There is considerable evidence that dietary proteins, in general, are more satiating than carbohydrate and fat( Reference Anderson and Moore 1 – Reference Westerterp-Plantenga, Lemmens and Westerterp 5 ), and that among protein sources dairy whey protein is particularly satiating( Reference Bendtsen, Lorenzen and Bendsen 6 – Reference Veldhorst, Smeets and Soenen 8 ). In comparison with carbohydrate, ingestion of whey protein has been shown to reduce subsequent energy intake relative to glucose( Reference Bellissimo, Desantadina and Pencharz 9 , Reference Zafar, Waslien and AlRaefaei 10 ), sucrose( Reference Anderson, Tecimer and Shah 11 ) or maltodextrin( Reference Bertenshaw, Lluch and Yeomans 12 – Reference Chungchunlam, Henare and Ganesh 14 ). In our own earlier study with healthy, normal-weight human subjects( Reference Chungchunlam, Moughan and Henare 13 – Reference Lam, Moughan and Awati 15 ), we found that whey protein, when provided as the main fraction of a preload meal, suppressed subsequent food intake and increased subjective ratings of fullness relative to maltodextrin. Consumption of whey protein has been shown to result in a greater reduction in subsequent food intake and increase in subjective ratings of satiety compared with casein( Reference Hall, Millward and Long 16 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 17 ), egg albumen( Reference Anderson, Tecimer and Shah 11 , Reference Pal and Ellis 18 ), milk protein( Reference Diepvens, Haberer and Westerterp-Plantenga 19 ), soya protein( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 17 ), tuna( Reference Pal and Ellis 18 ) and turkey meat( Reference Pal and Ellis 18 ). However, little is known about the characteristics of whey that elicit such an effect on satiety. The satiating effect of whey protein may be attributed to an effect of the intact proteins themselves, to the bioactive peptides released during digestion( Reference Jahan-Mihan, Luhovyy and El Khoury 20 , Reference Moughan, Fuller and Han 21 ) or to the unique amino acid composition of whey protein.
Proteins release peptides and amino acids into the digestive lumen during digestion, and amino acids are transferred to the blood via amino acid transport systems present in the intestinal mucosa( Reference Alpers 22 , Reference Ten Have, Engelen and Luiking 23 ). A previous study by Mellinkoff et al. ( Reference Mellinkoff, Frankland and Boyle 24 ) showed that an increase in postprandial peripheral serum amino acid concentrations was associated with a reduction in subjective ratings of hunger. The satiating effect of whey protein may be related to its rapid digestion and amino acid absorption( Reference Hall, Millward and Long 16 , Reference Ten Have, Engelen and Luiking 23 , Reference Boirie, Dangin and Gachon 25 ), resulting in rapid increases in plasma amino acid concentrations( Reference Hall, Millward and Long 16 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 17 , Reference Chungchunlam, Henare and Ganesh 26 – Reference Nilsson, Holst and Bjorck 28 ). Moreover, whey protein is a rich source of branched-chain amino acids (BCAA) such as isoleucine, leucine and valine( Reference Etzel 29 , Reference Moughan 30 ), and this is reflected in the postprandial plasma BCAA concentrations observed after whey protein intake( Reference Hall, Millward and Long 16 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 17 , Reference Chungchunlam, Henare and Ganesh 26 – Reference Nilsson, Holst and Bjorck 28 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 31 ). The satiating effect of intact whey protein has not been compared with that of a free amino acid mixture (AAM) simulating its amino acid content.
The objective of the present study was to assess whether the satiating effect of whey protein is related to its mixture of intact proteins or to the unique amino acid composition of whey protein. The effects of a whey protein isolate (WPI) compared with its equivalent AAM given in the free form on subsequent ad libitum food intake and subjective ratings of appetite were studied in healthy, normal-weight, young women. Testing a hypothesis concerning the effects of amino acids requires an accurate assessment of the amino acid composition of the protein, and therefore multiple hydrolysis times combined with least-squares non-linear regression was used to obtain the amino acid composition of WPI in this study.
Methods
Subjects
A total of twenty women, aged 18–40 years, with a BMI ranging from 19 to 26 kg/m2, responded to a public advertisement and were invited to participate in the present study. Subjects were screened for exclusion criteria that included smoking, athletic training, a gastrointestinal disorder or eating disorder, dieting or taking medication known to affect appetite, not consuming breakfast every day, having a history of menstrual irregularities, pregnancy, lactation or trying to become pregnant. All subjects had their height and weight measured in the laboratory to ensure that they met the BMI criteria. Before the start of the study, participants tasted all test meals, and subjects who had intolerance to the test foods or disliked the test foods were not included in the study. Subjects completed the Three Factor Eating Questionnaire( Reference Stunkard and Messick 32 ) as a measure of dietary restraint (mean score 9 (sem 1), range 1–21). All volunteers provided their written informed consent. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Massey University Human Ethics Committee (application no. 11/47). The human trial was registered with the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) as ACTRN12615000344594.
Amino acid analysis of the whey protein isolate
It was necessary to determine with accuracy the amino acid composition of the WPI. The WPI was hydrolysed in concentrated 6-m-hydrochloric acid (HCl) containing 0·1 % (v/v) phenol. The hydrolysates were sealed in an O2-free environment and then hydrolysed at 110°C for 0, 2, 4, 6, 10, 14, 19, 24, 52, 92 and 144 h to determine amino acids (excluding tryptophan, cysteine, methionine, asparagine and glutamine). The hydrolysates were dried under vacuum and dissolved in sodium citrate buffer (pH 2·2). Cysteine and methionine were oxidised with performic acid to cysteic acid and methionine sulfone, respectively, before HCl hydrolysis. Tryptophan was determined following alkaline (sodium hydroxide) hydrolysis of the protein in a N2 environment at 110°C for 0, 2, 4, 6, 10, 14, 20, 24, 52, 92 and 144 h. After hydrolysis of the sample protein, the amino acids were separated and quantified using HPLC. The WPI powder used in the present study was analysed for amino acid composition( Reference Chung Chun 33 ) (Table 1) at the Riddet Institute (Palmerston North, New Zealand) using multiple hydrolysis times (0–144 h) combined with least-squares non-linear regression( Reference Robel and Crane 34 , Reference Darragh, Garrick and Moughan 35 ) as described in detail in the protocol of Rutherfurd & Gilani( Reference Rutherfurd and Gilani 36 ).
Table 1 Amino acid composition (g/100g powder, A 0) of the whey protein isolate determined using a least-squares non-linear regression model after multiple hydrolysis times compared with standard 24-h hydrolysis values (Mean values with their standard errors)
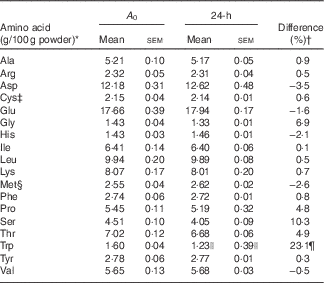
* Samples were analysed in triplicate.
† Difference (%)=((A 0 value–24-h value)/A 0 value)×100.
‡ Detected as cysteic acid after performic acid oxidation followed by acid hydrolysis.
§ Detected as methionine sulfone after performic acid oxidation followed by acid hydrolysis.
|| 20-h hydrolysis value for tryptophan.
¶ Difference (%)=((A 0 value−20-h value)/A 0 value)×100.
Test foods
The breakfast meal consisted of toasted wholemeal bread and a selection of margarine, strawberry jam and either coffee or tea plus white sugar and full-fat milk. The same subject-specific breakfast was provided in packed containers before each study day, and participants were instructed to consume all the food items provided. Breakfast consumption of each participant was recorded on each study day. The average metabolisable energy (ME) content of the breakfast meal was 1503 kJ with 57, 11 and 32 % of ME being derived from carbohydrate, protein and fat, respectively.
The preloads consisted of toast and an orange-flavoured spread. The two test preload spreads consisted of an orange-flavoured marmalade (Jok’N’Al Orange marmalade; Joknal Products Ltd, 255 kJ ME, 0·3 g protein, 15·0 g carbohydrate and 0 g fat/100 g serve) to which either a WPI or a mixture of free amino acids simulating the amino acid composition of whey protein (AAM) was added. A 50-g serve of protein was provided in the preload on the basis of the amount of protein observed to induce satiety in our previous studies( Reference Chungchunlam, Moughan and Henare 13 , Reference Chungchunlam, Henare and Ganesh 14 ). The WPI preload spread contained 60 g of dairy WPI (WPI 894; Fonterra Co-operative Group Ltd) mixed with 190 g of orange-flavoured marmalade, and was prepared in the laboratory 1 week before the study start and stored in a refrigerator. The AAM preload spread contained 50 g of the mixture of free amino acids and 200 g of orange-flavoured marmalade, and was freshly prepared on each test day. The AAM composition was equivalent to that of the WPI (Table 1) and was obtained by mixing pure, crystalline, free amino acids (Evonik Degussa GmbH). All amino acids were single l-amino acids of food grade, except for glycine and methionine (dl-methionine). The amino acid content of l-cysteine was adjusted for the presence of HCl and water, and the amino acid content of l-lysine was adjusted for the presence of HCl. The amounts of l-asparagine and l-aspartic acid (5·10 and 7·08 g, respectively, per 100 g WPI powder) as well as the amounts of l-glutamine and l-glutamic acid (6·40 and 11·25 g, respectively, per 100 g WPI powder) added to the AAM were estimated on the basis of the ratio contained in the WPI. To improve palatability, each preload spread (250 g) was accompanied with a slice (45 g) of toasted low-protein bread (Pavilion original sliced gluten-free bread; Pavillion Foods, 444 kJ ME, 1·2 g protein, 22·7 g carbohydrate and 1·2 g fat/45 g serve) and 100 ml of water. The bread was common to both preload diets and contributed a low amount of protein (1·2 g protein per preload meal). The amino acid composition of the bread was not determined. The WPI-enriched preload meal (295 g, 1849 kJ ME) contributed 52·8 g protein, 53·9 g carbohydrate and 1·8 g fat, and the AAM-enriched preload meal (295 g, 1790 kJ ME) contributed 51·8 g protein, 52·7 g carbohydrate and 1·2 g fat. The two test preloads were approximately isoenergetic (approximately 1800 kJ) and similar in the proportional energy provided by protein (48·6–49·2 % of ME), carbohydrate (47·7–48·3 % of ME) and fat (2·5–3·7 % of ME).
A lunchtime test meal was served in private cubicles 120 min following consumption of the preload meals and comprised of a homogeneous, single-item, hot fried-rice meal and bottled spring water. The hot fried-rice test meal included white rice, minced chicken meat, eggs, peas, maize, carrots, chicken stock, salt, sugar and vegetable oil. The fried-rice test meal contained 36·8, 8·5, 5·8 and 1·0 g of available carbohydrate, crude protein, crude fat and total dietary fibre, respectively, per 100 g, with a calculated ME content of 982 kJ/100 g.
Experimental procedure
In a single-blind, completely randomised block design, each woman subject served as her own control by participating in 2 session days, with a minimum of 2 d between test days. On the evening preceding each test day, subjects were instructed to abstain from strenuous physical activity and alcohol consumption, and to consume only water after 22.00 hours. Women participated during the early phase of their menstrual cycle (menstruation and follicular phase). On the morning of the test day, each subject consumed a subject-specific breakfast meal at home. The participants were instructed to consume all the food items provided at least 3 h before the test session appointment. Throughout the morning, participants were instructed to refrain from consuming anything except water.
Subjects arrived at the Human Nutrition Unit, Massey University, approximately 12.00 to 13.00 hours. Subjects who did not comply with restrictions on the evening before and the morning were re-scheduled for another test day. Upon arrival at the laboratory, a baseline questionnaire to assess ratings of appetite (hunger, desire to eat, prospective food consumption and fullness) and nausea was completed. A test preload meal was served to be consumed within 15 min. Following complete ingestion of the preload meal (time 0 min), subjects completed a questionnaire assessing the palatability of the preload meal. Ratings of appetite and nausea were measured at 0, 15, 30, 45, 60, 75, 90 and 120 min. Following the 120-min measurement, subjects were seated in individual cubicles to consume the lunchtime test meal within 15 min. Subjects were instructed to consume the test meal at will (ad libitum) until such point where they felt comfortably full. Subjects rated the likeability of the fried-rice test meal immediately after consumption using a questionnaire. Subjects also completed questionnaires to assess subjective ratings of appetite and nausea 15 and 30 min after consumption of the lunchtime test meal. This experimental protocol has been described in detail previously( Reference Chungchunlam, Moughan and Henare 13 , Reference Chungchunlam, Henare and Ganesh 14 , Reference Chung Chun 33 ).
Measures of satiety
The amounts of the fried-rice test meal and water, which were provided without restriction at lunch, were measured before and after consumption using an electronic scale to the nearest 0·01 g. Subjects rated the palatability of the preload drink (overall likeability, pleasantness of taste, likeability of texture and overall sweetness) and overall likeability of the fried-rice test meal using a 10-cm visual analogue scale (VAS) immediately after consumption. The VAS was end-anchored with ‘dislike extremely’ and ‘like extremely’ for ratings of overall likeability and likeability of texture and with ‘not at all’ and ‘extremely’ for ratings of pleasantness of taste and sweetness. Each subject was instructed to fill in a booklet of questionnaires to assess subjective ratings of appetite (hunger, desire to eat, prospective food consumption and fullness) and nausea. Ratings were made on a 10-cm VAS labelled at each end with extremes: ‘not at all’ and ‘extremely’ for hunger, fullness and nausea; ‘very weak’ and ‘very strong’ for desire to eat; and ‘nothing at all’ and ‘a large amount’ for prospective consumption. VAS-rated appetite and nausea scores were collected immediately before consuming the test preload (baseline), during a 120-min period following consumption of the test preload (0, 15, 30, 45, 60, 75, 90 and 120 min) and 15 and 30 min following consumption of the lunchtime ad libitum test meal.
Statistical analysis
Statistical analyses were performed using Statistical Analysis Systems statistical software package version 9.2 for WINDOWS (SAS Institute). Power analysis, based on results from previous studies( Reference Chungchunlam, Moughan and Henare 13 , Reference Chungchunlam, Henare and Ganesh 14 ), indicated that a sample size of thirteen female subjects had sufficient power of 80 % at a level of significance of 0·05 to allow the detection of differences in ad libitum test meal intake. Each variable was examined for normal distribution and the presence of statistical outliers( Reference Ott and Longnecker 37 ). Comparisons between the two preload meals were evaluated using two-tailed paired t tests, pairing on subject, for VAS-rated palatability scores of preload meal and test meal (cm), water (g), test meal (g and kJ ME) and total energy (preload+test meal, kJ ME) intakes. A P value<0·05 was considered statistically significant. Duncan’s multiple-range test was used for making multiple pair-wise treatment comparisons. A repeated-measures ANOVA was used to evaluate the effect of preload, time of rating and their interaction (preload×time) on VAS-rated feelings of hunger, desire to eat, prospective food consumption, fullness and nausea. In the case of a significant interaction between preload and time (P<0·05), paired multiple comparisons were used to examine the preload and time combinations. VAS-rated feelings of appetite and nausea were also reported as net incremental AUC (net iAUC) from 0 to 120 min using the trapezoidal rule and evaluated using two-tailed paired t tests, pairing on subject. Results are expressed as means with their standard errors. Pearson’s correlation analysis was performed to test the relationship between energy intake (kJ ME) at the lunchtime ad libitum test meal and palatability of the preload as well as VAS-rated feelings of appetite and nausea.
Results
Amino acid analysis of the whey protein isolate
The estimated protein-bound amino acid content determined using the least-squares non-linear regression model (A 0) and the mean amino acid content after 24 h (20 h for tryptophan) of hydrolysis for each amino acid are presented in Table 1. The greatest difference was observed for tryptophan, which was underestimated by 23·1 % after 20 h of alkaline hydrolysis. Glycine, proline, serine and threonine were also underestimated by 6·9, 4·8, 10·3 and 4·9 %, respectively. The standard 24-h hydrolysis method overestimated the amino acid content of aspartic acid (3·5 %), glutamic acid (1·6 %), histidine (2·1 %) and methionine (determined as methionine sulfone) (2·5 %). These results highlight that when accurate amino acid compositional data are needed, the previously validated( Reference Robel and Crane 34 , Reference Darragh, Garrick and Moughan 35 ) multiple hydrolysis method should be used.
Subjects and nausea
All twenty women were similar in age (24·2 (sem 0·8) years) and BMI (22·7 (sem 0·4) kg/m2). Subjects did not report any adverse effects upon consuming the preload meals. Following ingestion of the preload meals (from 0 to 120 min), VAS-rated feelings of nausea (P>0·05, data not shown) and net iAUC for nausea (29·7 (sem 59·5) v. −2·3 (sem 34·6) cm, 120 min, for the WPI and AAM preloads, respectively, F 1,19=0·52, P=0·41) did not differ significantly between the two preload conditions. Pearson’s correlation indicated that there was no relationship between postprandial ratings of nausea (0–120 min) or net iAUC for nausea and energy intake at the lunchtime ad libitum test meal (P>0·05, data not shown).
Palatability of preload meals and test meal
There were significant differences found in VAS ratings for overall likeability (F 1,19=11·72, P=0·0029), pleasantness of taste (F 1,19=12·11, P=0·0025) and overall sweetness (F 1,19=8·14, P=0·0102) between the two preloads. The AAM preload meal was rated as being the least liked (1·5 (sem 0·4) cm), least pleasant in taste (1·4 (sem 0·3) cm) and least sweet (3·3 (sem 0·6) cm) compared with the WPI preload meal (3·9 (sem 0·7) cm, 4·3 (sem 0·7) cm and 5·3 (sem 0·6) cm, respectively). There was no effect of preload on the ratings for likeability of texture (4·4 (sem 0·6) cm and 3·3 (sem 0·5) cm for the WPI and AAM preload meals, respectively, F 1,19=2·77, P=0·11). Pearson’s correlation analysis showed that the VAS scores for overall likeability (r −0·17, P=0·28), pleasantness of taste (r −0·14, P=0·35), likeability of texture (r −0·01, P=0·91) and overall sweetness (r −0·01, P=0·95) of the preload meals were not correlated to energy intake at the subsequent test meal. The palatability of the lunchtime ad libitum test meal did not differ between the WPI and the AAM preload meals (F 1,19=0·13, P=0·71), with a mean overall likeability score of 8·6 (sem 0·2) cm.
Ad libitum test meal intake and total energy intake
There were no significant differences in ad libitum water intake (F 1,19=1·00, P=0·33), with the amount of water ingested at lunch being 393·2 (sem 35·7) and 363·5 (sem 45·6) g for the WPI and the AA preload meals, respectively. There was no significant effect of preload (F 1,19=1·47, P=0·24) on the amount (g) and energy intake (kJ ME) of the ad libitum fried-rice test meal consumed at lunch. The amount and energy intake of the test meal ingested following consumption of the WPI preload were 268·5 (sem 27·3) g and 2636·6 (sem 268·5) kJ, respectively, and for the AAM preload 238·4 (sem 27·3) g and 2340·6 (sem 223·1) kJ, respectively. When the energy intake from the preload meal was added to the ad libitum food test meal energy intake at lunch, total energy intake did not differ significantly between the two preload meals (4385·0 (sem 271·1) and 4069·7 (sem 223·7) kJ for the WPI and AAM preload meals, respectively, F 1,19=2·11, P=0·16).
Subjective ratings of appetite
When the total testing period (approximately 3 h) was examined, there was no significant interaction between preload and time for the VAS-rated feelings of hunger (F 10,370=0·47, P=0·91), desire to eat (F 10,370=0·14, P=0·99), prospective food consumption (F 10,370=0·25, P=0·99) or fullness (F 10,360=0·26, P=0·98) (Fig. 1). There was no significant main effect of preload (P>0·05), but, as expected, there was a significant main effect of time (P<0·0001) observed for each rating of appetite. When the ratings determined following consumption of the preload meal (0–120 min) were adjusted for baseline ratings, there was no significant interaction between preload and time observed for the VAS-rated feelings of hunger (F 7259=0·59, P=0·76), desire to eat (F 7259=0·32, P=0·94), prospective food consumption (F 7259=0·37, P=0·91) or fullness (F 7252=0·47, P=0·85). The appetite ratings did not differ significantly between preloads (P>0·05), but were influenced by time (P<0·0001). The VAS-rated feelings of hunger, desire to eat, prospective food consumption and fullness expressed as net iAUC following consumption of the two preload meals (0–120 min) are shown in Fig. 1. There were no significant differences found in the net iAUC responses for the ratings of hunger (F 1,19=2·75, P=0·11), desire to eat (F 1,19=0·07, P=0·79), prospective food consumption (F 1,19=0·62, P=0·44) or fullness (F 1,19=0·01, P=0·91) between the two preload conditions. Pearson’s correlation analysis was performed to examine the relationship between energy intake at the subsequent test meal and subjective ratings of appetite immediately before consumption of the ad libitum test meal (120 min). Energy intake of the test meal was positively related to ratings of hunger (r 0·33, P=0·0324) and prospective food consumption (r 0·38, P=0·0144), but was not correlated to ratings of desire to eat (r 0·22, P=0·15) and fullness (r −0·10, P=0·53).

Fig. 1 Subjective visual analogue scales (VAS) ratings of hunger, desire to eat, prospective food consumption and fullness before (baseline, B) and after consumption of a preload meal enriched with either a whey protein isolate (WPI, ![]() ) or a free amino acid mixture (AAM,
) or a free amino acid mixture (AAM,
![]() ) simulating the amino acid composition of WPI (AAM), and 15 and 30 min following consumption of the ad libitum test meal. Values are means (n 20) women, with their standard errors. There was no significant interaction between preload and time (P>0·05), and there was no significant main effect of preload (P>0·05), but each VAS-rated feeling differed by time (P<0·0001). Inset: net incremental AUC from 0 to 120 min in response to ingestion of the two preload meals. There was no significant main effect of preload for each subjective rating (P>0·05).
) simulating the amino acid composition of WPI (AAM), and 15 and 30 min following consumption of the ad libitum test meal. Values are means (n 20) women, with their standard errors. There was no significant interaction between preload and time (P>0·05), and there was no significant main effect of preload (P>0·05), but each VAS-rated feeling differed by time (P<0·0001). Inset: net incremental AUC from 0 to 120 min in response to ingestion of the two preload meals. There was no significant main effect of preload for each subjective rating (P>0·05).
Discussion
Little is known about the aspects of whey protein that mediate its established( Reference Bellissimo, Desantadina and Pencharz 9 – Reference Diepvens, Haberer and Westerterp-Plantenga 19 , Reference Pal, Radavelli-Bagatini and Hagger 38 ) relative satiating effect, but many studies have implicated a role of the pattern of amino acids absorbed( Reference Gietzen 39 – Reference Harper, Benevenga and Wohlhueter 43 ) and the action of specific amino acids( Reference Butler, Davies and Gehling 44 – Reference Morris, Li and MacMillan 51 ). The aim of the present study was to evaluate whether the unique amino acid composition of whey protein plays a role in satiety, by comparing the effects of a preload meal containing intact WPI or a mixture of free amino acids simulating the amino acid composition of the WPI (AAM) on subsequent ad libitum food intake and subjective ratings of appetite in twenty women, where the amino acid composition of whey protein was known with a high degree of accuracy.
This study required the delivery of free amino acids simulating the amino acid composition of whey protein. This meant that the amino acid composition of whey protein needed to be determined with accuracy. The chemistry and stability of amino acids during acid hydrolysis used in amino acid analysis differ among amino acids, and the 24-h analysis time (20-h for tryptophan) represents a compromise relative to the optimal hydrolysis time across amino acids, leading to the maximal release of most amino acids from the protein while minimising amino acid degradation. The hydrolysis and degradation of amino acids occur simultaneously during hydrolysis, and this needs to be recognised when determining the amino acid composition of proteins. By determining the amino acid content using multiple hydrolysis times, and using a previously validated( Reference Robel and Crane 34 , Reference Darragh, Garrick and Moughan 35 ) least-squares non-linear regression to model the relationship between amino acid yield and hydrolysis time, the actual protein-bound amino acid content of a protein source can be estimated accurately. This method was used in the present study.
Dietary products based on free amino acids are generally low in palatability and may induce adverse responses because of their bitter taste( Reference Kirimura, Shimizu and Kimizuka 52 , Reference Nishimura and Kato 53 ). The use of an orange-flavoured marmalade spread to mask the taste of free, crystalline amino acids was effective, with subjects reporting no adverse effects or differences in ratings of nausea following consumption of the amino acid-based diet. Although the preload meal containing the free amino acids was perceived as being the least liked (overall likeability), least pleasant in taste and least sweet in relation to the WPI preload meal, no correlation between the perceived sensory characteristics of the preload meal and subsequent food energy intake was observed, thus indicating that these dietary characteristics did not influence the outcomes of the study.
Ingestion of a preload meal containing similar amounts of carbohydrate and energy but enriched with WPI or AAM resulted in similar ad libitum intakes of water (g) and food (g and kJ ME) at a test meal 120 min after ingestion of the preload. Total energy intake (preload+test meal energy intakes) also did not differ between the preload meals. With respect to subjective ratings of hunger, desire to eat, prospective food consumption and fullness (VAS scores and net iAUC), there were no significant differences between the two preload meals. The VAS scores for the subjective ratings of appetite were found to be related, as would be expected, with subsequent food energy intake. The effect of intact whey protein on subsequent food intake and subjective ratings of appetite was reproduced when whey protein was replaced in the preload by free amino acids. The form of delivery of the amino acids did not influence the measures of satiety. These results suggest that the previously observed satiating effect of whey protein is related to its unique amino acid composition. Several studies have implicated a role of absorbed amino acids on voluntary food intake. A deficit of specific amino acids in the diet has been shown to result in a decrease in food intake( Reference Gietzen 39 – Reference Harper, Benevenga and Wohlhueter 43 ). Amino acids may play a role in the reduction of food intake and stimulation of satiety( Reference Mellinkoff, Frankland and Boyle 24 , Reference Fromentin, Darcel and Chaumontet 54 ).
This is the first study to compare the effects of intact whey protein and a mixture of free amino acids simulating the amino acid composition of whey protein on satiety in humans. One of the limitations of the present study is that we were unable to measure the rate of gastric emptying in subjects themselves and the concentrations of amino acids in the peripheral plasma, which may have helped in elucidating the postprandial kinetics of the two preload meals and would have extended the interpretation of the results. Nevertheless, in an accompanying study with the growing rat, stomach-emptying rates, determined using magnetic resonance spectroscopy, for the two experimental diets used here were found to be similar (see online Supplementary Material), suggesting similar rates of appearance of amino acids in the peripheral blood. The similar effect of preload meals enriched with whey protein or its equivalent free amino acids in inducing satiety, as observed in the present study, provides novel information that total amino acids (TAA), rather than constituent proteins and derived peptides, may be involved in the satiating response to whey protein. Several studies have reported more rapid increases in plasma TAA and BCAA following consumption of whey protein in comparison with other protein sources( Reference Hall, Millward and Long 16 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 17 , Reference Diepvens, Haberer and Westerterp-Plantenga 19 , Reference Boirie, Dangin and Gachon 25 , Reference Nilsson, Stenberg and Frid 27 , Reference Nilsson, Holst and Bjorck 28 ). In our earlier study( Reference Chungchunlam, Henare and Ganesh 26 ), whey protein elicited a greater increase in plasma BCAA relative to maltodextrin, and a significant correlation (P<0·05) between plasma concentrations of BCAA and satiety-related hormones, glucagon-like peptide-1 and pancreatic polypeptide, was observed. With regard to free amino acid meals, Nilsson et al.( Reference Nilsson, Holst and Bjorck 28 ) found that the increase in circulating levels of insulin following consumption of a drink containing whey protein appeared to be mimicked by ingestion of a drink containing equivalent amounts of free isoleucine, leucine, valine, lysine and threonine. These amino acids, naturally abundant in whey protein, may play a role in the reduction of food intake and stimulation of satiety( Reference Fromentin, Darcel and Chaumontet 54 ). Several studies have demonstrated a similar profile of circulating amino acids when proteins and an equivalent mixture of free amino acids were consumed( Reference Adibi and Mercer 55 – Reference Silk, Chung and Berger 58 ). However, the literature on the effects of free amino acids on food intake and satiety in human subjects is limited. Ingestion of a capsule containing methionine, phenylalanine, tryptophan and valine reduced food intake 30 min later compared with a placebo capsule containing magnesium trisilicate( Reference Butler, Davies and Gehling 44 ). Some amino acids thought to be involved in the regulation of food intake and body weight include leucine( Reference Garlick 45 – Reference Layman and Walker 47 ), phenylalanine( Reference Ballinger and Clark 48 , Reference Pohle-Krauza, Navia and Madore 49 ) and tryptophan( Reference Latham and Blundell 50 , Reference Morris, Li and MacMillan 51 ). Although satiety and metabolic responses to the consumption of particular free amino acids have been studied, the contribution of the overall pattern of amino acids is less well known and needs further study.
In conclusion, consumption of both the whey protein-containing diet and the free amino acid-containing diet influenced measures of satiety to a similar extent in normal-weight adult women. Thus, the parent protein and a free AAM simulating the amino acid composition of the parent protein induce the same degree of satiety. Importantly, this suggests that the mechanism involved in the effect of whey protein on satiety is mediated via the constituent amino acids, rather than the whole protein or bioactive peptides released during digestion or some combination of these. Although this study provides information concerning the effectors, it does not address possible mechanisms of the effect, and therefore the latter postulate awaits confirmation at the mechanistic level. These observations warrant further investigation by assessing the rate of whey protein digestion, the dynamics of uptake of dietary amino acids, the appearance of circulating amino acids and their potential mechanistic association with satiety responses. Concomitantly, there is a need for further studies to compare the satiating effects of whey protein with other protein sources. It is known that proteins differ in their amino acid composition, but it is not clear whether such differences are the underlying causes for reported differences between proteins in the promotion of satiety.
Acknowledgements
The authors thank the participants of the present study. The authors also thank Dr Derek Haisman and Vikas Mittal for their assistance in developing the preload meals, Maria-Tine Biersteker, Natascha Strobinger, Mehak Dhillon and Sumon Saha for their technical assistance, Dr Shane Rutherfurd for his advice on amino acid determination and the stomach-emptying rat trial, and Dr Carlos Montoya, Dr Jason Hindmarsh and Trent Olson for their assistance with the stomach-emptying rat trial.
The present study was funded by the Riddet Institute, a New Zealand government-supported Centre of Research Excellence. The authors are thankful to Fonterra Co-operative Group Ltd (New Zealand) for providing free samples of the whey protein isolate and Evonik Industries AG (Germany) for their generous donation of individual, pure, free amino acids.
The authors’ contributions are as follows: S. M. S. C. oversaw the design and conduct of the study, analysed and interpreted the data, and led the drafting of the manuscript; S. J. H. assisted with the conduct of the study and contributed to the writing of the manuscript; S. G. contributed to data analysis and helped with manuscript writing; P. J. M. contributed to the study design and manuscript writing. S. M. S. C. and P. J. M. had primary responsibility for the final content.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516003767





