Atherosclerosis, the primary pathophysiological mechanism for coronary artery disease (CAD) development, is a multifactorial process that results from a interaction between modifiable and non-modifiable cardiovascular risk factors(Reference Lim, Vos, Flaxman and Danaei1). Atherosclerotic lesion initiation, progression and complication are complex and involve lipoproteins, vascular wall components, blood cells and the immune system(Reference Golia, Limongelli and Natale2). The inflammatory process participates in CAD genesis and may be perpetuated by low-grade chronic inflammation, associated with overweight and other factors(Reference Chistiakov, Grechko and Myasoedova3). Among the pro-inflammatory cytokines related to atherosclerosis development, IL-6 plays a central role in the spread of the inflammatory cascade(Reference Hartman and Frishman4).
The Mediterranean dietary pattern and the consumption of its main components, such as nuts and extra-virgin olive oil, act beneficially on cardiovascular risk factors and can suppress markers in inflammatory pathways related to atherosclerosis(Reference Estruch5). Clinical studies conducted in humans demonstrate that nuts and olive oil consumption may improve markers of systemic inflammation, such as IL-6 and C-reactive protein (CRP)(Reference Mena, Sacanella and Vazquez-Agell6–Reference Fernandes, Fialho and Santos8), and also reduce the incidence of cardiovascular events such as acute myocardial infarction(Reference Estruch, Ros and Salas-Salvadó9). On the other hand, other randomised trials conducted with similar populations (healthy individuals and at high cardiovascular risk) have found null effects(Reference Baer and Novotny10–Reference de Oliveira, Kovacs and Moreira12) regarding the effect of these foods on inflammation, demonstrating that the relationship between nuts, olive oil and inflammatory markers warrants further investigation.
Studies that have shown benefits of Mediterranean diet and its components on cardiometabolic health are mainly concentrated in European populations(Reference Storniolo, Casillas and Bulló13–Reference Casas, Urpi-Sardà and Sacanella15) and in primary cardiovascular disease prevention. Among Spanish individuals with high cardiovascular risk, the Mediterranean diet pattern supplemented with nuts or extra-virgin olive oil resulted in lower concentrations of inflammatory markers in comparison to baseline data(Reference Casas, Sacanella and Urpí-Sardà14) and to a control low-fat diet as well(Reference Casas, Urpi-Sardà and Sacanella15). However, few studies that have evaluated the consumption of nuts and olive oil in the context of a Mediterranean diet or not have been conducted among individuals with established cardiovascular disease(Reference Chen, Holbrook and Duess16–Reference Fitó, Cladellas and de la Torre20) and in non-European populations. In addition, the effects of consumption of nuts and olive oil on systemic inflammation among patients on continuous use of specific drug therapy such statins and antiplatelet agents, which are known to modulate inflammatory markers(Reference Kwon, Kang and Kang21,Reference Hadi, Mohammad and Ajeena22) , are not well established(Reference Chen, Holbrook and Duess16–Reference Fitó, Cladellas and de la Torre20).
Extra-virgin olive oil and nuts produced and cultivated in Brazil are similar to those from Europe, providing a high content of bioactive compounds and unsaturated fatty acids(Reference Crizel, Hoffmann and Zandoná23,Reference de Carvalho24) with potential benefits on the inflammatory profile. A Mediterranean dietary pattern is not feasible in many countries because most of the foods that composes it are not widely available, may be expensive or are not part of local eating habits. However, specific components or foods with similar anti-inflammatory properties may be evaluated in the cardiovascular context. Thus, the aim of this study was to investigate the effects of supplementing a healthy diet with pecan nuts or extra-virgin olive oil on inflammatory markers in patients with stable CAD.
Methods
Study design and ethical approvals
This is a subanalysis of the GENUTRI study, for which detailed protocol(Reference Portal, Markoski and Quadros25) and main outcome results(Reference Campos, Portal and Markoski26) were previously published. Briefly, this is a randomised, pragmatic, parallel clinical trial, with a 1:1:1 allocation rate, lasting 12 weeks, conducted at Instituto de Cardiologia do Rio Grande do Sul, Porto Alegre, Brazil. The study was approved by the Research Ethics Committee of the institution under protocol number 534.850 (CAAE 26591514.8.0000.5333), conducted according to the Declaration of Helsinki and registered on the ClinicalTrials.gov website under number NCT02202265. The study was conducted between August 2014 and June 2016(Reference Campos, Portal and Markoski26).
Participants
Inclusion criteria were diagnosis of stable CAD (defined by the presence of unstable angina or acute myocardial infarction for more than 60 d(27)) and age between 40 and 80 years. Exclusion criteria were psychiatric illnesses, extreme obesity (body mass index [BMI] ≥ 40 kg/m2), life expectancy less than 6 months, pregnancy or lactation, kidney disease undergoing dialysis, use of a wheelchair, uncontrolled hypothyroidism and hyperthyroidism, heart failure, use of dietary supplements, chronic use of anti-inflammatory and immunosuppressive drugs and participation in other clinical studies.
Randomisation and blinding
A block randomisation sequence was generated by the website www.randomization.com and the numbers were placed in individual opaque and sealed envelopes. Considering the characteristic of the intervention, the study was not fully blinded(Reference Portal, Markoski and Quadros25,Reference Campos, Portal and Markoski26) .
Study interventions
Participants were randomised to one of three groups: control group (CG), which received a healthy diet according to the nutritional guidelines(Reference Portal, Markoski and Quadros25,Reference Campos, Portal and Markoski26) ; pecan nut group (PNG), which received the guideline-oriented diet supplemented with 30 g/d of pecan nut or olive oil group (OOG), which received the guideline-oriented diet supplemented with 30 ml/d of extra-virgin olive oil(Reference Kris-Etherton and Innis28). Diets were calculated individually for weight reduction or maintenance, according to BMI at baseline (to weight management if BMI < 25 kg/m2:25 kcal/kg/d; to weight loss if BMI > 25 kg/m2:20 kcal/kg/d)(Reference Portal, Markoski and Quadros25), and participants received a folder with general guidelines on healthy eating, in addition to a table describing the foods to be avoided, eaten in moderation or consumed daily. The CG was instructed not to consume pecans, other nuts and olive oil during the study period; PNG was instructed not to eat olive oil and OOG was instructed not to consume pecans and other nuts. Participants who received the supplements were also instructed not to share with family, friends or with any other person (consumption should be individual).
The nutritional composition of the three diets prescribed, its similarities and differences are described in Table 1. The fatty acids and total polyphenols composition of the pecan and olive oil offered in the study and details about the origin and methods for conservation and daily consumption of the foods were previously published in details(Reference Portal, Markoski and Quadros25,Reference Campos, Portal and Markoski26) .
Table 1 Example of the nutritional composition of a diet with ˜2000 kcal/d prescribed to the study groups
(Percentages)

PNG, pecan nut group; OOG, olive oil group; CG, control group.
% TE, percentage of total energy. Adapted from Campos VP, et al. (Reference Campos, Portal and Markoski26).
Data collection and follow-up
In the initial assessment, participants were submitted to a standardised questionnaire that included socio-demographic, clinical (current and past), lifestyle data and level of physical activity(Reference Portal, Markoski and Quadros25). The medical diagnoses of dyslipidaemia, hypertension and type 2 diabetes mellitus were defined according to the current guidelines at the beginning of the study(Reference Portal, Markoski and Quadros25). Body weight (kg), height (cm) and waist circumference (cm) were obtained, and BMI (kg/m2) was calculated.
Food consumption was assessed using a 24-h dietary recall (24hR)(29). The total energy intake (TEI, kcal/d) was calculated, as well as the following nutrients (in grams and in percentages of TEI): carbohydrates, proteins, total fats, saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA) and total dietary fibres. Dietary cholesterol was determined in milligrams. The Avanutri Revolution© software (Avanutri) was used to calculate the nutritional value of diets, according to tables of chemical composition of food and databases with homemade recipes. Participants were reassessed monthly during a 3-month period (i.e. at 4, 8 and 12 weeks). The adherence rate was assessed by both the presence in consultations and according to the 24hR applied in each follow-up visit.
Biochemical analyses
Blood samples were collected at the first and last evaluation, after 12 weeks. The plasma samples were obtained by centrifugation at 3000 rpm for 15 min at 4ºC and stored at –80ºC until use. The cytokines IL-2, IL-4, IL-6, IL-10 and interferon-γ (all in pg/ml) were simultaneously evaluated from the plasma aliquots, using the Cytometric Beads Array with the detection reagent Th1/Th2 (BD Biosciences) according to the manufacturer’s instructions. Flow cytometry data were acquired on the BD FACSCanto II device (BD Biosciences). Quantitative results were generated using FCAP Array v3.0.1 software (BD Biosciences). Ratios between pro-inflammatory cytokines and anti-inflammatory cytokines (IL-6/IL-10 ratio, IL-2/IL-4 ratio and interferon-γ/IL-4 ratio) were calculated.
The levels of high-sensitivity C-reactive protein (hs-CRP, in mg/l) were evaluated by the turbidimetry technique (Roche Cobas Integra 400 Plus Chemistry Analyzer®) and the levels of fibrinogen (in mg/dl) by the coagulometric method (Sysmex CA-600 systems®).
Outcomes
The primary outcome was changes in IL-6 concentrations (pg/ml) after 3 months of follow-up between the groups. Changes in other IL levels, hs-CRP, fibrinogen and in all ratios were considered secondary outcomes.
Sample size
This study is an exploratory analysis, taking into account that the sample size was primarily calculated for the main outcome of GENUTRI study (LDL-cholesterol)(Reference Campos, Portal and Markoski26). Considering a significance level of 5 % and power of 80 %; a sd of 2·5 in olive oil, a sd of 0·9 in nuts and a sd of 2·7 in the CG changes in IL-6 after 12 weeks(Reference Esposito, Marfella and Ciotola30,Reference Willett, Howe and Kushi31) and a minimum difference of 1·5 pg/dl between groups, thirty up to forty-nine individuals would be necessary for each group(Reference Mena, Sacanella and Vazquez-Agell6,Reference Urpi-Sarda, Casas and Chiva-Blanch32) .
Statistical analysis
Statistical Package for Social Sciences (SPSS), version 17.0 for Windows, and R.4.0.2 (R Core Team, 2020) software were used for statistical analysis. Categorical variables were shown as absolute numbers and proportions; continuous variables were shown as mean and standard deviation (sd) or standard error (se) and median (interquartile range). Kolmogorov–Smirnov test was used to evaluate the normality of the variables. ANOVA and Kruskal–Wallis test were used for comparisons between variables with normal and non-normal distribution, respectively. Pearson’s χ 2 test (between-group analysis) and McNemar’s test (intragroup analysis) were used for comparisons between proportions. Repeated measures of nutrients intake were assessed according to treatment group and time by the generalised estimating equation, with normal probability distribution for symmetric variables and gamma probability distribution for asymmetric variables, followed by Bonferroni’s test. All dietary variables (according to 24hR) were adjusted for TEI by the residual method(Reference Willett, Howe and Kushi31). Mixed effect models (normal or gamma probabilities) adjusted for sex, type of statin use and relative body weight variation were used for inflammatory markers evaluation at baseline and at the end of follow-up according to groups. Analyses were performed on an intention to treat basis. Missing data were dealt with by multiple imputation, and sensitivity analyses including only individuals who completed the protocol were made to confirm the results. The level of significance in two-tailed tests was set at 5 %.
Results
As previously described(Reference Campos, Portal and Markoski26), 370 individuals were recruited between August 2014 and January 2016, and 166 did not meet the inclusion criteria or showed no interest in participating. In total, sixty seven individuals with stable CAD were randomised to the CG, sixty eight to the PNG and sixty nine to the OOG. Figure 1 shows the flow chart of the study, including all withdrawals and deaths. At the end of the study, CG showed an adherence rate of 66 %, PNG 84 % and OOG 86 %. Detailed information on adverse effects and adverse events according to study groups has been previously published(Reference Campos, Portal and Markoski26).

Fig. 1 Flow chart of the study. NSAID, non-steroidal anti-inflammatory drugs; ITT, intention-to-treat. Adapted from Campos VP, et al. (Reference Campos, Portal and Markoski26).
Table 2 shows the characteristics of all participants at baseline, and OOG exhibits a significant lower prevalence of hypertension (P = 0·03). In comparison with the participants who completed the study, those who withdrew showed lower concentrations IL-2, IL-6, IL-10 and interferon-γ and higher values of IL-6/IL-10 ratio and IL-2/IL-4 ratio at baseline. However, among patients who did not complete the study, there was no significant difference between basal levels of inflammatory markers according to study group (online Supplementary Tables S1 and S2).
Table 2 Baseline characteristics of participants according to study group*
(Mean values and standard deviations; median and interquartile range; number and percentages)
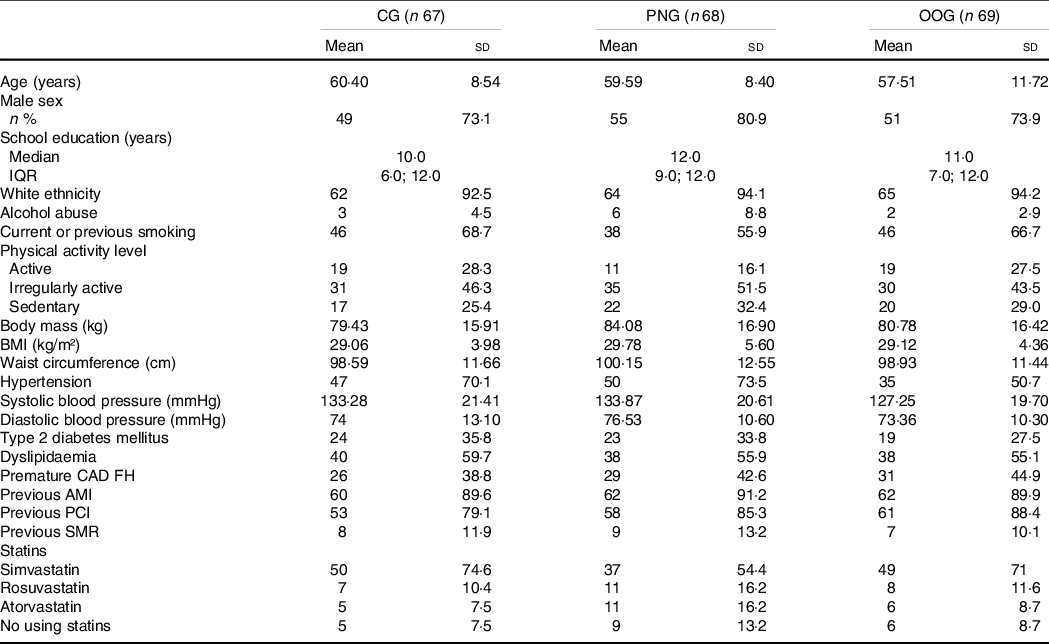
CG, control group; PNG, pecan nut group; OOG, olive oil group; BIM, body mass index; CAD FH, family history of coronary artery disease; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; SMR, surgical myocardial revascularisation.
* Adapted from Campos VP, et al. (Reference Campos, Portal and Markoski26).
Initial and final values of IL-4, IL-2/IL-4 and IL-6/IL-10 ratios were significantly different according to statin use. Individuals using atorvastatin showed higher levels of IL-4 and lower levels of IL-2/IL-4 ratio before intervention (P values 0·009) in comparison with those not using statins or those using either simvastatin or rosuvastatin. Those who were not using statins showed lower levels of IL-4 and fibrinogen (P values 0·036 and 0·016, respectively) after interventions when compared with those using statin. And those using atorvastatin and rosuvastatin showed lower values of IL-2/IL-4 and IL-6/IL-10 ratios after intervention (P values 0·039 and 0·009, respectively) when compared with those using no statin or simvastatin (online Supplementary Tables S3). There was no difference with respect to means of other inflammatory markers according to statin use.
As previously shown(Reference Campos, Portal and Markoski26), there was no difference among study groups regarding the number of patients who changed the dose or type of statin or other drugs (insulin, antihypertensive, antiplatelet and anti-diabetic drugs), and there was no intragroup difference regarding levels of physical activity at the end of follow-up. With respect to dietary intake, in grams, there was no difference between groups regarding carbohydrates, proteins, total fats, SFA and PUFA, dietary fibre and cholesterol at the end of the study, neither in energy intake. MUFA, in grams, was significantly higher in PNG and OOG in comparison with CG after follow-up (P < 0·01) (Table 3). However, as previously published(Reference Campos, Portal and Markoski26), when carbohydrates, protein and fats were converted to % of TEI, PNG and OOG showed a significant increase in MUFA intake compared with baseline (P < 0·001), but final percentages of MUFA were not different between groups (P = 0·41).
Table 3 Dietary profile of the participants according to follow-up time and study group*,†
(Mean values and standard deviation)

CG, control group; PNG, pecan nut group; OOG, olive oil group; TEI, total energy intake; CHO, carbohydrates; PTN, proteins; TF, total fats; SFA, saturated fatty acids; PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; Chol, dietary cholesterol; DF, dietary fibre.
a, b Different letters indicate between-group differences at the end of the study (P < 0·01). Generalised estimating equation (GEE) with normal probability distribution (symmetric variables) and gamma probability distribution (asymmetric variables), followed by Bonferroni test and adjusted for TEI using the residual method.
* P-values regarding differences between groups at the end of the study: TEI: 0·81; carbohydrates: 0·18; proteins: 0·57; total fats: 0·30; SFA: 0·98; PUFA: 0·69; MUFA: < 0·01; cholesterol: 0·38; dietary fibres: 0·75.
† Adapted from Campos et al. (Reference Campos, Portal and Markoski26).
Table 4 shows the mean values of inflammatory markers before and after the interventions (crude and adjusted). After adjustment for sex, statin used and relative body weight variation, there was no difference between the groups regarding all inflammatory markers at the end of the study. IL-4 (difference: 0·896 (se = 0·266) pg/dl; 95 % CI 0·371, 1·421; P = 0·001), IL-6 (difference: –0·593 (se = 0·159) pg/dl; 95 % CI –0·905, –0·280; P < 0·001), IL-10 (difference: –0·337 (se = 0·12) pg/dl; 95 % CI –0·571, –0·102; P = 0·005) and IL-2/IL-4 ratio (difference: –0·114 (se = 0·025); 95 % CI –0·163, –0·065; P < 0·001) showed significantly differences in CG at the end of the study when compared with baseline. PNG had significantly reductions in IL-6 (difference: –0·335 (se = 0·143) pg/dl; 95 % CI –0·615, –0·054; P = 0·020) and IL-2/IL-4 ratio (difference: –0·062 (se = 0·027); 95 % CI –0·115, –0·01; P = 0·021) at the end of the study in comparison with baseline values. And OOG showed lower values of IL-6 (difference: –0·325 (se = 0·143) pg/dl; 95 % CI –0·605, –0·044; P = 0·023), IL-6/IL-10 ratio (difference: –0·080 (se = 0·038); CI95 % –0·155, –0·005; P = 0·007) and fibrinogen (difference: –31·1 (se = 10·2) mg/dl; 95 % CI –51·1, –11·1; P = 0·003) in the intragroup comparison. Sensitivity analysis including only individuals who completed the protocol is presented in online Supplementary Table S4, confirming the lack of difference between study groups at the end of the study and showing the same results regarding intragroup analysis of the primary outcome.
Table 4 Initial and final means (crude and adjusted) of inflammatory markers after 12 weeks of follow-up according to study group‡
(Mean values and their standard errors)

* Crude means.
** Means adjusted for type of statin in use, sex and weight variation. Mixed effects models with normal probability distribution (symmetric variables) and γ probability distribution (asymmetric variables), followed by Bonferroni test. CG, control group; PNG, pecan nut group; OOG, olive oil group; IL, interleukine; INF-γ, interferon-γ; hs-CRP, high-sensitivity C-reactive protein.
† Significant intra-group comparisons (P values < 0·05).
‡ P-values for comparison between groups (two by two) of the inflammatory markers obtained from adjusted mixed models at the end of the study. IL-2: CG – PNG: 0·72, CG – OOG: 0·94, PNG – OOG: 0·87; IL-4: CG – PNG: 0·53, CG – OOG: 0·36, PNG – OOG: 0·97; IL-6: CG – PNG: 0·41, CG – OOG: 0·39, PNG – OOG: 0·99; IL-10: CG – PNG: 0·72, CG – OOG: 0·10, PNG – OOG: 0·35; INF-γ: CG – PNG: 0·81, CG – OOG: 0·94, PNG – OOG: 0·57; IL-6:IL-10 ratio: CG – PNG: 0·87, CG – OOG: 0·83, PNG – OOG: 0·48; IL-2:IL-4 ratio: CG – PNG: 0·29, CG – OOG: 0·05, PNG – OOG: 0·74; INF- γ:IL-4 ratio: CG – PNG: 0·83, CG – OOG: 0·70, PNG – OOG: 0·42; us-CRP: CG – PNG: 0·60, CG – OOG: 0·98, PNG – OOG: 0·42; Fibrinogen: CG – PNG: 0·96, CG – OOG: 0·35, PNG – OOG: 0·18.
Discussion
In this study, we observed that there was no difference between a healthy diet with or without 30 g/d of pecan nuts or 30 ml/d of olive oil on inflammation in individuals with established CAD – all three dietary approaches may modulate specific inflammatory markers in a 12-weeks follow-up period. As far as we know, our study is one of the few conducted in the setting of secondary cardiovascular prevention considering nuts and extra virgin olive oil as intervention in a non-Mediterranean diet setting; in addition, pecan nuts have been scarcely explored, mainly on markers of systemic inflammation and in individuals with previous CVD. And finally, we evaluated specific inflammatory markers poorly explored in the context of nuts, olive oil consumption and secondary prevention.
Healthy eating patterns are associated with lower concentrations of inflammatory markers, as they are sources of MUFA, PUFA, dietary fibre, vitamins, minerals and antioxidants(Reference Calder, Ahluwalia and Brouns33,Reference Galland34) . The beneficial effects on inflammatory markers related to extra virgin olive oil are due to phenolic compounds and the high proportion of unsaturated/saturated fatty acids, with oleic acid (MUFA) being its main fatty acid(Reference Urpi-Sarda, Casas and Chiva-Blanch32); those related to nuts are unsaturated fatty acids, L-arginine, minerals, phenolic compounds, dietary fibres and phytosterols(Reference Urpi-Sarda, Casas and Chiva-Blanch32,Reference Bitok and Sabaté35) . We probably did not detect differences between the groups in our study because there were no differences regarding the intake of nutrients that may modulate inflammatory markers such as MUFA (in % of TEI), PUFA and dietary fibres. Interestingly, the CG showed reductions in IL-6, IL-10 and increases in IL-4 levels. There are similar findings among Europeans who followed a low-calorie Mediterranean diet for 3 months, where levels of IL-10 decreased and IL-4 increased(Reference Sofi, Dinu and Pagliai36); and among Norwegians where a low-fat diet for 3 months reduced IL-6 levels(Reference Heggen, Klemsdal and Haugen37).
Similar results regarding inflammation and different types and dosages of nuts compared with control diets have been shown considering studies conducted in healthy individuals(Reference Baer and Novotny10), with type 2 diabetes mellitus(Reference Hou, Ojo and Wang11), or those metabolically at-risk(Reference Casas-Agustench, López-Uriarte and Bulló38,Reference McKay, Eliasziw and Chen39) . In addition, systematic reviews with meta-analysis on primary cardiovascular prevention have assessed the effect of nuts on inflammation. Neale et al. (Reference Neale, Tapsell and Guan40) found no statistically significant results regarding the effect of nuts on CRP and IL-6 in comparison with control diets. However, the results for CRP levels appeared to be dose dependent: when studies were grouped according to nut dose, an effect estimate near zero was found for those that included < 50 g of nuts/d, while an effect estimate of −0·34 mg/l (−0·63 to–0·06) was found when 50 g or more were used. This result suggests that the dose of our intervention (30 g/d) may not have been sufficient to differentiate the results from a healthy diet without nuts supplementation. Another systematic review(Reference Mazidi, Rezaie and Ferns41) found no statistically significant difference in relation to the consumption of nuts on CRP, IL-10 and IL-6 levels in comparison with control diets.
Regarding olive oil, studies evaluating the effects of 20–50 ml/d of this food on inflammation conducted with obese individuals(Reference de Oliveira, Kovacs and Moreira12,Reference Kruse, von Loeffelholz and Hoffmann42) , those with atherosclerosis(Reference Widmer, Freund and Flammer43) and with non-alcoholic fatty liver disease(Reference Rezaei, Akhlaghi and Sasani44) showed null results in comparison with control diets as well. Systematic review with meta-analysis(Reference Fernandes, Fialho and Santos8) evaluated the effect of regular olive oil consumption (interventions with oral capsules were excluded) on CRP and IL-6 in primary prevention. In total, thirteen studies were included in qualitative synthesis, nine of which were conducted in European populations and seven studies were included in the meta-analysis. Regarding the effect of olive oil on CRP and IL-6, considering two studies that used a low-fat diet as a comparator, an estimated effect (MD) of –1·40 (–1·81 to –0·99; P < 0·01; I 2 0 %) and -0·60 (–0·64 to –0·56; P < 0·01; I2 0 %), respectively, was observed. In contrast, these results were not significant when evaluating the effect of olive oil among unhealthy individuals (at high cardiovascular risk and with metabolic syndrome). Since our population is in fact unhealthy, it may explain our failure to demonstrate an effect regarding the reduction of CRP when considering the supplementation of olive oil.
Studies that focused on nuts and olive oil in individuals with CAD are scarce and present conflicting results. Among North Americans, almond supplementation (85 g/d) for 6 weeks did not alter CRP and IL-6 levels when compared with the control diet(Reference Chen, Holbrook and Duess16). In Spanish individuals, 50 ml/d of virgin olive oil daily for 3 weeks reduced IL-6 and CRP compared with baseline(Reference Fitó, Cladellas and de la Torre20). Considering nuts and olive oil intake in the context of a Mediterranean diet, hs-CRP levels did not change in comparison with a control diet in Brazilians(Reference Thomazella, Góes and Andrade17) and in Germans(Reference Michalsen, Lehmann and Pithan18) with CAD. In contrast, CRP levels were reduced after 1 year of follow-up in a large study conducted in Europe when Mediterranean diet was compared with a low-fat diet; however, stratifying individuals by endothelial dysfunction, there was no statistically significant difference between the groups in CRP(Reference Yubero-Serrano, Fernandez-Gandara and Garcia-Rios19) suggesting that more severe cardiac patients may not benefit from dietary interventions compared with less severe ones.
In addition to its lipid-lowering effect, statins have the capability to reduce vascular inflammation and effectively decrease markers of inflammation(Reference Golia, Limongelli and Natale2,Reference Owens45) . As most of our patients used statins, the intensive pharmacological treatment alone may reduce the levels of inflammatory markers and, therefore, any potential effect of the diet on specific inflammatory markers such IL-2, hs-CRP and interferon-γ could have been masked. Drug therapies have demonstrated reduction in the recurrence of cardiovascular events through anti-inflammatory drugs(Reference Ridker, Everett and Thuren46), which intervene on innate immunity. Our findings are in accordance with other authors(Reference Yubero-Serrano, Fernandez-Gandara and Garcia-Rios19,Reference Fitó, Cladellas and de la Torre20) and suggest that alternative therapies such as healthy diets with a low content of SFA and cholesterol and a high content of bioactive compounds and unsaturated fatty acids could be used as adjuvants, contributing to cardiometabolic health in medicated individuals.
Regarding the effect of olive oil supplementation on fibrinogen, the literature is scarce and remains inconsistent in relation to this result. Similar to our findings, a significant reduction in fibrinogen with the consumption of olive oil was observed among women who had higher baseline values for this marker(Reference Oosthuizen, Vorster and Jerling47), and no changes in fibrinogen levels were found when supplementing olive oil among patients with HIV(Reference Kozić Dokmanović, Kolovrat and Laškaj48). However, a higher adherence to the Mediterranean diet (defined in this study by the highest tertile of a specific diet score), which includes consumption of olive oil, reduced fibrinogen levels by 6 % among healthy individuals in comparison with a lower adherence (lowest tertile)(Reference Chrysohoou, Panagiotakos and Pitsavos49).
Studies that have evaluated the effect of nuts and olive oil on other inflammatory markers, other than CRP,(Reference Mena, Sacanella and Vazquez-Agell6–Reference Fernandes, Fialho and Santos8,Reference Baer and Novotny10,Reference de Oliveira, Kovacs and Moreira12,Reference Casas, Sacanella and Urpí-Sardà14,Reference Chen, Holbrook and Duess16–Reference Michalsen, Lehmann and Pithan18,Reference Fitó, Cladellas and de la Torre20,Reference Esposito, Marfella and Ciotola30,Reference Casas-Agustench, López-Uriarte and Bulló38–Reference Mazidi, Rezaie and Ferns41,Reference Widmer, Freund and Flammer43) fibrinogen,(Reference Baer and Novotny10,Reference Michalsen, Lehmann and Pithan18,Reference Yubero-Serrano, Fernandez-Gandara and Garcia-Rios19,Reference Oosthuizen, Vorster and Jerling47–Reference Chrysohoou, Panagiotakos and Pitsavos49) and IL-6(Reference Mena, Sacanella and Vazquez-Agell6,Reference Fernandes, Fialho and Santos8,Reference Baer and Novotny10,Reference Hou, Ojo and Wang11,Reference Casas, Sacanella and Urpí-Sardà14–Reference Chen, Holbrook and Duess16,Reference Yubero-Serrano, Fernandez-Gandara and Garcia-Rios19,Reference Fitó, Cladellas and de la Torre20,Reference Esposito, Marfella and Ciotola30,Reference Casas-Agustench, López-Uriarte and Bulló38,Reference Neale, Tapsell and Guan40,Reference Kruse, von Loeffelholz and Hoffmann42–Reference Rezaei, Akhlaghi and Sasani44) are scarce. In addition, studies that evaluated the supplementation of these foods have different protocols. Most differ in terms of duration, population evaluated, supplementation dose, control or comparator group, inflammatory markers evaluated, among others. These questions can interfere with the interpretation and comparison of our results with other findings. Despite many epidemiological studies have reported the promising effects of foods rich in antioxidants on cardiovascular prevention, there are still many unclear points with regard to experimental studies for treatment of atherosclerotic diseases(Reference Saita, Kondo and Momiyama50).
Finally, in our study, subjects using atorvastatin had higher levels of IL-4 before the intervention. As an anti-inflammatory cytokine, the literature is controversial regarding serum levels of IL-4 in patients with CAD(Reference Li, Zong and Zhang51,Reference Szodoray, Timar and Veres52) . In patients with ST segment elevation myocardial infarction, higher levels of IL-4 were observed after 30 d of the event when compared with baseline, and there was a positive correlation between baseline IL-4 levels and amount of infarcted mass in 30 d. These findings suggest an important role for this cytokine, which possibly attenuates the myocardial inflammatory process through greater cell differentiation in less inflammatory phenotypes(Reference Coste, França and Izar53).
We also observed a reduction in the IL-6/IL-10 and IL-2/IL-4 ratios among participants who used high-potency statins. Cheng et al. (Reference Cheng, Ding and Xia54) demonstrated that atorvastatin was able to affect the Th1/Th2 response in humans with heart failure. However, other authors showed no immunomodulatory effect by atorvastatin on the Th1/Th2 balance in human T cells in vitro (Reference Coward and Chow55). On the other hand, rats after acute myocardial infarction treated with atorvastatin showed higher levels of IL-10 and reduced levels of IL-6(Reference Stumpf, Petzi and Seybold56), and hyperlipidaemic rats treated with rosuvastatin showed increased levels of IL-4(Reference Saadat, Mohamadian Roshan and Aslani57).
As we have previously reported(Reference Campos, Portal and Markoski26), our study has several limitations and strengths. Among them, we used only a 24hR to estimate the participant’s food intake, which may not demonstrate the participant’s actual food intake despite be recommended as an approach for examining the effects of a dietary intervention(29). We did not control the eventual use of anti-inflammatory medication or other specific drugs during the study, which may have influenced the results if used near blood samples’ collection. The adherence rate of < 80 % showed in the CG may have induced a bias in our main results, even considering methods for handling with missing data. The participants allocated to the supplemented groups may have shown greater motivation compared with the CG, and, therefore, they have had better adherence to the dietary prescription(Reference Moreira, Most and Howard58). We did not ask participants to return the empty food packages for a better control of supplements intake. The amount of supplements, duration of the study and sample size may not have being enough to detect a possible difference between groups. We also did not analyse the ventricular function and the extent of CAD among patients, situations that can possible influence the levels of inflammatory markers(Reference Shirazi, Bissett and Romeo59,Reference Van Linthout and Tschöpe60) and the levels of tumour necrosis factor α as well. And finally, we did not evaluate samples of healthy individuals as controls of our experiments. Among the strengths of this study, we can cite the intention-to-treat analysis and the proximity to ‘real life’ by supplementing the diets with locally produced foods(Reference Campos, Portal and Markoski26). And despite we did not show differences between study groups, in our study, a healthy diet feasible and supplemented with foods locally produced was able to modify inflammatory markers.
Conclusion
There was no significant effect of including pecan nuts or extra virgin olive oil to a healthy diet on inflammatory markers in individuals with CAD. Further studies are needed to better define the modulation of the inflammatory profile of patients with established ischaemic heart diseases in response to dietary interventions.
Acknowledgements
We acknowledge Dr. Ricardo Bruch for laboratory analysis; the staff from the Hemodynamic Service and the Laboratory of Cellular and Molecular Cardiology of the Instituto de Cardiologia do Rio Grande do Sul; the companies Olivas do Sul, Divinut and Pecanita for the supplies of olive oil and nuts; Andréa Wieck-Ricachenevsky and Carine Prado for Cytometric Beads Array training and Lucas Damiani and Luciana Ishihara for statistical support.
This study was funded by the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq), the Rio Grande do Sul Research Foundation (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul; FAPERGS) and the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES).
A. M. designed the study. A. M. and A. C. B-F. performed the statistical analysis. C. W. participated in data collection and follow-up. A. M. and C. W. wrote the draft of the manuscript. M. M. M. and C. W. performed biochemical analyses. C. B. A. G., M. M. M., V. L. P., A. S. Q., A. C. B-F. and A. M. reviewed the manuscript. All authors read and approved the final version of the article.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521001513








