The gastrointestinal tract is a primary endocrine organ. More than twenty different regulatory peptide hormones are secreted by the gut. These gut hormones play key roles in appetite regulation and energy homeostasis(Reference Murphy and Bloom1). Ghrelin is so far the only orexigenic hormone. Ghrelin is released from the stomach during fasting and might signal directly to the hypothalamus or through the vagus nerve to stimulate food intake. Ghrelin exists in two distinct isoforms: acetylated and desacetylated ghrelin, both of which are derived from the same precursor preproghrelin. Besides regulating short-term appetite, ghrelin participates in long-term energy homeostasis. For instance, chronic administration of ghrelin in rodents results in prolonged hyperphagia and weight gain(Reference Tschop, Smiley and Heiman2, Reference Wren, Small and Ward3). In vitro, ghrelin appears to mediate fat deposition, with the lipogenic effects in part mediated by the Y1 receptor(Reference Kos, Harte and O'Hare4). Obestatin is a peptide encoded by the ghrelin gene, whose physiological function remains obscure(Reference Gourcerol, Coskun and Craft5–Reference Zhang, Ren and Avsian-Kretchmer8).
Other appetite-regulating peptides from the gut, such as glucagon-like peptide-1 (GLP-1)(Reference Abbott, Monteiro and Small9), peptide tyrosine–tyrosine (PYY)(Reference Acuna-Goycolea and van den Pol10, Reference Batterham, Cowley and Small11) and cholecystokinin (CCK)(Reference Gibbs, Young and Smith12), characteristically relay a sense of ‘fullness’ resulting in postprandial satiation and meal termination. They are released into the circulation after a meal. GLP-1 is a potent incretin – central or peripheral administration strongly stimulates insulin release. It exists in several forms, but the most common circulating form is GLP-17–36. PYY occurs in two forms: PYY1–36 and PYY3–36. Most circulating PYY immunoreactivity is in the amino-terminally truncated form, PYY3–36. The chronic administration of PYY3–36 results in weight loss in obese rodents(Reference Adams, Lei and Jodka13, Reference Chelikani, Haver and Reidelberger14). Transgenic mice overexpressing PYY are protected against diet-induced obesity (DIO) and genetic obesity(Reference Boey, Lin and Enriquez15). PYY3–36 and GLP-1 can directly stimulate anorectic pathways in the hypothalamus and brainstem, and may also act through the vagus nerve. The arcuate nucleus is important in integrating the gut hormone energy homeostasis signals. Neuropeptide Y/agouti-related peptide neurons and pro-opiomelanocortin neurons signal to the paraventricular and other hypothalamic nuclei to increase or decrease appetite, respectively(Reference Murphy and Bloom1).
Levin et al. (Reference Levin and Keesey16, Reference Levin, Triscari and Sullivan17) discovered that when Sprague–Dawley rats are fed a high-fat (HF) diet, some rats develop obesity, while others remain lean. This phenomenon has also been confirmed in our laboratory(Reference Zhao, Wang and Ma18). Since the diet-induced obese (DIO) rat model closely resembles the polygenic nature of human obesity(Reference Buettner, Scholmerich and Bollheimer19, Reference Speakman, Hambly and Mitchell20), it has been extensively used in obesity research(Reference Levin and Keesey16, Reference Levin, Triscari and Sullivan17, Reference Furnes, Zhao and Chen21). During the obesity induction phase, rats have free access to either a HF and high-energy diet or a control (CON) diet (low-fat (LF) and low-energy). After the DIO and diet-resistant (DR) phenotypes are established, the effects of switching from a HF diet back to a LF diet become an interesting topic of study. Levin et al. (Reference Levin, Triscari and Hogan22) found that when switched to a LF diet, the DR rats eat 13 % less, gain 55 % less weight and have 49 % lower food efficiency, whereas the DIO rats eat 4 % less but have comparable weight gain and efficiency with the CON rats. Huang et al. (Reference Huang, Yu and Li23) learned that the replacement of a HF diet with a LF diet is associated with a lowered fasting plasma total PYY concentration in DIO mice, which are changed to a LF diet. Moreover, Aziz et al. (Reference Aziz, Kenney and Goulet24) found that modifying the starch type in the diet can affect some gut hormones. However, to our knowledge, no study has been carried out to systematically examine the effects of changing dietary fat content on gut hormones. Thus, we aimed to examine the long- and short-term effects of changing dietary fat content on some plasma gut hormone concentrations in the DIO and DR rats.
Methods
Animals and diets
All experimental procedures were approved by the Animal Ethics Committee of Harbin Medical University and conducted in compliance with the guidelines for animal use.
Adult male Sprague–Dawley rats (n 144; body weight 170–190 g) were purchased from the Vital River Laboratories (Beijing, China). They were housed individually in wire cages with controlled environmental conditions (temperature 22 ± 1°C, relative humidity 60 %, light cycle from 08.00 to 20.00 hours). The rats were fed standard laboratory chow (Keaoxieli, Beijing, China) for the first week to adapt to a new environment. In the following experimental period, the rats were given either a modified control AIN-93G diet (LF diet, 16·4 kJ/g) or a HF diet (22·0 kJ/g) (Table 1). All micronutrients, proteins and fibre in the HF and LF diets were balanced in terms of energy. The only variation was the relative energy contribution of fats and carbohydrates, with 12 and 60 % from fat. Equal parts of the insoluble fibre (cellulose) and soluble fibre (inulin) were provided to closely resemble the natural ingredients in the diet. The HF and LF diet formulations, which were made from semi-synthetic materials, were in the powder form.
Table 1 Composition of the diets*
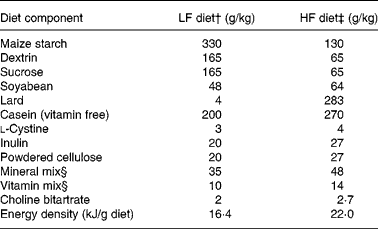
LF, low fat; HF, high fat.
* In the diets, all micronutrients, proteins and fibre were balanced by energy, the only variation was the relative energy contribution of fats and carbohydrates, with 12 and 60 % kJ from fat.
† Containing 11·9 % fat, 67·3 % carbohydrates and 20·8 % protein by energy.
‡ Containing 59·4 % fat, 19·8 % carbohydrates and 20·8 % protein by energy.
§ AIN-93G mineral and vitamin mixes(Reference Reeves, Nielsen and Fahey38).
Experimental protocol
The experiment comprises three phases: HF DIO phase; dietary intervention phase; refeeding phase (Fig. 1).

Fig. 1 Three phases of the study: high-fat (HF) diet-induced obesity phase; dietary intervention phase; fasted-refed phase. LF, low fat; DIO, diet-induced obesity; DR, diet resistant; CON, control; DIOHF, DIO on HF diet; DIOLF, DIO on LF diet; DRHF, DR on HF diet; DRLF, DR on LF diet.
HF DIO phase (weeks 0–6)
After the acclimatisation period, the rats were placed on a HF diet. After 2 weeks, rats with intermediate weight gains (n 24) were switched to a LF diet and designated as controls, while the other rats were continued on a HF diet. At the end of week 6, the rats with higher weight gains were designated as DIO rats (n 48), the rats with lower weight gains were designated as DR rats (n 48) and the remaining rats were excluded from the study.
Dietary intervention phase (long-term effects, weeks 7–10)
After 6 weeks, the DIO and DR rats were further divided in random into two groups each (n 24). One group continued on a HF diet (DIO rats on HF diet (DIOHF) and DR rats on HF diet (DRHF), while the other was switched to a LF diet (DIO rats on LF diet (DIOLF) and DR rats on LF diet (DRLF) for 4 weeks. The CON rats continued on a LF diet.
Refeeding phase (short-term effects, last day of the experiment)
After 10 weeks, each group was randomly divided into three subgroups (n 8): fasted; fasted-refed HF; fasted-refed LF groups. In the fasted group, the rats were deprived of food for 13 h (19.00–08.00 hours) with free access to water. In the fasted-refed group, rats in each subgroup were deprived of food for 12 h (19.00–07.00 hours) and refed the HF/LF diet (200 kJ) for 1 h (07.00–08.00 hours), respectively.
Energy intake and body weight
The 24 h food intake was measured every day. Food was weighed at 09.00 hours, and the remaining food and spillage were collected and weighed at the end of a 24 h period. Energy intake (kJ) was determined by multiplying food weight (g) and energy density (kJ/g) of each diet together. Body weight (g) was recorded weekly.
Preparation of plasma samples
Rats were anaesthetised with an intraperitoneal injection of sodium pentobarbital (40 mg/kg per body weight) at 08.00 hours. For the determination of ghrelin, obestatin, CCK, PYY and GLP-1 concentrations, blood samples were collected from the abdominal aorta and immediately transferred into chilled polypropylene tubes containing EDTA-2Na. These tubes were gently rocked several times immediately after blood collection to get an even mixture and to prevent coagulation. Blood from the tubes was transferred to the centrifuge tubes containing 0·6 trypsin-inhibiting units (60 μl) of aprotinin per ml blood (Applichem GmbH, Darmstadt, Germany) and gently rocked for several times to inhibit the activity of proteinases. Blood samples were centrifuged at 1600 g for 15 min at 4°C. Plasma were collected and stored at − 80°C until the assay.
Assays of gut hormones
Plasma total ghrelin concentrations were measured using an ELISA kit (Linco Research, St Charles, MO, USA). The theoretical minimum detection concentration of this assay was 0·02 ng/ml total ghrelin. The intra- and inter-assay variations reported by the manufacturer were < 5 and < 14 %, respectively. Plasma obestatin, CCK26–33, PYY3–36 and GLP-17–36 concentrations were determined using fluorescent ELISA kits (Phoenix Pharmaceuticals, Belmont, CA, USA). The sensitivities of these assays were 38·9 pg/ml obestatin, 9·5 pg/ml CCK26–33, 10·7 pg/ml PYY3–36 and 20·8 pg/ml GLP-17–36, respectively. The intra-assay variation was 5–10 %, and the inter-assay variation was < 15 %.
Statistical analysis
Data were presented as means with their standard errors. All statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA), with α = 0·05.
Weekly body-weight and energy intake data generated from the HF DIO phase were analysed by a two-way repeated-measures ANOVA (phenotype × time), followed by Tukey's post hoc test. The other data generated from this phase were analysed by a one-way ANOVA, followed by Tukey's post hoc test.
Weekly body-weight and energy intake data generated from the dietary intervention phase were analysed by a three-way repeated-measures ANOVA (phenotype × dietary intervention × time), followed by Tukey's post hoc test. Total cumulative energy intake and white adipose tissue (WAT) data were analysed by a two-way ANOVA (phenotype × dietary intervention), followed by Tukey's post hoc test. The data for plasma ghrelin, obestatin, CCK, PYY and GLP-1 were analysed by a three-way ANOVA (phenotype × dietary intervention × refeeding status), followed by Tukey's post hoc test.
The relationships between fasting plasma ghrelin, obestatin, CCK, PYY, GLP-1 concentrations, dietary intervention and body weight were examined using bivariate correlations. Independent variables potentially influencing body weight were tested by a multiple linear regression analysis.
Results
High-fat diet-induced obesity phase (weeks 0–6)
A two-way repeated-measures ANOVA revealed significant main effects of phenotype (P < 0·0001) and time (P < 0·0001), as well as a significant interaction between phenotype and time (P < 0·0001), on body weight and energy intake. After 2 weeks on a HF diet, a distinct phenotype of body weight was observed (Fig. 2(a)): the DIO rats had a higher body weight gain than the CON and DR rats (P < 0·0001). By the end of week 6, the DIO rats had a higher body weight gain than the DR (307·3 (sem 3·7) v. 233·4 (sem 3·8) g; P < 0·0001) and CON rats (307·3 (sem 3·7) v. 252·1 (sem 4·9) g; P < 0·0001). The DIO rats consumed significantly more food than the DR and CON rats (Fig. 2(b)). The total cumulative energy intake over this 6-week period was higher in the DIO rats than in the DR (16·9 (sem 0·1) v. 15·0 (sem 0·1) MJ; P = 0·001) and CON rats (16·9 (sem 0·1) v. 15·4 (sem 0·2) MJ; P = 0·001). When the diet of the CON rats was switched from HF to LF, their weekly energy intake decreased by 19·0 %. After 2 weeks, the energy intake in the CON rats increased by 14·6 %.

Fig. 2 Body-weight changes (a) and energy intake (b) during the diet-induced obesity phase in rats that were fed a low-fat (LF; control (CON), n 24) or a high-fat (HF) diet (diet-induced obesity (DIO), n 48 and diet-resistant (DR), n 48). Values are means, with standard errors represented by vertical bars. ▾, CON; ●, DIO; ○, DR. DIO was different from DR and CON: *P < 0·05, **P < 0·01 and ***P < 0·001, respectively. DR was different from CON: †P < 0·05, ††P < 0·01 and †††P < 0·001, respectively.
Dietary intervention phase (weeks 7–10)
Body weight, energy intake and white adipose tissue
At the beginning of this phase, half of the DIO and DR rats were switched to a LF diet (DIOLF and DRLF) for 4 more weeks, while the other half continued on a HF diet (DIOHF and DRHF). The analysis by a three-way repeated-measures ANOVA revealed significant effects of phenotype (P < 0·0001) and time (P < 0·0001), as well as interactions between phenotype and time (P < 0·0001) and between intervention and time (P < 0·0001), on body weight. The DIO rats had a significantly higher body weight than the DR and CON rats over the interventional period. In weeks 8 and 9, the body-weight gain of the DIOLF rats was significantly lower than that of the DIOHF rats. However, this difference disappeared when the weekly energy intake was considered as a covariate. The body-weight gain of the DRLF rats was similar to that of the DRHF rats over the interventional period (Fig. 3(a)).

Fig. 3 Body-weight changes (a) and energy intake (b) during the dietary intervention phase in the rats that were fed a low-fat (LF; control (CON, ■), diet-induced obese rats on low-fat diet (DIOLF, ○) and diet-resistant rats on low-fat diet (DRLF, △)) or a high-fat diet (diet-induced obese rats on high-fat diet (DIOHF, ●) and diet-resistant rats on high-fat diet (DRHF, ▾)). Values are means, with standard errors represented by vertical bars, for n 24. DIOHF and DIOLF were different from CON, DRHF and DRLF: **P < 0·01 and ***P < 0·001, respectively. DRHF and DRLF were different from CON: †P < 0·05, ††P < 0·01 and †††P < 0·001, respectively. DIOHF was different from DIOLF, CON, DRHF and DRLF: ‡P < 0·05 and ‡‡‡P < 0·001, respectively. DRLF was different from DIOLF, CON and DRHF: §P < 0·05. CON and DIOLF were different from DRHF and DRLF: ‖P < 0·05.
There were significant effects of time (P < 0·0001), phenotype (P < 0·0001) and intervention (P = 0·001), as well as an interaction between time and intervention (P < 0·0001), on weekly energy intake. The DIO rats had a significantly higher energy intake than the CON and DR rats. The DIOLF and DRLF rats decreased their energy intake by 11 and 9 %, respectively, over the first week on a LF diet. After 2 weeks, their energy intake recovered to the pre-interventional level (Fig. 3(b)).
The effects of phenotype (P = 0·007) and dietary intervention (P = 0·047) on WAT (P < 0·05) were observed. The DIO rats had more WAT than the DR rats (46·3 (sem 2·7) v. 33·7 (sem 2·7) g; P = 0·002). Moreover, switching from a HF diet to a LF diet resulted in lower WAT (43·9 (sem 2·7) v. 36·7 (sem 2·2) g; P = 0·044). There was no interaction between phenotype and dietary intervention on WAT.
Plasma gut hormone concentrations
There were main effects of phenotype (P < 0·0001), dietary intervention (P < 0·0001) and refeeding status (P < 0·0001), as well as an interaction between dietary intervention and refeeding status (P < 0·0001), on plasma ghrelin levels. The DIO rats had significantly lower plasma ghrelin concentrations than the DR (P < 0·0001) and CON (P < 0·0001) rats. Switching from a HF diet to a LF diet resulted in significantly higher plasma ghrelin concentrations than consuming a HF diet (P < 0·0001). In addition, post-LF plasma ghrelin concentrations were significantly lower than fasting concentrations (P < 0·0001) in each group. However, the postprandial plasma ghrelin concentrations were suppressed by a HF diet only in the DRHF and DIOHF rats (P < 0·0001; Fig. 4(a)).

Fig. 4 Plasma ghrelin (a), obestatin (b), cholecystokinin (CCK) (c), peptide tyrosine–tyrosine (PYY) (d) and glucagon-like peptide 1 (GLP-1) (e) concentrations in the control (CON, □), diet-resistant rats on high-fat diet (DRHF, ▨), diet-resistant rats on low-fat diet (DRLF, ![]() ), diet-induced obese rats on high-fat diet (DIOHF, ▧) and diet-induced obese rats on low-fat diet (DIOLF,
), diet-induced obese rats on high-fat diet (DIOHF, ▧) and diet-induced obese rats on low-fat diet (DIOLF, ![]() ) rats that were fasted for 12 h (fasting) and fasted-refed for 1 h with a LF (post-LF) or a HF (post-HF) diet. Values are means (n 6–8), with standard errors represented by vertical bars. * Mean values were significantly different (P < 0·05).
) rats that were fasted for 12 h (fasting) and fasted-refed for 1 h with a LF (post-LF) or a HF (post-HF) diet. Values are means (n 6–8), with standard errors represented by vertical bars. * Mean values were significantly different (P < 0·05).
There was a main effect of refeeding status (P < 0·0001) and an interaction between phenotype and refeeding status (P = 0·0001) on plasma obestatin levels. Post-HF plasma obestatin concentrations significantly increased compared with fasting concentrations in the DRLF, DRHF and CON rats. However, the rats that were refed a LF diet did not have significantly higher plasma obestatin concentrations than the fasted rats (Fig. 4(b)).
There were main effects of intervention (P = 0·013) and refeeding status (P < 0·0001) on plasma CCK levels. Switching from a HF diet to a LF diet resulted in a lower plasma CCK concentration (P = 0·013). In addition, the rats that were refed with both the HF and LF diets had significantly higher plasma CCK concentrations (P < 0·0001) than the fasted rats (Fig. 4(c)).
There were main effects of phenotype (P < 0·0001) and refeeding status (P < 0·0001) on plasma PYY levels. The DIO rats had significantly higher plasma PYY concentrations than the DR (P < 0·0001) and CON (P < 0·0001) rats. Moreover, the rats refed with both the HF and LF diets had significantly higher plasma PYY concentrations than the fasted rats (P < 0·0001; Fig. 4(d)).
There was a main effect of refeeding status (P < 0·0001) on plasma GLP-1 levels. The rats refed with a HF diet had significantly higher plasma GLP-1 concentrations than the fasted rats (P < 0·0001) and the rats refed with a LF diet (P = 0·014; Fig. 4(e)).
Correlations of fasting plasma ghrelin, obestatin, cholecystokinin, peptide tyrosine–tyrosine, glucagon-like peptide-1 concentrations and dietary intervention with body weight
Fasting plasma ghrelin concentrations were negatively correlated with body weight (r − 0·589, P < 0·0001, n 8). Fasting plasma CCK (r 0·408, P = 0·015, n 8) and PYY (r 0·285, P = 0·047, n 8) concentrations were positively correlated with body weight. Multiple regression analysis indicated that only the fasting plasma ghrelin concentration (β = − 0·501, P = 0·020, n 8) was an independent predictor of body weight.
Discussion
The present study is the first to highlight the long- and short-term effects of changing dietary fat content on gut hormones in DIO and DR rats. Switching from a HF diet to a LF diet in DIO and DR rats for 4 weeks can result in less fat mass, a higher fasting and post-HF plasma ghrelin concentration and a lower postprandial plasma CCK concentration. The short-term effects of changing dietary fat content were that post-HF plasma ghrelin concentrations were higher than post-LF concentrations in DRLF and DIOLF rats, and post-HF plasma obestatin concentrations were higher than post-LF concentrations in CON, DRHF and DRLF rats.
Similar to previous observations(Reference Levin and Keesey16, Reference Zhao, Wang and Ma18), the DIO rats showed significantly higher body weight, energy intake and WAT than the DR and CON rats. Replacing a HF diet with a LF diet significantly reduced energy intake in the DIO, DR and CON rats. However, their energy intake recovered to previous levels after 2 weeks. The DIOLF rats demonstrated a lower body weight than the DIOHF rats during the following 2 weeks after switching from a HF diet to a LF diet. However, this difference disappeared when the analysis was performed with weekly energy intake as a covariate. At the end of this experiment, switching from a HF diet to a LF diet reduced WAT, but not body weight, in the DIO and DR rats. These findings confirmed that diet and genetic background interacted to establish high- (DIO) and low (DR)-body-weight set points, which were then defended against subsequent changes in diet composition and/or energy availability(Reference Levin, Triscari and Hogan22). Because of the short-term effects of gut hormones on appetite and the long-term effects on energy homeostasis, gut hormones are speculated to play key roles in the above-mentioned changes in energy intake, body weight and WAT.
In the present study, fasting and post-HF plasma ghrelin concentrations increased after switching from a HF diet to a LF diet in the DIO and DR rats. In line with the previous studies(Reference Cummings, Weigle and Frayo25, Reference Cummings, Purnell and Frayo26), the present findings showed that postprandial plasma ghrelin concentrations were suppressed by a LF diet in each group. However, postprandial plasma ghrelin concentrations were suppressed by a HF diet only in the DRHF and DIOHF rats. The CON, DRLF and DIOLF rats, which adapted to a LF diet, had higher postprandial plasma ghrelin concentrations than the DRHF and DIOHF rats, which, in turn, adapted to a HF diet, after being refed an isoenergetic HF diet. These results support previous reports showing that carbohydrate suppresses ghrelin more potently than fat and protein(Reference Erdmann, Topsch and Lippl27, Reference Monteleone, Bencivenga and Longobardi28). Ghrelin is a physiological meal initiator(Reference Cummings, Purnell and Frayo26). The high postprandial plasma ghrelin concentration cannot effectively induce satiety. Consequently, the CON, DRLF and DIOLF rats, whose postprandial plasma ghrelin has not been suppressed by a HF diet, will ingest food more than their physiological energy need when they were refed the HF diet. This can partly explain why rats tended to eat more on a HF diet than a LF diet and why switching from a HF diet to a LF diet reduced the total cumulative energy intake in both the DIO and DR rats.
Furthermore, fasting and post-HF plasma ghrelin concentrations increased after switching from a HF diet to a LF diet in the DIO and DR rats. Therefore, plasma ghrelin concentrations may be affected by body weight and diet composition and may in turn regulate energy intake, body weight and WAT. It has been shown that ghrelin is expressed in human abdominal subcutaneous adipocytes; moreover, ghrelin isoforms appear to mediate fat deposition with the lipogenic effects(Reference Kos, Harte and O'Hare4). These data support our finding that plasma ghrelin concentration was associated with WAT.
Zhang et al. (Reference Zhang, Ren and Avsian-Kretchmer8) reported that obestatin reduces food intake, antagonising the effects of ghrelin. However, some studies have demonstrated that obestatin has no effects on ghrelin-induced hunger or gastric transit(Reference Gourcerol, Coskun and Craft5–Reference Seoane, Al-Massadi and Pazos7). Furthermore, it remains obscure whether the postprandial plasma obestatin level changes in response to a meal. One study has found that plasma obestatin levels do not change in response to a meal in humans(Reference Huda, Durham and Wong29). In contrast, another study has found that plasma obestatin levels increase significantly in fasting rats compared with rats fed an ad libitum diet(Reference Guo, Ren and Zheng30). In the present study, post-LF plasma obestatin concentrations did not change significantly compared with fasting plasma obestatin concentrations in the rats of each group. However, post-HF plasma obestatin concentrations significantly increased compared with fasting concentrations in the DRLF and DRHF rats, but not in the DIOLF and DIOHF rats. Therefore, it seems that the dietary fat content and phenotype of rats have interaction effects on the postprandial plasma obestatin concentration. Obestatin is speculated to have a potential role in the regulation of energy homeostasis.
The effects of CCK on the gastrointestinal system include inhibiting gastric emptying, food intake, and stimulating gall bladder contraction and pancreatic enzyme secretion(Reference Dockray31–Reference Moran and McHugh33). In line with the previous study(Reference Liddle, Goldfine and Rosen32), plasma CCK concentrations increased one- to twofold after the rats were refed with a HF diet or a LF diet in the present study. The DIO rats had a higher fasting plasma CCK concentration than the DR and CON rats. These results support the previous notion that CCK played a key role in appetite regulation and long-term energy homeostasis. However, no conclusive results were found to prove that an acute switching diet affected the plasma CCK concentration. Although the plasma CCK concentration was related to the rat phenotype, it was not safe to conclude that switching from a HF diet to a LF diet had long-term effects on the plasma CCK concentration.
PYY can delay gastric emptying and reduce gastric secretion(Reference Taylor34). In the present study, lowering dietary fat content by switching from a HF diet to a LF diet produced no effects on the fasting and postprandial plasma PYY concentrations. An acute changing dietary fat content seemingly had no effect on the plasma PYY concentration. These results were different from the report which shows that lowering dietary fat content can lower plasma total PYY in the DIO rats(Reference Huang, Yu and Li23). Differences in rodents, experimental design and the detected isoform of PYY may be the reasons for this divergence.
GLP-1 is a potent incretin, which suppresses meal-induced gastric acid and pancreatic juice secretion and delays gastric emptying(Reference Nauck, Niedereichholz and Ettler35, Reference Wettergren, Schjoldager and Mortensen36). It is believed to act as a satiety signal and possibly to play a key role in long-term energy homeostasis. In the present study, post-HF plasma GLP-1 concentrations increased significantly in the DRHF and DRLF rats but not in the CON, DIOHF and DIOLF rats. It is considered that GLP-1 has inhibitory effects on food intake(Reference Flint, Raben and Ersboll37). Therefore, the sensitive response of GLP-1 to a HF diet in the DR rats may explain why they ingested less energy and had lower body weights and WAT than the DIO rats, to a certain degree. However, the positive long- and short-term effects of changing dietary fat content on the plasma GLP-1 concentrations were not observed in the present study.
Several limitations of the present study should be explained. First, we only detected postprandial plasma gut hormone concentrations at one time point. In the present study, the primary aims were to research the long- and short-term effects of changing dietary fat content on gut hormones. The fasting, post-HF and post-LF plasma gut hormone concentrations can reflect these effects to some extent. In future studies, the time profile of gut hormone secretion will be obtained to determine the effects of changing dietary fat content on gut hormones in detail. Second, the causality between energy intake, body weight, WAT changes and plasma gut hormone concentrations cannot be confirmed in the present study. The agonist and antagonist of gut hormones may be helpful to clarify this problem. Third, although changing dietary fat content produced many effects on gut hormones, simply attributing these effects to dietary fat content per se is inappropriate. Energy intake, palatability of foods, stress, sex and activity, in addition to dietary composition, can affect gut hormones. Future studies should focus on the mechanisms underlying these effects. Fourth, only the main plasma isoform, but not all isoforms, of some gut hormones was detected. Fifth, no data on energy expenditure, activity level and resting energy levels, which may explain the different ‘phenotype’, were observed. However, some published data(Reference Levin and Keesey16, Reference Levin, Triscari and Sullivan17, Reference Levin, Triscari and Hogan22) can be referred to, which can help us to grasp the characteristics of HF DIO rats.
In conclusion, replacing a HF diet with a LF diet for 4 weeks resulted in lower WAT and regulated the plasma ghrelin and CCK concentrations in DIO and DR rats. Acute changing dietary fat content had effects on plasma ghrelin and obestatin concentrations. Moreover, it has been shown that gut hormones play key roles in energy homeostasis. Thus, we demonstrate that changing dietary fat content appears to play long- and short-term roles in the gut hormone profile, which may consequently influence energy intake and fat mass.
Acknowledgements
The present study was funded by the Natural Science Foundation of China 30872108. The authors' responsibilities were as follows: J. L. and S. W. designed the research; J. L., N. Z., Z. L., R. L. and C. L. conducted the research; J. L. analysed the data; J. L. wrote the manuscript; J. L. and S. W. had primary responsibility for the final content. All authors read and approved the final manuscript. There are no conflicts of interest.







