed to writing the article.
A. Sc. and A. P. have received research funding, consultancy, travel support and speaking fees from the nutrition and supplement industry. N. T., K. R., A. S. and I. H. declare no conflict of interest.












