Fruits and vegetables are excellent sources of fibres, vitamins and minerals, but they also contain components like polyphenols, terpenes and alkaloids that may provide substantial health benefits beyond basic nutrition(Reference Aggarwal and Shishodia1).
The Allium genus includes approximately 500 species. Commonly used Allium vegetables include onion, garlic, leeks, chives and scallions, which are used all over the world(Reference Sengupta, Ghosh and Bhattacharjee2). It has been shown that Allium species may help to prevent tumour promotion, CVD and ageing; all processes that are associated with free radicals(Reference Stajner, Miliç and Canadanoviç-Brunet3). Onions are regularly consumed forming part of the basic diet of many subjects. They have been recognised as an important source of valuable phytonutrients, such as flavonoids, fructo-oligosaccharides (FOS), thiosulphinates and other sulphur compounds(Reference Slimestad, Fossen and Vågen4). These compounds have been implicated in providing health-promoting as well as adverse attributes to onions. Other interesting biological onion properties reported are potential antioxidant, anticarcinogenic, antimutagenic, anti-asthmatic, immunomodulatory, antimicrobial and prebiotic characteristics(Reference Griffiths, Trueman and Crowther5).
The constant increase in onion consumption and production in many countries has triggered a worldwide disposal of onion wastes in large amounts. Onion wastes could be processed and stabilised in order to obtain useful onion by-products for the food industry. Several onion by-products were characterised in a previous study as potential functional food ingredients with antioxidant and antibrowning properties(Reference Roldán, Sánchez-Moreno and de Ancos6). An onion powder could be easily added into many foods to improve these technological properties, while also adding prebiotic or other health-related effects(Reference Corzo-Martínez, Corzo and Villamiel7).
Onion flavonoids, particularly the flavonol, quercetin and its glycosides, have been the target of a wide range of in vitro and in vivo investigations, including actions on redox homeostasis(Reference Price and Rhodes8–Reference Nemeth and Piskula12). Organosulphur compounds (OSC) are also becoming target of many investigations due to their potential chemopreventive and antioxidant effects, but their toxic properties in animals and birds are also a cause of concern and limit the usefulness of onion wastes as animal feeds(Reference Bianchini and Vainio13–Reference Stan, Kar and Stoner15).
Few, if any, in vivo studies have been done focusing on the fibre fractions in onions such as FOS. Onions have characteristic high contents of certain dietary fibres, particularly fructans and FOS(Reference Jaime, Martín-Cabrejas and Mollá16–Reference Shiomi, Benkeblia and Onodera19), but their chain length distribution differs from most other sources such as chicory. Inulin-type fructans have a potential role in colorectal cancer prevention, associated with their ‘bifidogenic’ prebiotic effect in animal models(Reference Pool-Zobel20). The use of FOS as food ingredients has triggered much research on their possible health effects. Functionally, they are used as non-digestible dietary fibre and technologically they are used for their texturing properties in many foodstuffs(Reference Benkeblia and Shiomi21). Recently, other interesting uses of FOS have been described, including their use as sweeteners for diabetics(Reference Mabel, Sangeetha and Platel22).
To the present knowledge, no studies have investigated the potential antioxidant and prebiotic in vivo effect of onion or onion by-products as FOS sources. Effects of onion components on gut health parameters including changes in pH, transit time, bacterial activities and SCFA production need to be investigated. Previous studies with onions have provided evidence for some adverse effects on Hb biosynthesis and anaemia due to the formation of Heinz bodies in birds and some animal species, including rodents. The effects seem to be caused by some of the sulphur compounds, but details of the mechanism are lacking. As a consequence, haeme and redox homeostasis might be affected, and we therefore determined hepatic gene expression of the rate-limiting gene in haeme biosynthesis along with expression of several antioxidant enzymes.
We present here a rat model study aimed to evaluate the potential effect of an onion by-product and two derived onion fractions, a soluble onion extract rich in FOS and polyphenols and an onion residue fraction, on selected effects related to onion-containing compounds, including anaemia, antioxidant defence, phase 2 enzyme induction, gut health and related gene expression.
Materials and methods
Chemicals
All chemical reagents used are analytical grade from Fluka (Steinheim, Germany), Merck (Darmstadt, Germany) and Sigma-Aldrich (Steinheim, Germany). Ethanol (96 %) was purchased from De Danske Spritfabrikker (Aalborg, Denmark). Water is MilliQ (Millipore, Bedford, MA, USA) with >18 MΩ resistivity.
Dietary substances
Onion powder was freeze-dried from an onion paste(Reference Roldán, Sánchez-Moreno and de Ancos6) at Instituto del Frío-CSIC in Madrid. The onion paste was a ‘Recas’ cultivar onion by-product that was pasteurised before shipment. It was kept at 4°C until the preparation of animal diets. In addition, two onion fractions were produced from the onion powder; an onion extract rich in FOS (water/ethanol soluble) and an onion residue (dry residue). They were produced at the National Food Institute, Technical University of Denmark as described later.
Fructan and fructo-oligosaccharides extraction
Fructans and FOS extraction from the onion powder was carried out following the modified Shiomi method with minor modifications described by Jaime et al. (Reference Jaime, Martínez and Martín-Cabrejas23). The total amount of onion powder used was 1·5 kg. The yield of this powder was 60 % onion extract rich in FOS and percentage of onion residue. The procedure used was the following: portions of 200 g freeze-dried, finely milled material was homogenised in 1 litre of 70 % ethanol and immediately heated to the boiling point for 10 min. Subsequently, the mixture obtained was centrifuged at 2930 g for 15 min at room temperature. The supernatant was decanted and the pellet was extracted one more time with 40 ml of 70 % boiling ethanol and centrifuged again after cooling. Supernatants were pooled and vacuum evaporated at 30–33°C to dryness obtaining an ethanolic extract. Pellets were combined as a residue fraction and lyophilised. The residue fraction contained 30 % by weight of the starting material.
Analysis for sugars, starch, fructo-oligosaccharides and quercetin in the onion fractions
Samples were extracted at 80°C with 80 % (v/v) ethanol, 20 % (v/v) ethanol and finally water. The combined extracts were lyophilised and redissolved in water. Soluble sugars were determined by standard methods(Reference Beutler and Bergmeyer24–Reference Outlaw, Tarczynski and Bergmeyer26). Starch was degraded to glucose units as previously described(Reference Nielsen, Skjærbæk and Karlsen27) and fructans were determined after fructanase treatment according to the protocol of the manufacturer (Megazyme Intl., Bray, Ireland). All assays were performed in microplates using a Spectra-Max 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA). High-performance anion exchange chromatographic analysis of fructo-oligosaccharide size distribution was performed as described for glucans(Reference Blennow, Bay-Smidt and Olsen28). For analysis of quercetin, each fraction was extracted twice with 96 % ethanol, 70 % ethanol, methanol and water, successively. The extracts were combined and concentrated by evaporation, added with genistein as an internal standard and analysed on a 2·1 mm × 10 cm C18 BEH column (1·7 μm particle size), using a UPLC-TQD system (Waters, Milford, MA, USA) operated in the multiple reaction mode. A gradient from 10 to 100 % acetonitrile–methanol (1:1) with 0·1 % formic acid with 0·7 ml/min flow rate was used, and quantification multiple reaction mode transitions for quercetin and genistein were 301>151 and 269>133, respectively. Analysis of each fraction was performed with and without preceding hydrolysis of glucosides in 1·2 m HCl at 90°C for 2 h.
Animals
Thirty-two male Fisher 344 rats were obtained from Charles River (Sulzfeld, Germany). The animals were housed 2 × 2 in Macrolon cages with stainless steel wire lids. During the study the animals were maintained on a 12 h light and 12 h dark cycle at an average temperature and relative humidity of 22 ± 1°C and 55 ± 5 %, respectively, and air was changed 8–10 times/h. Diets and tap water were provided ad libitum. Animals were divided into four groups of eight rats with equal mean body weights. After 12 d of adaptation to the control diet, the rats were fed either (1) control diet; (2) control diet added 10 % of onion by-product powder; (3) control diet added 7 % of onion extract or (4) control diet added 3 % onion residue for a period of 4 weeks until euthanisation (Table 1). Every diet was based on a purified rodent diet produced at the National Food Institute, Technical University of Denmark, according to Table 1. Animal experiments were carried out under the supervision of the Danish National Agency for the Protection of Experimental Animals. All animal study procedures have been approved by the Institutional Committee for Animal Experimentation, and the Institute has been approved for this type of experiment with rodents by the Danish Ministry of Justice.
Table 1 Composition of animal diets
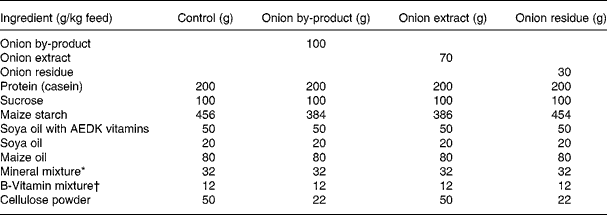
* Containing in mg/kg diet: 3000 Ca; 1900 P; 3600 K; 300 S; 2500 Na; 1500 Cl; 600 Mg; 34 Fe; 30 Zn; 10 Mn; 0·20 iodine; 0·15 Mo; 0·15 Se; 2·5 Si; 1·0 Cr; 1·0 F; 0·5 Ni; 0·5 B; 0·1 Li; 0·1 V; 0·07 Co.
† Composition in mg/kg: 5000 (IU) vitamin A; 1000 (IU) vitamin D3; 50 (IU) vitamin E; 5 thiamine; 6 riboflavin; 8 pyridoxol; 2 folic acid; 0·3 d-biotin; 0·03 vitamin B12; 20 pantothenate; 2600 cholinhydrogentartrat; 400 inositol; 40 nicotinic acid; 1 phylloquinine; 40 p-aminobenzoic acid; 1000 methionine; 2000 l-cystine.
Transit time measurement
One week before sacrifice, the rats were dosed with 5 % of carmine solution. Each animal was dosed with 0·7 ml/100 g body weight. At dosing, the time, animal number and animal weight were recorded. One piece of faeces was collected from each cage once before dosing. After dosing, faeces were examined every hour for the appearance of red colour. At the time red faeces were observed, time was recorded and faeces collected in a tube. Afterwards, 30 mg of the red faeces were suspended in 3 ml 0·1 m NaOH, centrifuged at 2000 g for 30 min, and absorbance was read at 450 and 550 nm.
Sacrifice and sampling
After 4 weeks on the experimental diets, the animals were fasted overnight. The next day, after recording the body weight, the rats were anaesthetised in CO2/O2 and sacrificed by decapitation. Immediately after the decapitation, samples of blood were collected as detailed later. The liver was removed, weighed and grinded in liquid N2 to a fine powder. Three portions of 30 mg each were stored separately for later analysis of antioxidant enzyme activities, gene expression and comet assay. The caecum was washed in ice-cold saline and weighed. The pH in the caecum content near the colon outlet was determined using a microelectrode (Knick, Portamess 751 calimatic pH meter, Berlin, Germany) equipped with a Hamilton biotrode (Reno, NV, USA). Caecum was opened and approximately 0·5 g of the content from the same area was sampled and stored at − 80°C until analysis for β-glucosidase (BGL) and β-glucuronidase (GUS) activity. Of the remaining content, one part (approximately 0·1 g) was suspended in nine parts of alkaline buffer (0·1 m Tris, pH 9·6, and 5 mg/ml malonic acid), centrifuged (14 000 g, 10 min, 4°C) and filtrated using a sterile 0·2 μm filter. Samples were kept at − 80°C until analysis for SCFA. The caecum was rinsed in 0·9 % NaCl and weighed again empty. After the measurement of pH, samples of caecal contents were taken and treated as described later. The caecum was weighed again empty. One millilitre of blood was collected into a PAXgene blood RNA tube for purification of RNA from the leucocytes (BD Denmark A/S, Brøndby, Denmark). The rest of the blood was collected in vacutainer tubes with heparin as anticoagulant. After 10 min of incubation on ice, samples were centrifuged at 1500 g for 10 min at 4°C. Plasma was removed for later analysis. The leucocytes were carefully aspirated into 10 % dimethyl sulphoxide in Histopaque-1077 (1:2) for comet analysis. The erythrocyte fraction was haemolysed by adding an equal volume of ice-cold water. All collected fractions were immediately frozen at − 80°C.
Hb and antioxidant enzymes
On the day of analysis, the 50 % haemolysates were thawed slowly on ice and diluted 2·5 × in water and sonicated 10 s on ice. For the analysis of Hb, glutathione reductase (GR; EC1.8.1.7) and catalase (EC1.11.1.6), the samples were further diluted to 40 × in 100 mm KH2PO4 buffer (pH 7·4) containing 1 mm dithiothreitol and 1 mm EDTA. Glutathione peroxidase (GPx1; EC1.11.1.9) was analysed in 400 × diluted samples. The 30 mg liver powder was homogenised in 1 ml PBS pH 7·4 for 20 s, and centrifuged at 10 000 g for 20 min at 4°C. The supernatant was used for measuring GR and GPx1 activities, together with total protein in 200 × diluted samples.
The enzyme activities of GR, GPx1 and catalase, including Hb and total protein, were determined spectrophotometrically on an Automated Roche/Hitachi 912 Analyzer (Roche Diagnostic A/S, Hvidovre, Denmark) at 37°C. The activity of GR was measured by following the consumption of NADPH at 340 nm by the method of Goldberg & Spooner(Reference Goldberg, Spooner and Bergmayer29). The GPx1 activity was determined by the coupled enzyme method described by Paglia & Valentine(Reference Paglia and Valentine30). The peroxidative activity of catalase was measured by the reaction of formaldehyde (HCHO) as described earlier elsewhere(Reference Johansson and Borg31).
RNA isolation and quantitative real-time PCR
Total RNA was isolated from 30 mg liver powder using Qiagen RNeasy Mini kit according to the protocol described by the manufacturer (Qiagen, Hilden, Germany). Reverse transcriptase reactions were performed using Random Hexamer and SuperScript II Reverse Transcriptase kit according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). Relative mRNA expression was quantified by Real-time PCR on an ABI 7900HT FAST System using the comparative ΔCt method according to ABI manual (TaqMan Gene Expression Master Mix Protocol, Applied Biosystems, Foster City, CA, USA). PCR amplification for each gene target was performed in triplicate with cDNA samples equivalent to 3 ng RNA. The eukaryotic 18S rRNA was used as an internal normalisation standard and data were expressed as fold difference in gene expression relative to a calibrator. Control group samples were pooled and used as a calibrant. TaqMan Gene Expression Assays used were the following: eukaryotic 18S rRNA endogenous control (catalogue number 4352930E); rat Alas1 (catalogue number Rn00675323_g1); rat Cat (catalogue number Rn00680386_m1); rat Gclc (catalogue number Rn00689048_m1); rat Gpx1 (catalogue number Rn00577994_g1); rat Gr (catalogue number Rn01482160_m1); rat Nqo1 (catalogue number Rn00566528_m1).
Comet assay
The single-cell gel electrophoresis (Comet assay) was performed according to the recommendations of Tice et al. (Reference Tice, Agurell and Anderson32), with some minor modifications. Observations were made at magnification of 400 × with a fluorescent microscope (Leica microsystems A/S, Herlev, Denmark) coupled via a CCD camera to an imagine analysis system (Kinetic Imaging 5.0, Bromborough, UK). The data were based on 100 randomly selected cells per sample, fifty cells per each of the two replicate slides. Positive and negative controls were included for each assay. The Caco-2 colon cancer cell line was used as negative control, and for positive control Caco-2 cells were exposed to 4 % of ethyl methanesulphonate in water. DNA damage was measured with the parameters of tail length, olive tail moment, tail extent moment and percentage tail DNA.
β-Glucosidase and β-glucuronidase enzymes
Samples of caecal content (0·2 g) were homogenised in 1 ml PBS, 0·1 % NaN3 pH 7·4, centrifuged at 10 000 g for 20 min at 4°C. The supernatant was used to determine the activity of bacterial BGL and GUS on an Automated Roche/Hitachi 912 Analyzer (Roche Diagnostic GmbH, Mannheim, Germany) at 37°C.
BGL (EC3.2.1.21) was assayed by determining the rate of hydrolysis of the substrate p-nitrophenyl-β-d-glucopyranoside. The amount of p-nitrophenol released was measured at 415 nm. p-Nitrophenol was used as standard. GUS (EC3.2.1.31) was measured by determining the rate of release of phenolphthalein from phenolphthalein-β-d-glucuronide at 540 nm with phenolphthalein as standard. The specific activity is defined as U/g caecal content.
SCFA in caecal content
Propionic and butyric acids in caecal contents were analysed using capillary electrophoresis with indirect UV detection as described previously(Reference Hansen, Baunsgaard and Autrup33).
Statistical analysis
The data were analysed for normal distribution using the Shapiro–Wilks' W test and for homogeneity of variance using Levene's test (P>0·05). Some data had to be log-transformed in order to meet these criteria. The normally distributed and variance homogenous data were analysed by ANOVA. If significant differences were found between groups, further comparisons were done using least-square means. Other data were analysed using the non-parametric Wilcoxon rank-sums test. We used the Statistical Analysis Systems statistical package v. 9.1 (SAS Institute, Cary, NC, USA) and consider a P-value below 0·05 significant.
Results
Onion powder and extracts
The contents of sugars, fructans and quercetin were determined in the onion powder and its fractions in order to determine the exposures in the different rat groups. Table 2 shows the measured contents in each of the fractions. Recovery of sugars after extraction was 96·3 % and recovery of quercetin was 105·6 %, whereas the apparent recovery of fructans was 156 %, indicating that extraction of fructans from the solid onion by-product was more efficient in the preparatory procedure than in the subsequent procedure used for quantitative analysis. A semiquantitative size distribution analysis of the fructans in the extract indicated that more than 90 % had ten fructose residues or less and more than 60 % had five residues or less. Very small amounts of longer chain fructans were present, very unlike the pattern seen for a reference chicory extract (data not shown). The quercetin was analysed before and after hydrolysis of glycosides, and 25–30 % of the quercetin was present as the aglycone in each fraction (data not shown). Starch was not found in any of the samples (data not shown). The results of these analyses show that as a percentage of the total recovered materials, an amount corresponding to 85 % of the sugars, 88 % of the fructans and 91 % of the flavonoid in the feed containing onion by-product was found in the feed with extract and the remaining 9–15 % in the feed with residue.
Table 2 Contents (mg/g) of sugars, fructans, quercetin and total DM in the onion by-product and its fractions
(Mean values and standard deviations)

* Estimated on the basis of measured water content in this fraction.
Hb and antioxidant enzymes
The concentration of Hb expressed as g/l of erythrocytes is shown in Fig. 1. There was a significant decrease (P < 0·05) in the Hb concentration in the rats fed with the three onion products compared with the rats in the control group. Antioxidant enzyme activities were measured in erythrocytes and in liver. There was a significant increase (P < 0·05) in GR and GPx1 activities in erythrocytes of rats fed with the onion extract rich in FOS. In contrast, hepatic GPx1 activity was significantly decreased (P < 0·01) in the onion extract fed rats, but not in the other onion groups compared with the control group. Hepatic GR was not affected by any of the three onion products (Table 3).

Fig. 1 Hb concentration of rats fed with an onion by-product and two derived onion fractions. Values are means of eight measurements performed in each rat group, with standard deviation depicted by vertical bars. * Significant difference between the onion groups and the control group at P < 0·05.
Table 3 Effect of an onion by-product and two derived onion fractions on rat erythrocytes and hepatic antioxidant enzyme activities
(Mean values and standard deviations, n 8)

GR, glutathione reductase; GPx1, glutathione peroxidase; CAT, catalase.
Mean values were significantly different from those of the control group: *P < 0·05, **P < 0·01.
Gene expression of phase 2 enzymes and haeme synthesis
The relative expression quantification method was used for gene expression quantification. The eukaryotic 18S rRNA was used as endogenous reference and data were expressed as fold difference in gene expression relative to a calibrant. Control group samples were pooled and used as calibrant. Rat β-actin was also used as endogenous reference, but gave the same results as obtained with eukaryotic 18S rRNA (not shown).
Hepatic gene expression revealed that Gr, Gpx1 and Cat were not affected in onion fed rats. The gene expression of the phase 2 enzyme Gclc involved in glutathione (GSH) synthesis was significantly upregulated by a factor of more than 2, but only in the rats given the onion residue. The expression of Nqo1, which is also a phase 2 enzyme, was numerically increased to the same extent in this fraction, but the increase was not significant (P = 0·14). We also explored whether hepatic haeme biosynthesis was affected by measuring the gene expression of Alas1, the rate-limiting step in porphyrin biosynthesis. Feeding onion by-product powder or any of the two onion fractions had no significant effect on expression of this gene (Table 4).
Table 4 Effect of an onion by-product and two derived fractions on rat hepatic gene expression of antioxidant enzymes†
(Mean values and standard deviations, n 5)
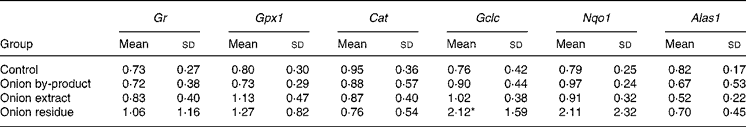
Gr, glutathione reductase; Gpx1, glutathione peroxidase; Cat catalase. Phase 2 enzymes: Gclc, γ-glutamate cysteine ligase catalytic subunit; Nqo1, NAD(P)H:quinone oxidoreductase; Alas1, 5-aminolevulinate synthase.
Mean value was significantly different from those of the control group: *P < 0·05.
† Gene expression of target genes is related to the endogenous reference 18S rRNA and to a calibrant (relative expression quantification).
DNA damage
Liver and leucocytes samples were used to measure DNA damage by the comet assay. At least 100 scores per sample were analysed, two samples from each animal were used. Internal positive and negative controls were included in each assay performed. Their values were within the laboratory historical control range. There were no significant differences (P>0·05) in any of the comet parameters analysed between the control and the three onion groups in liver and leucocyte samples (data not shown).
Caecum weight, pH and transit time
Data on animal caecal weights, caecal pH and transit time values are reported in Table 5. The caecal weight of rats fed with onion by-product was not significantly higher (P = 0·12) compared to the control group and the two onion fractions groups. Caecal pH was significantly decreased by the onion by-product and both fractions (P < 0·01). No significant difference (P>0·05) was found when gastrointestinal transit time was measured in the three onion groups compared to the control group.
Table 5 Caecal weight, caecal pH and transit time in rats fed with an onion by-product and two derived onion fractions
(Mean values and standard deviations, n 8)
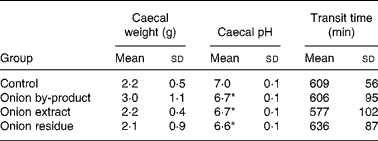
Mean value was significantly different from those of the control group: *P < 0·01.
Bacterial activities
BGL and GUS activities were measured in the caecal contents (Fig. 2). Both activities were significantly increased (P < 0·05) in the three onion groups compared with the control group.

Fig. 2 Effect of an onion by-product and two derived onion fractions on bacterial β-glucosidase (![]() , BGL) and β-glucuronidase (
, BGL) and β-glucuronidase (![]() , GUS) activities in caecal content. Values are means of eight measurements performed in each rat group, with standard deviation depicted by vertical bars. * Significant difference between the onion groups and the control group at P < 0·05.
, GUS) activities in caecal content. Values are means of eight measurements performed in each rat group, with standard deviation depicted by vertical bars. * Significant difference between the onion groups and the control group at P < 0·05.
SCFA
There was a significant increase (P < 0·05) in the formation of caecal propionate and butyrate in all onion fed rats (Fig. 3). The effect was significantly stronger in the onion residue fed group compared with the onion by-product and the onion extract fed groups.

Fig. 3 Effect of an onion by-product and two derived onion fractions on SCFA content: propionic (![]() ) and butyric (
) and butyric (![]() ) acids in caecum. Values are means of eight measurements performed in each rat group, with standard deviation depicted by vertical bars. * Significant difference between the onion groups and the control group at P < 0·05.
) acids in caecum. Values are means of eight measurements performed in each rat group, with standard deviation depicted by vertical bars. * Significant difference between the onion groups and the control group at P < 0·05.
Discussion
In the present study, we have investigated the biological responses of healthy rats fed with an onion by-product and two derived fractions. The onion powder was obtained from a pasteurised ‘Recas’ paste, which was chosen among a battery of stabilised onion by-products (juices, bagasses and pastes) from different cultivars. From a technological and nutritional point of view, stabilised onion by-products from the ‘Recas’ cultivar showed good characteristics to be developed as antioxidant food ingredients. Pasteurisation applied as stabilisation treatment and paste as a form of onion by-product kept the bioactive and technological characteristics of fresh onion. The by-product chosen in the previous study showed several advantages: a remarkable antioxidant activity; a high content of polyphenols; an excellent antibrowning effect(Reference Roldán, Sánchez-Moreno and de Ancos6).
We extracted the soluble fibre from this onion by-product powder in order to elucidate whether fructans and FOS are partly responsible for the potential health-promoting effects of onion. Our analyses show that a very large part of the water or ethanol soluble compounds, including sugars, FOS and flavonoids were successfully extracted into this fraction. For FOS, we succeeded mainly in extracting shorter chain length fructans including kestose. It is possible that some longer chain FOS remained in the residue fraction obtained after extraction, but onion is known to be mainly composed of short chain FOS, so we find this unlikely. The residue fraction seems therefore to be mostly composed of insoluble cell wall material. Quercetin was used as a marker for ethanol soluble onion compounds, and also these compounds were only left in small amounts in the residue. We have no direct data on the concentration of OSC in the fractions; but in a different study, we have analysed the urine from these rats by NMR and observed that organosulphur metabolites were distributed between the extract and the residue groups according to the fractions of onion materials in their diets (7:3, data to be published elsewhere), indicating that the doses to the rats in the three groups would be approximately 3:2:1 for onion by-product, extract and residue, respectively.
A significant decrease in the Hb concentration of the rats fed with onion compared with rats in the control group was a main finding (Fig. 1). In agreement with the present results, several studies using different animals as models concluded that onion supplementation resulted in dose-dependent reductions in erythrocyte counts and Hb levels involving oxidative damage to erythrocytes and consequent haemolytic anaemia and Heinz body formation(Reference Ostrowska, Gabler and Sterling34, Reference Yamamoto, Aoyama and Hamaguchi35). As far as we are aware, a similar response has not been described in human subjects, indicating that this toxic effect is not present or at least much weaker in human subjects. Onion OSC have been proposed to be responsible of this toxic effect in rats due to their ability to generate reactive oxygen species in the presence of GSH. Particularly, the relatively lipophilic dipropyl tri- and tetrasulphides and dipropenyl disulphide may be largely responsible for the onion toxicity observed in some animals(Reference Yamato, Hayashi and Kasai36, Reference Munday, Munday and Munday37). This would be in accordance with our observation that the residue was also toxic, so hot ethanol was not sufficient for full extraction of OSC.
With respect to onion antioxidant properties, several rat studies have related these properties with the onion antihyperglycaemic or antihypertensive effects(Reference Yamamoto, Aoyama and Hamaguchi35, Reference El-Demerdash, Yousef and El-Naga38). Some recent studies have described an enhancement of the total antioxidant capacity of plasma in rats fed with onions(Reference Son, Jung and Jeon39, Reference Park, Kim and Kim40). We found that antioxidant enzyme activities in erythrocytes showed a significant increase in GR and GPx1 activities when rats were fed with the onion extract rich in FOS (Table 3). This effect might be a result of erythrocyte GSH depletion leading to an increased demand for GSH-dependent enzymes and especially for reduced GSH. In agreement with this assumption, the hepatic gene expression of Gclc was higher after feeding with onion fractions (Table 4), but only significantly so in the residue fraction. GPx1 activity in liver tissue of rats fed with the onion extract was significantly decreased compared with the activities found in the rest of the groups (Table 3). We speculate that the antioxidant flavonols, quercetin and its glycosides may have acted as inhibitors.
Onions have also been proposed to be chemoprotective due to the ability of their OSC to increase activities of phase 2 detoxification enzymes and proteins, including GSH S-transferase, epoxide hydrolase, NAD(P)H:quinone oxidoreductase1 and UDP-glucuronosyltransferase(Reference Munday, Munday and Helmut41, Reference Guyonnet, Belloir and Suschetet42). Moreover, the combination of OSC and the glycosides of quercetin found in onions have been reported to exert chemoprotective action by enhancing the phase 2 enzymes and inhibiting phase 1 enzyme activities such as cytochromes P450. It has been shown by others that consumption of onion decreased the activity of CYP2E1(Reference Teyssier, Amiot and Mondy43).
Hepatic gene expression evaluated in the present study revealed an upregulation of the phase 2 enzyme Gclc in rats fed with the onion residue fraction, but not in rats fed with the onion by-product or the onion extract fraction giving higher doses of OSC. By contrast, none of the other antioxidant and phase 2 genes evaluated in the present study was significantly up- or downregulated by onion supplementation, although the expression of Nqo1 apparently increased with a pattern similar to that of Gclc (Table 4). If the OSC compounds are responsible for Gclc induction, our data indicate that the more lipophilic OSC may be the more efficient enzyme inducers since only the residue fraction was significantly active. Expression of hepatic Alas1 did not follow these same patters and was unaffected by feeding rats with onion products, indicating that toxicity is not due to global downregulation of haeme biosynthesis. On the other hand, upregulation to compensate for haeme loss does not take place in the liver, but may happen in the red bone marrow. Additional studies with further fractionation will have to be performed in order to study which onion compounds with or without thiol groups in their composition play a role in onion toxicity or in modulating phase 2 enzyme activities.
Since GSH depletion might cause increased formation of reactive chemical species, including oxygen radicals, the potential genotoxicity of onion was also evaluated by performing the comet assay. Our results show that the onion products did not decrease the background level of DNA damage (data not shown). Therefore, it could be inferred that the three onion products were not genotoxic. Recent studies have indicated that OSC protect human-derived cells against oxidative DNA damage(Reference Arranz, Haza and García44, Reference Belloir, Singh and Daurat45). With the present study, it cannot be verified that these onion products possess an antigenotoxic effect.
We observed a significant lowering effect by feeding the onion by-product or either of the two onion fractions on rat caecal pH without an effect on transit time (Table 5). A marked effect was also shown on bacterial BGL and GUS activities (Fig. 2) and on gut fermentation to SCFA (Fig. 3).
BGL and GUS enzymes are the principal glycosidases produced by the intestinal microbiota, which hydrolyse glycosidic bonds in the gut originating either from the diet or from compounds excreted with the bile. As a result, there is a release of aglycones or metabolites some of which are potentially toxic or carcinogenic. The health significance of increases in BGL or GUS is uncertain. Interestingly, slightly higher BGL and GUS activities were found when rats were fed with the onion extract fraction compared with the other rat groups fed with onion by-product or the residue. A significant increase in caecal lactobacilli and bifidobacteria could partly explain the increase in the BGL activity due to the fact that these two colonic bacterial genera possess higher levels of BGL activities. However, the Bifidobacterium genus expresses a low GUS activity, and consequently other bacterial genera might be involved in the observed rise in GUS activity(Reference De Preter, Raemen and Cloetens46). Moreover, in accordance with Lara-Villoslada et al. (Reference Lara-Villoslada, de Haro and Camuesco47), short-chain FOS with a high content of kestose promoted a more favourable intestinal microbiota increasing caecal lactobacilli and bifidobacteria counts as well as SCFA production in healthy rats. Thus, we expect similar results in the present study since we confirmed the findings of others of a high presence of this trisaccharide in the onion extract(Reference Jaime, Martínez and Martín-Cabrejas23).
SCFA are important products formed by fermentation of inulin-type fructans with rat or human gut microbiota. We observed increased levels of propionate and butyrate with all three products, including the group of rats fed with the low FOS onion residue (Fig. 3), indicating that the insoluble fibres contribute significantly to the fermentation. Butyrate has been shown to increase apoptosis in colon cell lines and to protect from genotoxic carcinogens by enhancing expression of genes involved in detoxification(Reference Pool-Zobel and Sauer48). Our results, therefore, show that onion by-products possess additional fermentable fibres compared to FOS-rich additives. Decreased pH is often seen as a consequence of caecal fermentation, and we have previously found that decreased pH was the best predictor of subsequent lower risk of colon cancer in rats fed sugars, oligofructose and inulin(Reference Jacobsen, Poulsen and Dragsted49). Overall, these effects in the caecum would clearly indicate that increased fermentation of onion fibres is taking place, and that this has altered the functionality and possibly also the composition of the gut microbiota leading to a healthier phenotype.
Conclusion
The present study represents a first step assuring the safety of onion by-products as a food ingredient. Feeding rats with an onion by-product and two derived onion fractions did not involve any genotoxic risk despite our reproduction of the well-known effect of onion on anaemia in rodents. The soluble fraction of the onion by-product seems to affect in vivo antioxidant properties, whereas the residue fraction caused phase 2 induction. Moreover, the onion by-product and the two derived onion fractions exert prebiotic properties as evidenced by decreased pH, increased butyrate production and altered gut microbiota enzyme activities. Additional model studies would have to be done with additional well-characterised subfractions in order to further explore these effects and to relate them with specific onion components. Human studies would be needed to ascertain that the effects are related to improved health.
Acknowledgements
The present study was supported by funding from the Spanish Ministry of Science and Innovation (AGL2003-09 138-C04-01; 200670I08; Consolider-Ingenio Programme 2010, FUN-C-FOOD, CSD2007-00 063; and AGL2008-04 798-C02-01/ALI) and from the Danish Ministry of Food, Agriculture and Fisheries (NuBI, 3304-FVFP-060696-01). E. R.-M. wishes to thank the Spanish Ministry of Science and Innovation for a Predoctoral Fellowship. We thank Vibeke Kegel and Margit Wagtberg Hansen for their excellent technical assistance. The cooperation of Anis Arnous with the onion fractions' freeze-drying process is also gratefully appreciated. The author's responsibilities were as follows: E. R.-M., C. S.-M. and M. P. C. provided the onion by-product and produced the onion powder. C. S.-M. proposed the evaluation of onion by-product bioactivity, suggesting the collaboration between the groups. T. H. N. and T. B. analysed the products for contents of sugars, FOS and quercetin. L. O. D. planned the rat study. M. P. was responsible for the animal study protocol and diets. E. R.-M. was responsible for the onion by-product extraction into two fractions, comet assay and antioxidant enzymes' analyses. B. N. K. was responsible for gene expression, bacterial enzymes and antioxidant enzymes' analyses. M. L. B. supervised the comet assay performance and results' data. M. H. was responsible for the SCFA and rat caecal pH; S. L. for the transit time measurement. L. O. D. provided statistical support. E. R.-M. wrote the first draft of the manuscript. L. O. D. and C. S.-M. helped E. R.-M. with the first manuscript draft. M. P. C., C. S. -M., and L. O. D. supervised E. R. -M. manuscript drafts. All authors approved the final manuscript. All authors declared that they had no conflict of interest.










