Fetal metabolism and development depend on nutrients crossing the placenta and, therefore, on maternal nutritional status and diet during pregnancy. The essential fatty acids and their derivatives, long-chain PUFA (LC-PUFA), are essential constituents of membranes, especially in the brain and retina(Reference Koletzko, Lien and Agostoni1), and must be provided to the fetus.
Given the importance of LC-PUFA in fetal development, in recent years, growing interest has been given to maternal fatty acid status. It is well known from interventional studies that LC-PUFA supplementation to pregnant women significantly increases LC-PUFA concentrations in both plasma and erythrocyte phospholipids (PL) in the mother as well as in the fetus(Reference Helland, Saugstad and Saarem2–Reference Sanjurjo, Ruiz-Sanz and Jimeno7). Few studies have assessed the effects of supplementation on plasma and erythrocyte PL in the same population(Reference Montgomery, Speake and Cameron8, Reference Otto, van Houwelingen and Hornstra9). It is generally accepted that LC-PUFA in plasma PL reflect tissue LC-PUFA status. Recent discussion has centred on whether erythrocyte membrane PL might be preferred to assess fatty acid status(Reference Baylin and Campos10, Reference Sun, Ma and Campos11). In addition, a positive correlation between folate and DHA levels has been reported in human subjects(Reference Umhau, Dauphinais and Patel12). Although the detailed mechanism remains unclear, the effect of folate supplementation on fatty acid status might be attributable to the increased methionine availability present after supplementation, which stimulates the metabolism of essential fatty acids(Reference Sugiyama, Kumazawa and Zhou13, Reference Pita and Delgado14).
The present study was performed to investigate the effects of supplementation with fish oil (FO) and/or 5-methyltetrahydrofolate (5-MTHF) from week 20 of gestation until delivery on the fatty acid profile of plasma and erythrocyte PL of pregnant women during the course of pregnancy and fetal LC-PUFA status. We also aimed to elucidate which is the best compartment for the assessment of the fatty acid status of pregnant women and their neonates.
Methods
Study design
Information regarding study design, recruitment, inclusion criteria, dietary intervention, and collection of data and biological material has been reported elsewhere(Reference Krauss-Etschmann, Shadid and Campoy15). Briefly, a multi-centre, randomised, double-blind controlled trial was conducted. A total of 315 healthy pregnant women were recruited before gestation week 20 at three different European centres (Ludwig Maximilians University, Munich (Germany), the University of Granada (Spain) and the University of Pecs (Hungary)). Women who consumed FO supplements since the beginning of pregnancy or vitamin B12 or folate supplements after gestation week 16 were excluded from the study. They were randomly assigned to four different groups and received daily a dietary supplement, from gestation week 20 until delivery, consisting of FO (500 mg DHA+150 mg EPA; Pronova Biocare), 400 μg 5-MTHF (BASF), both or placebo together with vitamins and minerals in amounts meeting the recommended intakes during the second half of pregnancy for European women. The mothers were physically examined at the 20th and 30th week of gestation and data on the course of pregnancy and dietary intake were collected by means of standardised questionnaires. At delivery, information about delivery and data on the newborn were collected. At the 20th and 30th week of gestation, 10 ml of maternal venous blood were collected into EDTA, and at delivery, 12 ml of maternal venous blood as well as 12 ml of venous cord blood were collected. The main outcome variables were fatty acid relative concentrations (wt%) in plasma and erythrocyte membrane PL of cord and maternal blood.
The study protocol was approved by the Medical Ethics Committees of all centres participating in the study. Written informed consent was obtained from all participants. The present trial was registered at www.clinicaltrials.gov (registration no. NCT01180933).
Biochemical analyses
Sample collection procedure
At the 20th week (before supplementations) and at the 30th week of gestation, 10 ml of maternal blood were collected into EDTA-containing tubes by venepuncture after an overnight fast. At delivery, 12 ml of maternal blood were obtained as described for the samples at the 20th and 30th week of pregnancy. Further, 12 ml of placental venous cord blood samples were collected into EDTA-containing tubes at delivery after clamping of the umbilical cord and before parturition of the placenta by puncture and aspiration from a long cord segment. Blood was centrifuged at 3500 rpm for 10 min at room temperature within 2 h. Plasma samples were frozen in liquid N2 at − 196°C in 100 and 200 μl aliquots and stored at − 80°C until fatty acid analyses. Blood cells were washed three times in isotonic NaCl solution. The last sediment was haemolysed in 1 ml of distilled water for 20 min at room temperature, and then 2 ml ice-cold isopropylic alcohol with 0·5 % butylated hydroxytoluene were added as an antioxidant and the samples were stored at − 80°C until further analyses.
Analyses of fatty acids in maternal and umbilical cord plasma and erythrocyte phospholipids
Total lipid extraction from plasma was performed according to the method of Kolarovic & Fournier(Reference Kolarovic and Fournier16). Briefly, 0·5 ml plasma plus 0·5 ml water were vortexed for 30 s with 100 μl of internal standard (0·857 g diheptadecanoyl phosphatidylcholine (PC)/l dissolved in chloroform). Then, lipids were extracted four times, initially with n-hexane–2-propanolol (3:2, v/v) containing 25 mg/l of butylated hydroxytoluene as an antioxidant and then three times with pure hexane. The pooled extracts were dried under vacuum and dissolved in 200 μl hexane–methyl-tert-butyl-diethyl ether–acetic acid (100:3:0·3, by vol.) and PL were isolated by liquid chromatography using aminopropyl columns (Sep Pak Cartridges; Waters) as described by Agren et al. (Reference Agren, Julkunen and Penttila17).
Lipids from erythrocytes were extracted by adding 3 ml chloroform and two internal standards (phosphatidylethanolamine (PE)-heptadecanoate and PC-pentadecanoate esters dissolved in methanol). The mixture was shaken on a vortex and the lower layer was aspirated and evaporated under a N2 stream. The dry lipid extract was resolved in chloroform and added to silica gel plates (Merck 60, 10 × 20 cm). The runner solvent for the first run was hexane–diethyl ether–chloroform–acetic acid (21:6:3:1, by vol.). The plate was dried under a hood at ambient temperature and ran again with chloroform–methanol–water (65:25:4, by vol.). For positioning PC and PE ester bands, the proper oleates were ran in parallel in every plate. The bands were stained with dichlorofluorescein, visualised under UV light and scrapped for transmethylation.
Fatty acid methyl esters from individual fractions were obtained by reaction with 3 m-HCl–methanol at 84°C for 40 min according to the method of Lepage & Roy(Reference Lepage and Roy18).
The quantification of fatty acid methyl esters from plasma PL was performed by GC using a gas chromatograph (HP5890 Series II; Hewlett Packard) equipped with a flame ionisation detector. Chromatography was performed using a 60 m-long capillary column with a 0·32 mm internal diameter and 0·20 μm thickness and impregnated with SP-2330 FS (Supelco). The injector and the detector were maintained at 250 and 275°C, respectively. N2 was used as the carrier gas and the split ratio was 29:1. Fatty acid methyl esters were identified by comparison of retention times with those of known standards.
Analysis of fatty acid methyl esters from erythrocyte PE and PC was performed by high-resolution capillary GLC (model 9001 GC; Finnigan/Tremetrics) with a split injection, an automatic sampler (A200SE; CTC Analytic) and a flame ionisation detector with a DB-23 cyanopropyl column of 60 m length (J & W Scientific). The conditions during the analysis were as follows: temperature of the injector at 80°C for 0·1 min, temperature increase by 180°C/min up to 280°C, temperature of the column area at 60°C for 0·2 min, temperature increase by 40°C/min up to 180°C, a 5 min hold period, temperature increase by 1·5°C/min up to 200°C, an 8·5 min hold period, temperature increase by 40°C/min up to 240°C and a 13 min hold period. The constant linear velocity was 0·3 m/s (referred to 100°C). For identification of sample peaks, we used two commercially available fatty acid methyl ester calibration mixtures (Supelco 37 FAME mix and NU-CKECK GLC reference 463) containing the fatty acids measured in the present study.
Results are expressed as percentages by weight (wt%) of total detected fatty acids with 14–24 C atoms.
Statistical analyses
The Kolmogorov–Smirnov or Shapiro–Wilk test was used to test the normality of variables. Baseline characteristics were compared among the intervention groups by using the Kruskal–Wallis test for continuous data and the χ2 test for ordinal data. A three-factor repeated-measures ANOVA with the intervention group as the between-subject factor and pregnancy time points (gestation week 20, gestation week 30 and delivery) as within-subject factors was performed to compare the effects of supplementation with time. Differences in cord blood fatty acid levels were assessed separately by using ANOVA for normally distributed variables and the Kruskal–Wallis test for not normally distributed variables. If significant effects were observed, multiple comparisons with Bonferroni correction were performed.
Spearman's correlations (ρ) were performed to determine the correlations between plasma and erythrocyte relative levels of fatty acids and between maternal fatty acid status at delivery and neonatal fatty acid status at birth. P values less than 0·05 were considered as significant.
Statistical analyses were performed using SPSS statistical software (version 15.0; SPSS Institute).
Results
Sample description
Information regarding sample description, compliance, dropouts and baseline characteristics of study participants has been reported previously(Reference Krauss-Etschmann, Shadid and Campoy15). Briefly, 315 healthy women with single pregnancies were recruited before the 20th week of gestation. Of these, four did not fulfil the inclusion criteria and were therefore excluded. Furthermore, forty-one women did not complete the study; the main reasons for dropping out were non-compliance, relocation and bad taste of the supplement (FO: 10·4 %; FO+5-MTHF: 16·9 %; 5-MTHF: 16·6 %; placebo: 10 %; P= 0·47). Compliance was good; 89·5 % of the subjects in the second trimester of pregnancy and 87·4 % in the third missed less than 5 d supplementation. Overall, 270 maternal blood samples could be drawn at the 30th week of pregnancy and 243 at delivery. The available cord blood samples for analyses were 220.
The baseline characteristics of the study participants are shown in Table 1. Maternal clinical or sociodemographic characteristics were similarly distributed in the four intervention groups (P>0·05). There were no significant differences with respect to the course of pregnancy or delivery complications between the groups (P>0·05). The habitual basal dietary intake of energy, fatty acids and nutrients of the participating mothers was similar in the four groups throughout the study (P>0·05) except for the supplementation (Table S1, available online).
Fatty acid patterns in maternal plasma and erythrocyte phospholipids during pregnancy and in umbilical plasma and erythrocyte phospholipids at delivery
The mean values of fatty acids in maternal plasma and erythrocyte PL during pregnancy and in umbilical plasma and erythrocyte PL at delivery are shown in Tables 2–4.
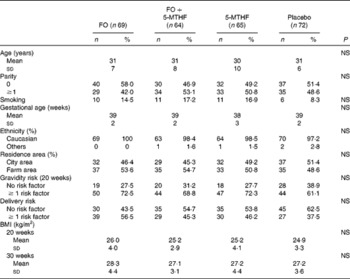
Table 2 Relative fatty acid composition (wt%) of maternal and neonatal plasma phospholipids (Mean values and standard deviations)
FO, fish oil; 5-MTHF, 5-methyltetrahydrofoate; AA, arachidonic acid.
a,b,cMean values within a column with unlike superscript letters were significantly different between the intervention groups (P< 0·05).
A,B,CMean values within a row with unlike superscript letters were significantly different between the pregnancy time points (P< 0·05).
* n 57.
† n 51.
‡ n 56.
§ n 53.
∥ Concentration of 18 : 3n-3 in cord plasma phospholipids was too low to be detected.
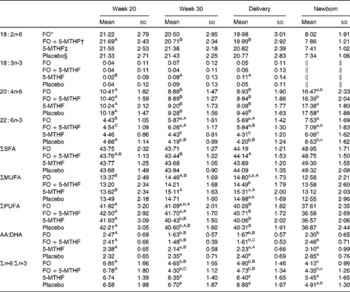
Table 3 Relative fatty acid composition (wt%) of maternal and neonatal erythrocyte phosphatidylcholine (Mean values and standard deviations)
FO, fish oil; 5-MTHF, 5-methyltetrahydrofoate; AA, arachidonic acid.
a,b,c Mean values within a column with unlike superscript letters were significantly different between the intervention groups (P< 0·05).
A,B,C Mean values within a row with unlike superscript letters were significantly different between the pregnancy time points (P< 0·05).
* n 36.
† n 31.
‡ n 31.
§ n 30.
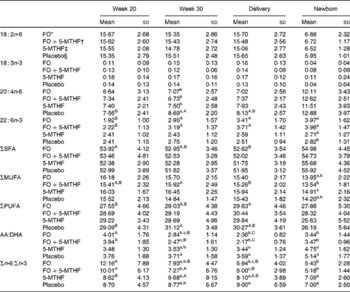
Table 4 Relative fatty acid composition (wt%) of maternal and neonatal erythrocyte phosphatidylethanolamine (Mean values and standard deviations)
FO, fish oil; 5-MTHF, 5-methyltetrahydrofoate; AA, arachidonic acid.
a,b,c Mean values within a column with unlike superscript letters were significantly different between the intervention groups (P< 0·05).
A,B,C Mean values within a row with unlike superscript letters were significantly different between the pregnancy time points (P< 0·05).
* n 46.
† n 42.
‡ n 33.
§ n 39.
DHA in maternal plasma PL and erythrocyte PC significantly increased during the second half of pregnancy in the FO and FO+5-MTHF groups (P< 0·001) compared with the placebo and 5-MTHF groups. DHA in maternal erythrocyte PE significantly increased during the second half of pregnancy in all intervention groups. The increments observed in the FO-supplemented groups (FO: 3·54 (sd 2·63) and FO+5-MTHF: 3·87 (sd 2·46)) were significantly higher than those observed in the not FO-supplemented groups (5-MTHF: 1·44 (sd 1·75) and placebo: 1·15 (sd 2·04)) (P< 0·001). DHA levels significantly increased between the 20th and 30th week of pregnancy in plasma PL and erythrocyte PC but no increment was observed between the 30th week of pregnancy and delivery. In contrast, DHA levels in erythrocyte PE continued to significantly rise between the 30th week of gestation and delivery (Figs. 1 and 2).
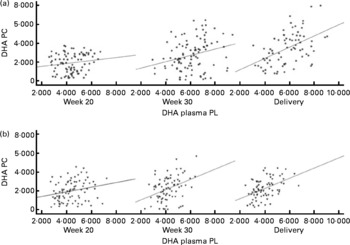
Fig. 1 Scattered data graph of DHA percentage concentrations in plasma and erythrocyte membrane phosphatidylcholine (PC) during the course of pregnancy in (a) the fish oil (FO)-supplemented (FO and FO+5-methyltetrehydrofolate) and (b) not supplemented (placebo and 5-methyltetrahydrofolate) groups. (a) Week 20: R 0·154, P= 0·152; week 30: R 0·275, P= 0·005; delivery: R 0·485, P< 0·001. (b) Week 20: R 0·234, P= 0·031; week 30: R 0·372, P= 0·001; delivery: R 0·467, P< 0·001. PL, phospholipid; DHA PC, DHA levels in erythrocyte membrane PC.
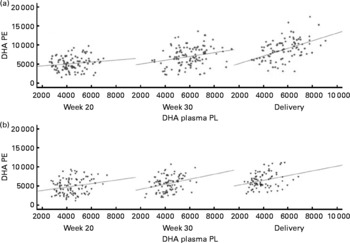
Fig. 2 Scattered data graph of DHA relative concentrations in plasma and erythrocyte membrane phosphatidylethanolamine (PE) during the course of pregnancy in (a) the fish oil (FO)-supplemented (FO and FO+5-methyltetrahydrofolate) and (b) not supplemented (placebo and 5-methyltetrahydrofolate) groups. (a) Week 20: R 0·148, P= 0·133; week 30: R 0·252, P= 0·008; delivery: R 0·473, P< 0·001. (b) Week 20: R 0·219, P= 0·028; week 30: R 0·276, P= 0·008; delivery: R 0·292, P= 0·007. PL, phospholipid; DHA PE, DHA levels in erythrocyte membrane PE.
The degree of these changes was different in the various lipid classes, with higher percentage changes in erythrocyte PE (FO: +67·8 % and FO+5-MTHF: +71·8 %) and PC (FO: +77·6 % and FO+5-MTHF: 67·1 %) than in plasma PL (FO: +28·4 % and FO+5-MTHF: +28·6 %) (P< 0·001).
No differences in maternal or fetal fatty acid levels in both plasma or erythrocyte PL were observed between the groups supplemented with 5-MTHF and the groups that did not receive 5-MTHF supplements (Tables 2–4).
Correlations between fatty acid relative levels (percentage of total fatty acids) in plasma and erythrocyte phospholipids
Significant correlations were observed between DHA levels in maternal plasma and their levels in erythrocytes (PC and PE). Correlation coefficients in the FO-supplemented groups did not differ from those observed in the not FO-supplemented groups (P>0·05; Figs. 1 and 2; Table S2, available online).
DHA levels in cord plasma significantly correlated with their levels in cord erythrocytes. Correlation coefficients for the association were r 0·354 (P< 0·001) and r 0·348 (P< 0·001) for erythrocyte PC and PE, respectively.
Correlations between maternal and neonatal fatty acid relative levels in plasma and erythrocyte phospholipids at delivery
DHA and arachidonic acid (AA) percentage levels in cord blood correlated significantly with fatty acid levels in maternal blood at delivery in both plasma and erythrocyte PL (Fig. 3). Correlation coefficients were higher in erythrocyte PE compared with those observed in plasma or erythrocyte PC (P< 0·05; Table S3, available online).
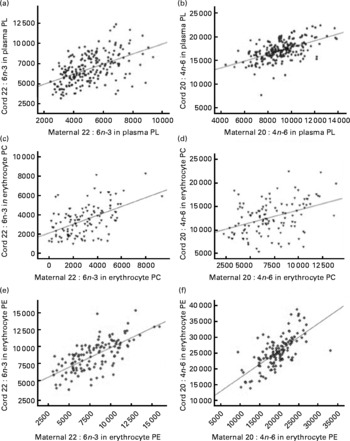
Fig. 3 Linear regression models for the relationship between maternal (a, c, e) DHA and (b, d, f) arachidonic acid levels expressed as a percentage of total fatty acids (wt%) at delivery and their relative levels in cord plasma and erythrocyte membrane (c, d) phosphatidylcholine (PC) and (e, f) phosphatidylethanolamine (PE). (a) R 0·522, P< 0·001; (b) R 0·559, P< 0·001; (c) R 0·516, P< 0·001; (d) R 0·404, P< 0·001; (e) R 0·686, P< 0·001; (f) R 0·690, P< 0·001. PL, phospholipid.
Discussion
It is well known from interventional studies that mothers consuming n-3 LC-PUFA supplements have higher n-3 LC-PUFA levels in both plasma and erythrocytes during pregnancy and at delivery(Reference Dunstan, Mori and Barden3, Reference Sanjurjo, Ruiz-Sanz and Jimeno7–Reference Otto, van Houwelingen and Hornstra9, Reference Krauss-Etschmann, Shadid and Campoy15, Reference Helland, Saugstad and Smith19). Higher levels of n-3 LC-PUFA have also been reported in umbilical cord plasma and erythrocyte PL in supplemented mothers(Reference Krauss-Etschmann, Shadid and Campoy15, Reference Helland, Saugstad and Smith19, Reference Barden, Dunstan and Beilin20). Furthermore, DHA levels in cord blood plasma and erythrocytes have been demonstrated to correlate with maternal blood DHA levels(Reference Dunstan, Mori and Barden3, Reference Smuts, Borod and Peeples6, Reference Matorras, Perteagudo and Sanjurjo21, Reference Vlaardingerbroek and Hornstra22). In agreement with previous interventional studies, FO(Reference Dunstan, Mori and Barden3, Reference Sanjurjo, Ruiz-Sanz and Jimeno7–Reference Otto, van Houwelingen and Hornstra9, Reference Krauss-Etschmann, Shadid and Campoy15, Reference Helland, Saugstad and Smith19) supplementation in the second half of pregnancy significantly increased DHA levels in maternal and umbilical plasma and erythrocyte PL. Supplementation did not significantly modify AA levels in umbilical erythrocyte membrane PL but AA levels in umbilical plasma PL were significantly lower in the FO-supplemented groups. Supplementation with 500 mg/d of DHA in populations with a mean daily DHA intake above the recommended 200 mg/d(Reference Koletzko, Lien and Agostoni1, Reference Franke, Verwied-Jorky and Campoy23) may lower AA in the fetus. The supplementation with a AA–DHA blend has proven to increase DHA levels without decreasing the levels of AA and may be a better way of avoiding the AA fall following n-3 LC-PUFA supplementation(Reference Otto, van Houwelingen and Hornstra9).
Human fatty acid status depends only partially on dietary intake. Genetic and metabolic factors, as well as lifestyle determinants, may affect fatty acid concentrations in human tissues. Furthermore, pregnancy may modify plasma and erythrocyte fatty acid concentrations as a result of the occurring metabolic changes. Several studies have been conducted in order to establish the most confident compartment for the assessment of the LC-PUFA status of individuals. A revised meta-analysis of intervention studies concluded that both plasma and erythrocyte PL appear to be good markers for DHA status in humans, but special consideration should be given to particular population subgroups(Reference Fekete, Marosvolgyi and Jakobik24). Only a few studies have evaluated the influence of n-3 PUFA supplements during the course of pregnancy in both erythrocytes and plasma in the same population(Reference Montgomery, Speake and Cameron8, Reference Otto, van Houwelingen and Hornstra9).
Studies on the kinetics of fatty acid incorporation into plasma PL and erythrocytes after DHA supplementation have shown a dose-dependent rapid increase of DHA in plasma PL with a subsequent steady-state concentration 1 month after the start of supplementation. Erythrocytes follow a similar pattern but it takes 4–6 months to reach equilibrium(Reference Katan, Deslypere and van Birgelen25–Reference Arterburn, Hall and Oken27). In addition, most of the PE in erythrocyte membranes is located in the inner layer, whereas PC is in the outer layer and can therefore interact more easily with circulating plasma lipids. Thus, PC reflects more directly the fatty acid composition of plasma PL, whereas the fatty acid composition of PE appears to be more dependent on a selective incorporation of LC-PUFA into PE. A study on DHA supplementation in healthy adult vegetarians showed strong correlations between erythrocyte PC and plasma PL fatty acid changes, while erythrocyte PE and plasma PL fatty acid changes were less closely related(Reference Geppert, Kraft and Demmelmair28). After the first 10 weeks of supplementation in the present study, DHA levels in maternal plasma and erythrocyte PC may have risen to their steady-state levels and consequently do not continue to increase in the last weeks of pregnancy. DHA in erythrocyte PE may need longer to reach the steady-state concentration and therefore continues to increase until delivery. Given the slow turnover of fatty acids in cell membranes, erythrocyte PL may be a more reliable measure of long-term fatty acid intake, whereas plasma PL appear more sensitive to short-term changes in the intake of LC-PUFA and may be more suitable to monitor the fatty acid status of individuals in intervention studies. Both plasma and erythrocyte fatty acids appear adequate to assess the fatty acid status in pregnant women and their neonates but the type of study is a major consideration when determining which body compartment reflects a better measure of fatty acid status.
It should be taken into account that studies on the kinetics of incorporation of fatty acids in plasma and erythrocyte PL have not been conducted in pregnant women. Most studies conducted in pregnancy have reported a similar evolution pattern of fatty acids in both plasma and erythrocyte and reported the positive correlations between maternal plasma and erythrocyte fatty acid levels and fatty acid changes throughout gestation(Reference Matorras, Perteagudo and Sanjurjo21, Reference Vlaardingerbroek and Hornstra22, Reference Courville, Keplinger and Judge29), which is consistent with the present results. In general, changes observed in erythrocyte membrane PL mirror those occurring in plasma PL but we observed that the degrees of DHA percentage changes were different in the various lipid fractions. Geppert et al. (Reference Geppert, Kraft and Demmelmair28) in their study in healthy adult vegetarians reported that relative changes of DHA 8 weeks after supplementation were greater in plasma and erythrocyte PC than in erythrocyte total lipids and erythrocyte PE, which contrast with the present finding of greater changes in erythrocyte PE and PC compared with plasma PL. Vlaardingerbroek & Hornstra(Reference Vlaardingerbroek and Hornstra22) observed in their study that the amount of most fatty acids continued to increase in erythrocytes after 32 week of pregnancy while the amounts in plasma hardly increased or even decreased, which is consistent with the present findings. These authors have suggested that the increment observed in erythrocyte PL could be explained by the replacement during pregnancy of erythrocyte PL classes with one ester-linked fatty acid moiety by PL classes carrying two ester-linked fatty acids, such as PC and PE(Reference Vaysse, Dureuil and Pilardeau30). As a result, plasma lipids could be more readily adsorbed to the erythrocyte membrane, which could explain the higher percentage increase of DHA in erythrocyte PC and PE. There is evidence suggesting that erythrocytes play an important role in the transport of DHA into the fetus(Reference Ruyle, Connor and Anderson31); the increment of PE and PC in erythrocyte membrane may be an adaptation mechanism during pregnancy to increase the transfer of LC-PUFA. However, further research is needed to elucidate the mechanism of how erythrocytes provide LC-PUFA to the fetus.
We observed positive significant correlations between plasma and erythrocyte DHA levels, which is consistent with previously reported data(Reference Matorras, Perteagudo and Sanjurjo21, Reference Vlaardingerbroek and Hornstra22, Reference Courville, Keplinger and Judge29). Our correlation coefficients were considerably lower than those reported in non-pregnant individuals(Reference Geppert, Kraft and Demmelmair28). Fatty acids bind to TAG in high proportion during pregnancy(Reference Herrera32), and the fact that we did not consider the LC-PUFA fraction bound to TAG in the present study could explain our lower correlation coefficients.
Correlation coefficients between maternal and cord fatty acid relative levels at delivery were significantly higher in erythrocyte PE compared with the correlation coefficients in plasma PL. Plasma levels of lipoproteins in cord serum are low, with HDL being the major lipoprotein and LDL and VLDL present in low and very low concentrations(Reference Legras, Durou and Ruelland33, Reference Nagasaka, Chiba and Kikuta34). Thus, in agreement with previously reported data(Reference Ruyle, Connor and Anderson31), it could be hypothesised that erythrocyte membrane PL are important carriers of LC-PUFA in fetal blood, which could explain the higher correlation coefficients between maternal and fetal fatty acid percentage levels in erythrocyte membrane PL. These results suggest that erythrocyte PL seem to be a more reliable biomarker for the prediction of fetal/neonatal fatty acid status on the basis of maternal fatty acid levels.
Both plasma and erythrocyte fatty acid levels seem to be suitable to assess maternal fatty acid status. More information on the physiological changes taking place in the erythrocyte membrane during gestation, as well as further knowledge about fetal metabolism and the kinetics of fatty acid accretion in fetal tissues, is needed. Nevertheless, fatty acid levels in erythrocyte PL seem to be a more reliable biomarker compared with those in plasma PL to predict the fetal/neonatal fatty acid status on the basis of maternal blood fatty acid levels.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512003716.
Acknowledgements
The authors thank all participating women for their collaboration and all colleagues in the study centres for their support. This study was carried out with partial financial support from the Commission of the European Communities, specific RTD Programme ‘Quality of Life and Management of Living Resources’, within the 5th Framework Programme (contract no. QLK1-CT-1999-00888 NUHEAL (Nutraceuticals for a healthier life)). This paper does not necessarily reflect the views of the Commission and in no way anticipates the future policy in this area. All authors have made substantive contributions to the study, and endorse the data and conclusions. B. V. K. is the recipient of a Freedom to Discover Award of the Bristol Myers Squibb Foundation, New York, NY, USA. The authors' contributions are as follows: M. V. E.-M. wrote the paper; C. C., B. V. K. and T. D. designed and coordinated the research; C. C. supervised the writing; T. D. was responsible for the erythrocyte membrane fatty acid analyses; M. C. R.-T. and A. G. were responsible for the fatty acid analyses in plasma PL; H. D. conducted the research; M. T. M. analysed the data. All authors certify that there is no actual or potential conflict of interest in relation to this article.