Weight loss (WL) occurs when a negative energy balance is sustained over time(Reference Hall and Guo1). However, despite its apparent simplicity, energy balance represents a complex and dynamic system in which its components (i.e. energy intake (EI) and energy expenditure (EE)) fluctuate over time(Reference Edholm, Adam and Healy2) and change in response to perturbations in either side of the equation(Reference Casanova, Beaulieu and Finlayson3,Reference Melby, Paris and Foright4) .
Although a clinically meaningful WL is usually achieved, levels of recidivism and weight regain are high(Reference Wadden, Neiberg and Wing5,Reference Greaves, Poltawski and Garside6) . It has been postulated that difficulties in maintaining a reduced body weight arise not only from a lack of adherence to dietary and physical activity (PA) recommendations(Reference Heymsfield, Harp and Reitman7) but also due to metabolic, psychological and behavioural compensatory responses that occur during periods of negative energy balance. Some of these proposed compensatory responses include reductions in EE(Reference Thomas, Bouchard and Church8), PA behaviours(Reference Levine, Eberhardt and Jensen9) and increases in EI(Reference Dulloo, Jacquet and Montani10). These compensatory responses may act to undermine adherence to the diet and/or PA recommendations, prompting an individual to regain the weight lost.
Adaptive thermogenesis (AT) represents a greater than predicted decrease in EE beyond what would be predicted from the changes in fat mass (FM) and fat-free mass (FFM) occurring during WL(Reference Dulloo, Jacquet and Montani10,Reference Major, Doucet and Trayhurn11) . It has been postulated to be a compensatory response that resists WL and promotes weight regain(Reference Fothergill, Guo and Howard12–Reference Tremblay, Royer and Chaput15), but its influence on longer-term weight management has been recently questioned(Reference Martins, Gower and Hill16). AT in resting EE (REE) has been previously documented in lifestyle(Reference Martins, Gower and Hill16–Reference Dulloo and Jacquet33) and surgical(Reference Wolfe, Schoeller and McCrady-Spitzer34–Reference Coupaye, Bouillot and Coussieu39) interventions. However, some studies have reported contrasting findings as they have not observed a significant value for AT(Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference Doucet, St-Pierre and Alméras32,Reference Hopkins, Gibbons and Caudwell40) .
Several narrative reviews examining the topic of AT in REE have been previously published(Reference Casanova, Beaulieu and Finlayson3,Reference Dulloo, Jacquet and Montani10,Reference Major, Doucet and Trayhurn11,Reference Tremblay, Major and Doucet14,Reference Tremblay, Royer and Chaput15,Reference Muller and Bosy-Westphal41–Reference Trexler, Smith-Ryan and Norton44) . However, no systematic reviews have been conducted specifically on this topic, and some of these narrative reviews have also focused exclusively on the occurrence of AT in REE during lifestyle interventions.
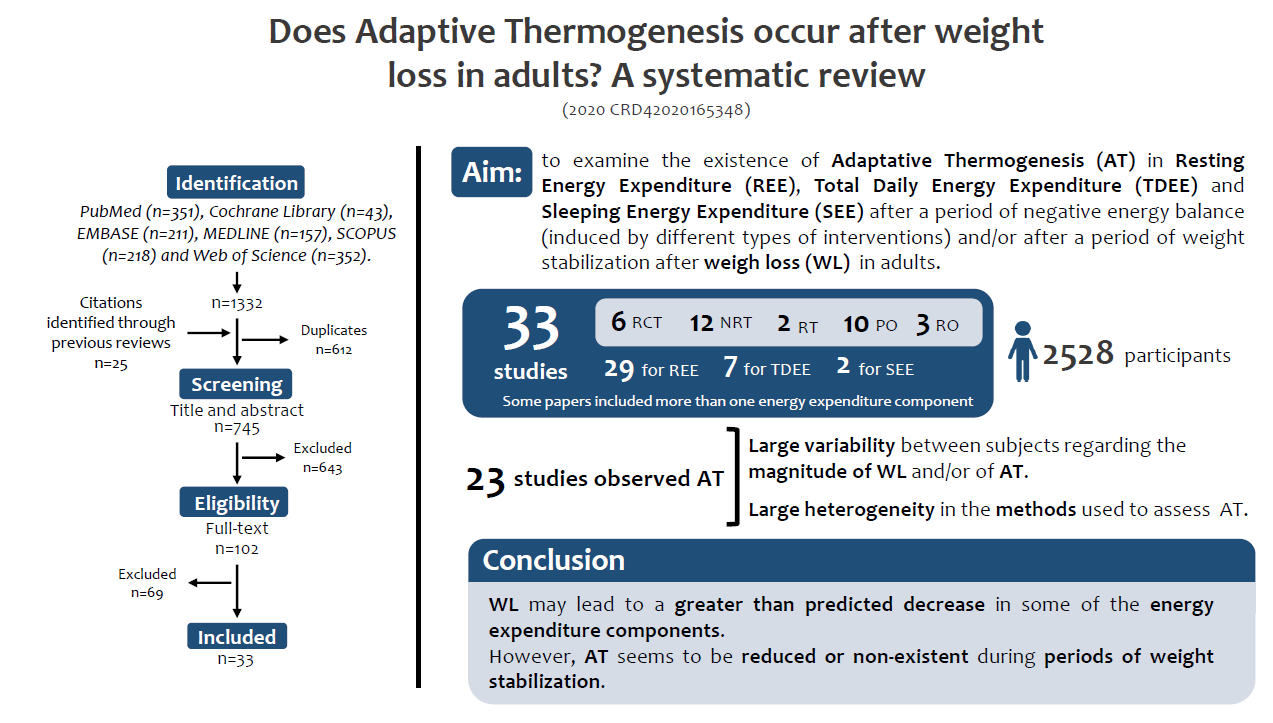
Therefore, this is the first systematic review examining the occurrence of AT in REE, total daily EE (TDEE) and sleeping EE (SEE) during or after WL induced by diet and/or exercise, bariatric surgery or pharmacological therapy, followed by weight stabilisation in adults.
Methodology
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines(Reference Liberati, Altman and Tetzlaff45) and was registered on PROSPERO (PROSPERO 2020 CRD42020165348).
Eligibility criteria
This systematic review included scientific articles published in peer-reviewed journals on or before 15 May 2020 that reported WL induced by diet and/or exercise, bariatric surgery or pharmacological therapy, and reported values for AT. All studies were evaluated according to the following inclusion criteria: (1) the study should include an intervention aimed to reduce weight that resulted in a statistically significant WL, (2) observational with follow-up or experimental study, (3) conducted in adults(>18 years), (4) a total sample size of at least ten participants, (5) intervention period of at least 1 week, (6) published in English, (7) objective measures of TDEE, REE and SEE (indirect calorimetry, metabolic chamber, doubly labelled water) and (8) objective measures of FM and FFM (dual-energy X-ray absorptiometry, air displacement plethysmography, bioelectrical impedance analysis and/or multicompartment molecular models) (e.g. four-compartment models, including combination of several techniques such as dual-energy X-ray absorptiometry, isotope dilution and air displacement plethysmography). Articles were excluded if they did not meet all of the inclusion criteria and/or had an exclusion criterion, such as the inclusion of participants with the following: (1) cancer, (2) thyroid diseases, (3) diabetes; (4) pregnancy or breast-feeding, (5) total parenteral nutrition, (6) organ transplant, (7) acute illnesses, such as infections or traumatic injury and (8) other medical conditions and/or the use of medications known to affect energy balance.
Information sources and search strategy
A comprehensive search of peer-reviewed articles published until 15 May 2020 (including online ahead of print publications) was conducted in the following electronic databases: PubMed, Cochrane Library, EMBASE, MEDLINE, SCOPUS and Web of Science. Searches included all meaningful combinations of the following sets of terms: (i) terms concerning the intervention(s) of interest (e.g. diet or energy restriction, bariatric surgery, PA or exercise, pharmacotherapy), (ii) terms representing the outcomes of interest (e.g. AT, metabolic adaptation, energy metabolism, REE, metabolic compensation), (iii) terms representing the population of interest (e.g. adults) and (iv) terms representing body composition components of interest (e.g. FM, fat-free mass, lean mass). Manual cross-referencing of the literature cited in prior reviews and hand-searches of the content were conducted to strengthen the systematic review. A search strategy example for PubMed is provided as an online Supplementary File 1.
Study selection and data processing
Based on the initial abstracts retrieved, duplicates were removed, and twenty-five were added from manual searching. Abstracts identified from the literature searches were screened for potential inclusion by two authors (C.L.N. and N.C.) and a third author (R.F.) when there was a disagreement between the first two. One-hundred and two articles were assessed for eligibility and thirty-three were included in this review. Data extraction was conducted by C.L.N. according to the PRISMA statement for reporting systematic reviews(Reference Liberati, Altman and Tetzlaff45) and included information about each article such as authors, year, study design, participants’ information (e.g. demographics and BMI), type of intervention (diet only, exercise only, diet + exercise, bariatric surgery or pharmacological), length of active intervention and/or the duration of follow-up, methodology, outcome measures and main results.
Study quality and risk of bias
To assess the study quality, the Quality Assessment Tool for Quantitative Studies checklist was used(Reference Armijo-Olivo, Stiles and Hagen46). This procedure was performed by two authors (C.L.N. and R.F.). The checklist evaluates six key methodological domains: study design, blinding, representativeness (selection bias), representativeness (withdrawals/dropouts), confounders and data collection. From the interpretation of the scores of each section (classified as strong, moderate or weak methodological quality), an overall score was given to each article. The quality assessment for each study is presented as online Supplementary File 2).
Results
A total of 1332 articles were retrieved by the aforementioned databases. From those, 612 duplicates were removed, and 25 articles identified through other sources were added, leading to a total of 745 articles for title and abstract screening. Six hundred and forty-three articles were excluded during title and abstract screening and 102 full texts were further assessed for eligibility. In this phase, sixty-nine were excluded (online Supplementary File 3) and thirty-three were included in this systematic review. The PRISMA flow chart of the study selection is presented in Fig. 1.
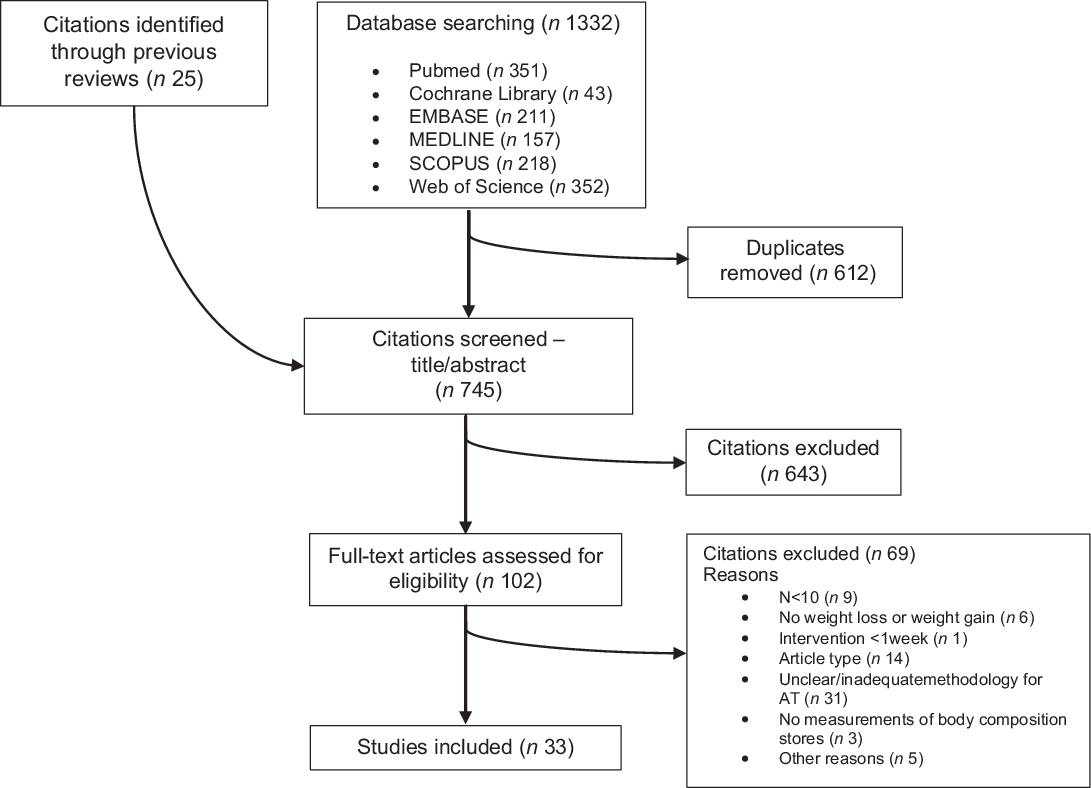
Fig. 1. Flow diagram of studies’ selection.
The studies included in this review comprised 2528 participants and were divided by each component of EE as follows:
-
REE – twenty-nine studies;
-
TDEE – seven studies;
-
SEE – two studies.
Some articles included more than one intervention type and/or assessed AT in more than one EE component.
From the included studies, six (20·7 %) were randomised controlled trials, two (6·9 %) were randomised trials without a control group (RT), twelve (41·4 %) were non-randomised trials, three (10·3 %) were retrospective observational studies and ten (34·5 %) were considered prospective observational studies. A summary of the results reported in each study, divided by study type and %WL, is presented in Table 1.
Table 1. Summary of the results
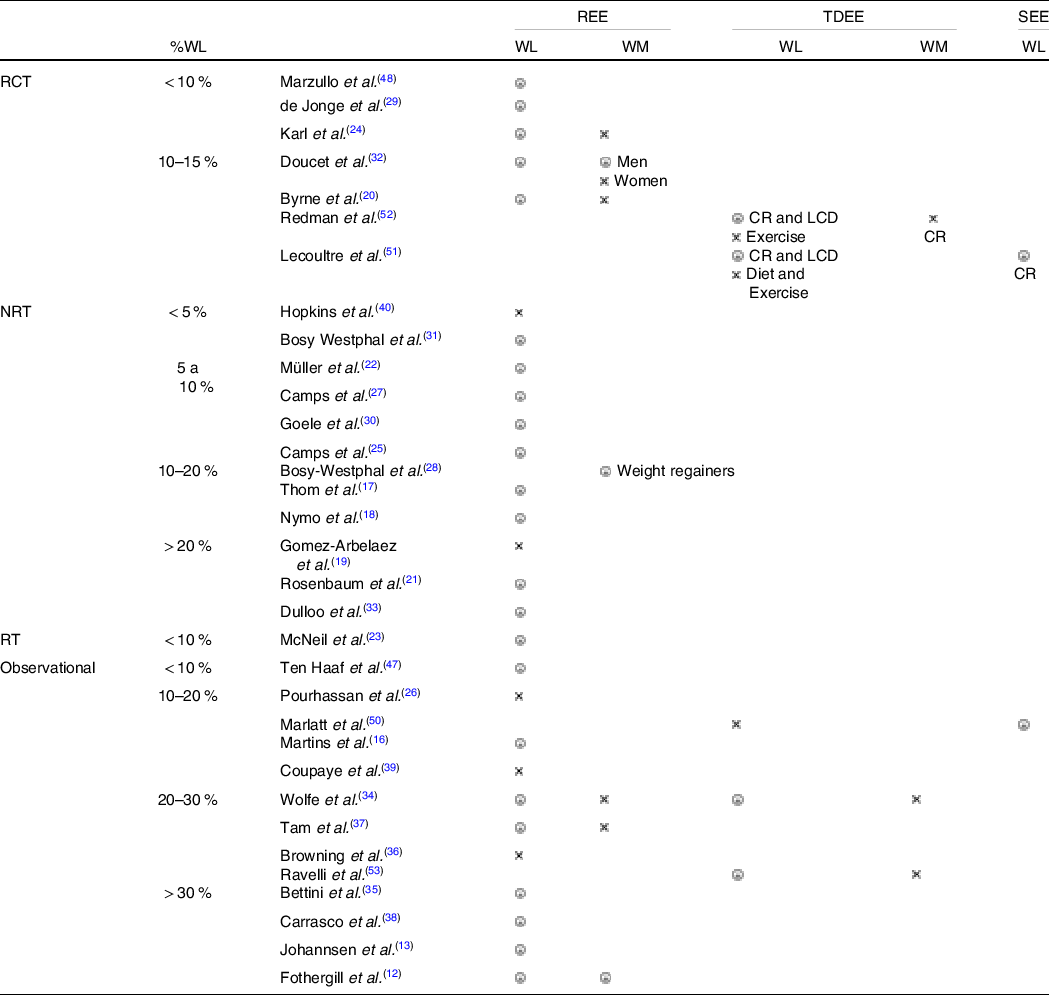
WL, weight loss; WM, weight maintenance; CR, caloric restriction; LCD, low-calorie diet; ![]() reported a higher-than-expected decrease for REE/TDEE/SEE (AT),
reported a higher-than-expected decrease for REE/TDEE/SEE (AT), ![]() did not report AT.
did not report AT.
Resting energy expenditure
A total of twenty-nine studies reporting changes in REE were included in this review(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16–Reference Hopkins, Gibbons and Caudwell40,Reference Ten Haaf, Verreijen and Memelink47,Reference Marzullo, Minocci and Mele48) (Table 2), divided into: randomised controlled trial = four (13·8 %), non-randomised trials = twelve (41·4 %), RT = two (6·9 %), prospective observational = eight (27·6 %), retrospective observational = three (10·3 %).
Table 2. Resting energy expenditure
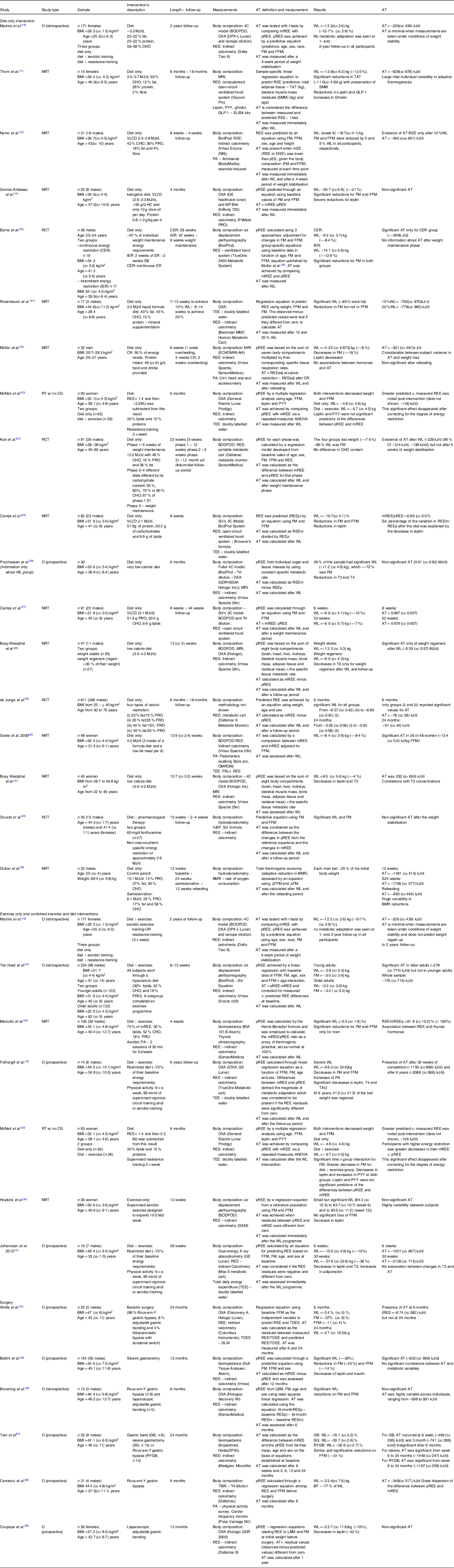
O, observational; NRT, non-randomised trial; RCT, randomised clinical trial; RT, randomised trial; CG, control group; FM, fat mass; FFM, fat-free mass; WL, weight Loss; AT, adaptive thermogenesis; CHO, carbohydrates; PRO, protein; CR, caloric restriction; VLCD, very low calorie diet; LCD, low calorie diet; CER, continuous energy restriction; IER, intermittent energy restriction; TDEE, total daily energy expenditure; BF, body fat; GB, gastric banding; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
Diet-only interventions
Eighteen studies using a diet-only intervention were included(Reference Martins, Gower and Hill16–Reference Dulloo and Jacquet33). From those, one used a pharmacological therapy together with caloric restriction(Reference Doucet, St-Pierre and Alméras32).
Participants’ characteristics
These studies involved 1780 participants (559 males). Only three studies had a mean BMI < 30 kg/m2(Reference Martins, Gower and Hill16,Reference Müller, Enderle and Pourhassan22,Reference Dulloo and Jacquet33) , while the majority of the studies included participants with obesity(Reference Thom, Dombrowski and Brosnahan17–Reference Rosenbaum and Leibel21,Reference McNeil, Schwartz and Rabasa-Lhoret23–Reference Camps, Verhoef and Westerterp27,Reference de Jonge, Bray and Smith29–Reference Doucet, St-Pierre and Alméras32) . The amount of weight lost varied between studies, with ten studies reporting a WL > 10 %(Reference Martins, Gower and Hill16–Reference Rosenbaum and Leibel21,Reference Camps, Verhoef and Westerterp25–Reference Camps, Verhoef and Westerterp27,Reference Dulloo and Jacquet33) and seven reporting moderate WL (< 10 %)(Reference Müller, Enderle and Pourhassan22–Reference Karl, Roberts and Schaefer24,Reference de Jonge, Bray and Smith29–Reference Doucet, St-Pierre and Alméras32) .
Diet type
Six studies used a very low-calorie diet(< 3·3 MJ/d) in order to lose weight(Reference Nymo, Coutinho and Torgersen18,Reference Gomez-Arbelaez, Crujeiras and Castro19,Reference Rosenbaum and Leibel21,Reference Pourhassan, Bosy-Westphal and Schautz26,Reference Camps, Verhoef and Westerterp27,Reference Doucet, St-Pierre and Alméras32) and five used a low calorie diet (3·3–5·0 MJ/d)(Reference Thom, Dombrowski and Brosnahan17,Reference Camps, Verhoef and Westerterp25,Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference Goele, Bosy-Westphal and Rumcker30,Reference Bosy-Westphal, Kossel and Goele31) . Other studies calculated the prescribed EI as a percentage of participant’s energy needs (calculated as measured REE × PAL): ˜67 %(Reference Dulloo, Jacquet and Montani10,Reference Karl, Roberts and Schaefer24) and 50 %(Reference Müller, Enderle and Pourhassan22). McNeil et al. multiplied each participant’s REE by 1·4 and then subtracted 3·3MJ from that result(Reference McNeil, Schwartz and Rabasa-Lhoret23).
The macronutrient distribution was different among studies. Three reported a high protein intake (> 25 % or > 1·2 g/kg)(Reference Thom, Dombrowski and Brosnahan17,Reference Nymo, Coutinho and Torgersen18,Reference Dulloo and Jacquet33) . A ketogenic diet was used by Gomez-Arbealez et al. (Reference Gomez-Arbelaez, Crujeiras and Castro19). Karl and colleagues used four types of diets differing in carbohydrate (CHO) content: 55 %, 60 %, 70 % or 80 % CHO(Reference Karl, Roberts and Schaefer24). Jonge et al. also divided the sample into four types of caloric restriction diets differing in fat and/or protein content: (i) 20 % fat/15 % protein (PRO), (ii) 20 % fat/25 % PRO, (iii) 40 % fat/15 % PRO and (iv) 40 % fat/25 % PRO(Reference de Jonge, Bray and Smith29). Dulloo et al. prescribed a 6·1 MJ/d diet, consisting of 25 % PRO, 17 % fat and 58 % CHO(Reference Dulloo and Jacquet33). Some studies did not report any information about the diet(Reference Martins, Gower and Hill16) or the macronutrient composition of the diet(Reference Martins, Gower and Hill16,Reference Byrne, Sainsbury and King20,Reference Pourhassan, Bosy-Westphal and Schautz26–Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference Goele, Bosy-Westphal and Rumcker30,Reference Bosy-Westphal, Kossel and Goele31) .
Methodology to assess adaptive thermogenesis
Thirteen studies used a predictive equation to estimate REE (pREE) and then calculated AT by comparing the pREE with a measured REE (mREE) using a statistical approach such as t test or ANOVA(Reference Martins, Gower and Hill16–Reference Rosenbaum and Leibel21,Reference McNeil, Schwartz and Rabasa-Lhoret23–Reference Camps, Verhoef and Westerterp25,Reference Camps, Verhoef and Westerterp27,Reference de Jonge, Bray and Smith29,Reference Doucet, St-Pierre and Alméras32,Reference Dulloo and Jacquet33) . Byrne et al. also used an additional two approaches: (i) an equation developed by Muller et al. (Reference Müller, Bosy-Westphal and Klaus49) to predict REE and (ii) adjusted REE to FM and/or FFM followed by a comparison between baseline and post-intervention adjusted baseline values(Reference Byrne, Sainsbury and King20). Bosy-Westphal et al. used the sum of seven tissue-level components obtained by MRI multiplied by their tissue-specific metabolic rates to predict REE and then subtracted the baseline REE with the post-intervention REE(Reference Müller, Enderle and Pourhassan22,Reference Pourhassan, Bosy-Westphal and Schautz26,Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference Bosy-Westphal, Kossel and Goele31) .
Adaptive thermogenesis
A significant value for AT was observed in fifteen studies(Reference Martins, Gower and Hill16–Reference Nymo, Coutinho and Torgersen18,Reference Byrne, Sainsbury and King20–Reference Camps, Verhoef and Westerterp25,Reference Camps, Verhoef and Westerterp27–Reference Bosy-Westphal, Kossel and Goele31,Reference Dulloo and Jacquet33) . Only three studies did not report a significant AT after WL(Reference Gomez-Arbelaez, Crujeiras and Castro19,Reference Pourhassan, Bosy-Westphal and Schautz26,Reference Doucet, St-Pierre and Alméras32) . Byrne et al. (Reference Byrne, Sainsbury and King20), which compared a continuous energy restriction v. an intermittent energy restriction, only reported AT for the continuous energy restriction group (˜209 kJ/d), which lost ˜8·4 % of their initial weight. For the intermittent energy restriction group, AT was not significant despite a greater WL (˜–12·9 %). Jonge et al. compared four types of caloric restriction diets varying in fat and/or protein (PRO) content(Reference de Jonge, Bray and Smith29). AT was only presented for the 20 % fat/15 %PRO and 20 %fat/25 %PRO groups, while the other two groups (40 % fat/15 %PRO and 40 % fat/25 %PRO) did not report AT despite significant WL. Despite the evidence for AT when measured immediately after the WL intervention, some intervention studies reported that this disappeared or was attenuated after a period of weight stabilisation (measured after the follow-up period)(Reference Karl, Roberts and Schaefer24,Reference Camps, Verhoef and Westerterp27,Reference de Jonge, Bray and Smith29) . Those three studies had participants with similar characteristics and methodologies to assess pREE (although de Jonge et al. created a regression equation without using FM and FFM as variables). Furthermore, Camps et al. also used a different methodology to assess AT (mREE/pREE).
Exercise only and combined exercise and diet interventions
Since only one article reported an exercise-only intervention(Reference Hopkins, Gibbons and Caudwell40), its results will be analysed with combined diet and exercise interventions, comprising seven articles(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16,Reference McNeil, Schwartz and Rabasa-Lhoret23,Reference Hopkins, Gibbons and Caudwell40,Reference Ten Haaf, Verreijen and Memelink47,Reference Marzullo, Minocci and Mele48) .
Participants’ characteristics
A total of 678 participants were involved (151 males). Only one study comprised participants with a BMI < 25 kg/m2(Reference Martins, Gower and Hill16). Half of the studies reported a > 10 % WL(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16) , while the others reported moderate amounts of WL (< 10 %)(Reference McNeil, Schwartz and Rabasa-Lhoret23,Reference Hopkins, Gibbons and Caudwell40,Reference Ten Haaf, Verreijen and Memelink47,Reference Marzullo, Minocci and Mele48) .
Intervention type
The study related to an exercise-only intervention(Reference Hopkins, Gibbons and Caudwell40) consisted of a supervised aerobic exercise designed to create an energy deficit of ˜10·5 MJ per week. The type of exercise was divided into aerobic(Reference Hopkins, Gibbons and Caudwell40,Reference Marzullo, Minocci and Mele48) , resistance training(Reference McNeil, Schwartz and Rabasa-Lhoret23) or both(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16) . One study did not add any information about the type of exercise(Reference Ten Haaf, Verreijen and Memelink47).
Methodology to assess adaptive thermogenesis
A predictive equation to estimate REE was created in five studies(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16,Reference McNeil, Schwartz and Rabasa-Lhoret23,Reference Ten Haaf, Verreijen and Memelink47) . Hopkins et al. also used a predictive equation to estimate REE but did not use their own sample but an independent population including women with overweight/obesity who did not participate in the intervention(Reference Hopkins, Gibbons and Caudwell40). All of the mentioned studies calculated AT by comparing pREE with mREE using a statistical approach such as t test or ANOVA. Marzullo et al. used the Harris-Benedict equation to estimate REE (pREE), dividing mREE by pREE to calculate a ratio(Reference Marzullo, Minocci and Mele48).
Adaptive thermogenesis
AT was reported in six studies(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16,Reference McNeil, Schwartz and Rabasa-Lhoret23,Reference Ten Haaf, Verreijen and Memelink47,Reference Marzullo, Minocci and Mele48) . Hopkins et al. study was the only study that did not report a significant value for AT(Reference Hopkins, Gibbons and Caudwell40), being the only exercise-only intervention in which participants lost a small amount of weight (–1·3 (sd 2·7) kg). Despite having AT after WL, one study reported an attenuation after 1–2 years of follow-up(Reference Martins, Gower and Hill16). The values for AT ranged between 126 and 418 kJ/d except for two studies(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13) . These studies reported significant weight losses (WL = –58·3(sd 24·9) kg(Reference Fothergill, Guo and Howard12) and WL = –57·6(sd 23·8) kg(Reference Johannsen, Knuth and Huizenga13)) and showed a larger AT (˜837–1255 kJ/d which increased during follow-up for ˜2092 kJ/d)(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13) .
Bariatric surgery
For bariatric surgery, six studies were included in this review(Reference Wolfe, Schoeller and McCrady-Spitzer34–Reference Coupaye, Bouillot and Coussieu39), with the study length ranging from 6 to 24 months.
Participants’ characteristics
A total of 294 participants (seventy-five males) underwent bariatric surgery. Baseline characteristics were similar among studies, with all including participants with obesity (mean BMI > 30 kg/m2). All of the studies presented a mean WL of ˜30 % except for those who underwent gastric banding (˜15–20 %).
Intervention type
The following weight reduction surgeries were conducted: Roux-en-Y gastric bypass(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Browning, Rabl and Campos36–Reference Carrasco, Papapietro and Csendes38) , sleeve gastrectomy(Reference Bettini, Bordigato and Fabris35,Reference Tam, Rigas and Heilbronn37) , gastric band(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Browning, Rabl and Campos36,Reference Carrasco, Papapietro and Csendes38,Reference Coupaye, Bouillot and Coussieu39) and biliopancreatic bypass with duodenal switch(Reference Wolfe, Schoeller and McCrady-Spitzer34).
Methodology to assess adaptive thermogenesis
A predictive equation was created and used for all the studies, calculating AT by comparing the pREE with a mREE using a statistical approach such as t test or ANOVA. Browning et al. calculated AT by a different approach ((6-monthREEp-baselineREEp)-(6-monthREEm-baselineREEm))(Reference Browning, Rabl and Campos36).
Adaptive thermogenesis
A significant value for AT was reported in four of the six studies(Reference Browning, Rabl and Campos36,Reference Coupaye, Bouillot and Coussieu39) . In two of these studies, AT only remained significant after 6 months, disappearing throughout time(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Tam, Rigas and Heilbronn37) . AT values were slightly lower for those who had gastric band surgery when compared with other surgeries such as sleeve gastrectomy or Roux-en-Y gastric bypass(Reference Tam, Rigas and Heilbronn37). Studies in which participants underwent gastric banding did not report significant values for AT(Reference Browning, Rabl and Campos36,Reference Coupaye, Bouillot and Coussieu39) . Both studies assessed AT by comparing the residuals (i.e. difference between measured REE and estimated based on the predictive equation) at baseline and after WL. A high variability between individuals was highlighted in two studies(Reference Browning, Rabl and Campos36,Reference Carrasco, Papapietro and Csendes38) .
Total daily energy expenditure
A total of five studies reporting changes in TDEE were included in this review(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Marlatt, Redman and Burton50–Reference Novaes Ravelli, Schoeller and Crisp53) , with two randomised controlled trials (40 %) and three prospective observational studies included (60 %) (Table 3).
Table 3 Total daily energy expenditure (TDEE)/24 h energy expenditure (24hEE)
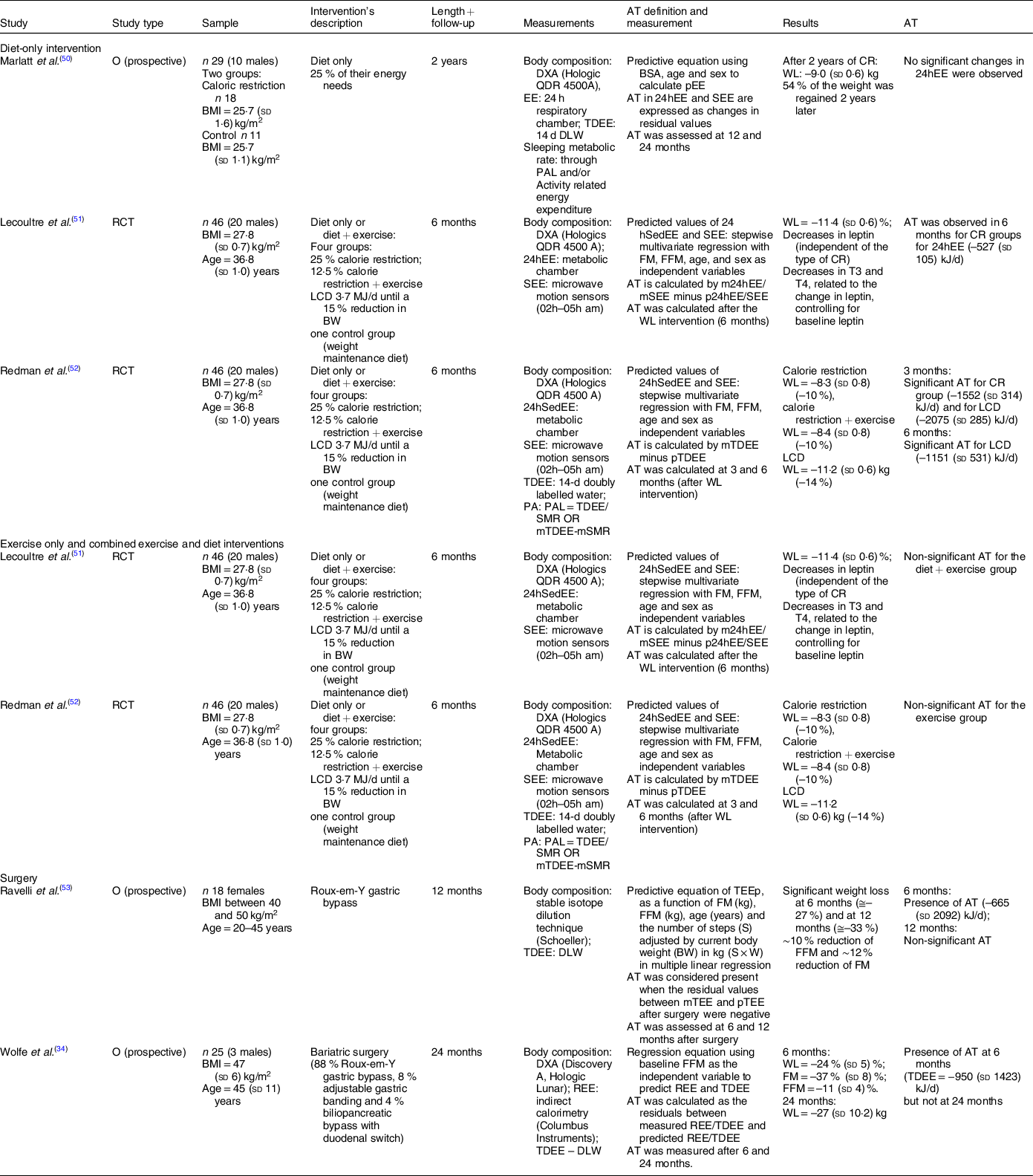
O, observational study; RCT, randomised clinical trial; TDEE, total daily energy expenditure; 24hEE, 24 h energy expenditure; SEE, sleeping energy expenditure; WL, weight loss; CR, caloric restriction; LCD, low calorie diet; FM, fat mass; FFM, fat-free mass.
From those, one was related to a diet-only intervention(Reference Marlatt, Redman and Burton50), two to a diet-only v. a combined diet and exercise intervention(Reference Lecoultre, Ravussin and Redman51,Reference Redman, Heilbronn and Martin52) and two to bariatric surgery(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Novaes Ravelli, Schoeller and Crisp53) . Due to the small number of studies, all intervention types were analysed together.
Participants’ characteristics
The five studies comprised 164 participants (fifty-three males). Participants from the studies related to lifestyle interventions had a BMI ranging from 25 to 30 kg/m2(Reference Marlatt, Redman and Burton50–Reference Redman, Heilbronn and Martin52). For studies that used bariatric surgeries, BMI was above 40 kg/m2(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Novaes Ravelli, Schoeller and Crisp53) . All of the studies reported a WL > 10 %.
Intervention type
Marlatt et al. created a caloric deficit of 25 % based on each participant’s energy needs(Reference Marlatt, Redman and Burton50), while the other two authors used two different approaches: (i) a low calorie diet (˜3·7 MJ/d) until each participant had reached a WL of 15 % of their initial weight or (ii) an individual diet based on individual EI targets(Reference Lecoultre, Ravussin and Redman51,Reference Redman, Heilbronn and Martin52) .
Methodology to assess adaptive thermogenesis
TDEE was assessed by doubly labelled water method(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Redman, Heilbronn and Martin52,Reference Novaes Ravelli, Schoeller and Crisp53) or by a metabolic chamber(Reference Marlatt, Redman and Burton50,Reference Lecoultre, Ravussin and Redman51) . A predictive equation was used to estimate TDEE (pTDEE) and AT was calculated by subtracting pTDEE from mTDEE.
Adaptive thermogenesis
AT was reported in four studies(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Lecoultre, Ravussin and Redman51–Reference Novaes Ravelli, Schoeller and Crisp53) . For lifestyle interventions, Redman et al. reported larger values for AT (˜–1255 to –2092 kJ/d)(Reference Redman, Heilbronn and Martin52), while Lecoultre reported lower values (–527 (sd 105) kJ/d)(Reference Lecoultre, Ravussin and Redman51). Marlatt et al. did not report any significant changes in TDEE(Reference Marlatt, Redman and Burton50). Both studies that used weight reduction surgeries(Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Novaes Ravelli, Schoeller and Crisp53) reported a significant AT after 6 months, but not after 12 months(Reference Novaes Ravelli, Schoeller and Crisp53) or 24 months(Reference Wolfe, Schoeller and McCrady-Spitzer34). Studies which did not find AT had a follow-up period and had similar methodologies to assess it, using a predictive equation with FM and FFM as variables and comparing the residual values.
Sleeping energy expenditure
Only two studies reporting changes in SEE were found(Reference Marlatt, Redman and Burton50,Reference Lecoultre, Ravussin and Redman51) (Table 4). One had a randomised controlled trial design and one was a prospective observational study.
Table 4. Sleeping energy expenditure

O, observational study; RCT, randomised clinical trial; TDEE, total daily energy expenditure; 24hEE, 24 h energy expenditure; SEE, sleeping energy expenditure; WL, weight loss; CR, caloric restriction; LCD, low calorie diet; FM, fat mass; FFM, fat-free mass.
Participants’ characteristics
The two studies comprised seventy-five individuals with a mean BMI between 25 and 30 kg/m2 (thirty males). Both studies reported a WL > 10 %.
Intervention type
Marlatt et al. generated a energy deficit of 25 % based on each participant’s energy needs(Reference Marlatt, Redman and Burton50), while Lecoultre et al. used two different approaches: i) a low calorie diet (˜3·7 MJ/d) until each participant had reached a WL of 15 % of their initial weight or ii) an individual diet based on individual EI targets(Reference Lecoultre, Ravussin and Redman51).
Methodology to assess adaptive thermogenesis
SEE was assessed in a respiratory chamber using microwave motion sensors. A predictive equation was created to estimate SEE (pSEE) and AT was calculated by subtracting pSEE from measured SEE.
Adaptive thermogenesis
Both studies reported significant and similar values for AT in SEE (˜–335 to –377 kJ/d).
Discussion
The aim of this systematic review was to examine whether AT occurs after WL and/or a period of weight stabilisation phase. Overall, significant values for AT were reported in twenty-seven of the thirty-three included studies. Most studies reported a large variability between subjects (e.g. when a standard deviation is higher than the respective mean) with regard to the magnitude of WL and/or AT.
Resting energy expenditure
The majority of the studies aimed to assess AT in REE. From those, twenty-three out of twenty-nine reported a significant value for AT in REE(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Martins, Gower and Hill16–Reference Nymo, Coutinho and Torgersen18,Reference Byrne, Sainsbury and King20–Reference Camps, Verhoef and Westerterp25,Reference Camps, Verhoef and Westerterp27–Reference Bosy-Westphal, Kossel and Goele31,Reference Dulloo and Jacquet33–Reference Bettini, Bordigato and Fabris35,Reference Tam, Rigas and Heilbronn37,Reference Carrasco, Papapietro and Csendes38,Reference Ten Haaf, Verreijen and Memelink47,Reference Marzullo, Minocci and Mele48) .
The reduction in REE after WL occurs mainly due to the losses of FFM and FM(Reference Bosy-Westphal, Kossel and Goele31,Reference Muller, Enderle and Bosy-Westphal42) . Furthermore, it is known that WL is accompanied by hormonal changes such as a decrease in circulating leptin and thyroid hormones, and these changes may contribute to AT(Reference Major, Doucet and Trayhurn11,Reference MacLean, Bergouignan and Cornier54,Reference Rosenbaum, Goldsmith and Haddad55) . Also, other factors may potentially contribute to AT such as changes in sympathetic nervous system activity and concentrations of insulin and catecholamines after WL(Reference Müller, Enderle and Pourhassan22). In this systematic review, some studies reported decreases in leptin(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13,Reference Thom, Dombrowski and Brosnahan17,Reference Gomez-Arbelaez, Crujeiras and Castro19,Reference Müller, Enderle and Pourhassan22,Reference McNeil, Schwartz and Rabasa-Lhoret23,Reference Camps, Verhoef and Westerterp25,Reference Bosy-Westphal, Kossel and Goele31,Reference Hopkins, Gibbons and Caudwell40) and in thyroid hormones(Reference Pourhassan, Bosy-Westphal and Schautz26,Reference Bosy-Westphal, Kossel and Goele31) . The administration of exogenous leptin and triiodothyronine may restore baseline hormone concentrations(Reference Rosenbaum, Goldsmith and Haddad55) and reverse the effects of AT. However, the role of these hormones on AT is still a matter of debate(Reference Müller, Enderle and Pourhassan22) as not all studies observe a relationship.
Intervention’s type and adaptive thermogenesis
Despite surgeries having a higher percentage of WL, they did not necessarily present higher values for AT, when compared with lifestyle interventions. Weight reduction surgeries differed in the degree of AT, with gastric banding being associated with a lower (or non-existent) AT and smaller amounts of WL (˜10–20 %) compared with sleeve gastrectomy and gastric bypass (˜30–40 %). No bariatric surgery’s studies have included assessments of AT in SEE. Although it remains unknown why different surgeries may lead to different magnitudes of AT, its technical procedure could be a potential explanation. In sleeve or gastric bypass surgeries, part of the stomach is removed, while in gastric banding procedures the stomach remains intact, which alter the hormonal responses which may be linked to AT(Reference Beckman, Beckman and Earthman56).
Although the studies performing bariatric surgeries reported the highest amounts of WL, the Biggest Loser’s participants reported similar changes in body weight by creating a large energy deficit(Reference Fothergill, Guo and Howard12,Reference Johannsen, Knuth and Huizenga13) . However, while in bariatric surgeries AT tended to disappear after a period of 6–24 months, on the Biggest Loser’s studies, AT not only remained present but also increased their value after 6 years. However, as some of the participants lost weight on the 2 weeks prior to the 6-year follow-up measurements, the state of energy balance (energy deficit) could have influenced the assessments of AT.
For lifestyle interventions, it is important to consider that different methodologies (macronutrient composition, degree of energy restriction and inclusion of exercise) to achieve a negative energy balance were utilised. Therefore, heterogeneity in the results reported in these lifestyle interventions was to be expected.
Exercise-only studies usually report lower than expected magnitudes of WL mainly due to compensatory increases in EI and decreases in EE(Reference Thomas, Bouchard and Church8). Therefore, there is a lack of exercise-only interventions including both a significant WL and assessments of AT. For this systematic review, only one study was included, which did not report a significant mean AT after a 12-week supervised exercise-only intervention(Reference Hopkins, Gibbons and Caudwell40), potentially explained by the smaller energy deficit.
Despite the large variability among studies, similar AT was found between bariatric surgeries and lifestyle interventions, regardless of total WL.
Relationship between the magnitude of weight loss and adaptive thermogenesis
It has been previously postulated that a relationship between total WL and degree of AT exists(Reference Johannsen, Knuth and Huizenga13,Reference McNeil, Schwartz and Rabasa-Lhoret23) . However, some studies have reported contradictory results(Reference Martins, Gower and Hill16,Reference Muller, Enderle and Bosy-Westphal42) . If a relationship between magnitude of WL and degree of AT existed, it would be plausible that bariatric surgery would lead to a greater AT as total WL is usually larger. However, only Tam et al. reported higher values for AT (> 1255 kJ/d)(Reference Tam, Rigas and Heilbronn37), when compared with lifestyle interventions. Interestingly, despite large WL (˜–20 %), two studies did not report a significant value for AT(Reference Browning, Rabl and Campos36,Reference Coupaye, Bouillot and Coussieu39) . Altogether, the findings from this analysis suggest that the amount of WL is not associated with the magnitude of AT, corroborating the results from previous studies(Reference Martins, Gower and Hill16,Reference Muller, Enderle and Bosy-Westphal42) .
The influence of the state of energy balance on adaptive thermogenesis
An important consideration when examining the presence of AT is to understand the state of energy balance participants are at the time of the measurements. It has been shown that the state of energy balance may be associated with AT(Reference Drummen, Tischmann and Gatta-Cherifi57). Notably, the majority of the included studies who did not report AT (in at least one group) had their participants EE measured under conditions of neutral energy balance (˜70 %)(Reference Müller, Enderle and Pourhassan22,Reference Karl, Roberts and Schaefer24,Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference de Jonge, Bray and Smith29,Reference Doucet, St-Pierre and Alméras32,Reference Wolfe, Schoeller and McCrady-Spitzer34,Reference Coupaye, Bouillot and Coussieu39,Reference Marlatt, Redman and Burton50,Reference Novaes Ravelli, Schoeller and Crisp53) . Furthermore, some studies reported a minimal AT when measurements were taken under conditions of weight stability(Reference Martins, Gower and Hill16,Reference Karl, Roberts and Schaefer24) . For instance, Martins et al. observed AT (˜209–251 kJ/d) after a 4-week weight stabilisation period(Reference Martins, Gower and Hill16). However, it is important to acknowledge that weight stability does not imply the presence of a neutral energy balance, as in the present study participants were under a very low energy-dense ketogenic diet (3·3 MJ/d)(Reference Martins, Gower and Hill16) which deplete glycogen stores. Therefore, participants could be in a negative energy balance and lose body fat while replenishing glycogen stores. Indeed, after 4 weeks of stabilisation, participants had lost an extra 0·8 kg of FM while gaining 0·9 kg of FFM.
Despite the potential influence of the state of energy balance on AT(Reference Drummen, Tischmann and Gatta-Cherifi57), most studies are not clear in reporting whether participants were assessed under similar states of energy balance, which could in part explain the conflicting and heterogeneous results. Therefore, in order to examine whether AT is present after WL, measurements should be conducted under conditions of neutral energy balance.
Methodological issues
The equivocal findings observed between studies may also be reflective of a lack of consistency regarding the definition and methods used to assess AT. In the present literature, the most common method is the use of regression models to predict REE. This method includes the utilisation of a previously validated equation or the development of an equation based on the baseline information from the population included in the study. Then, a comparison between measured and predicted REE is conducted to examine whether these are different. Therefore, examining the existence of AT is strongly dependent on the accuracy of the technique used to measure body composition. The four-compartment models, constructed from combinations of the reference methods(Reference Fuller, Jebb and Laskey58), are considered the gold standard method to assess FM(Reference Smith-Ryan, Mock and Ryan59,Reference Wilson, Mulligan and Fan60) . Since this model combines the use of several techniques, due to the assessment of bone mineral content (by dual-energy X-ray absorptiometry), total body water (isotopes dilution), body weight and body volume (air displacement plethysmography)(Reference Fuller, Jebb and Laskey58), it requires considerable time and cost and only a few studies used it. Therefore, the most common methods used in weight management research are two-compartment models, in which a stable density or hydration of FFM needs to be considered. Since FFM is composed of water, proteins, mineral and glycogen with different densities, any change in its composition during WL will alter the energy density of FFM. During WL, especially during an initial phase, a decrease in N, glycogen and Na leads to a negative water balance which changes the density of FFM, and thus compromising the FM obtained by densitometry methods(Reference Müller and Bosy-Westphal61).
Moreover, it is important to acknowledge that FFM represents a heterogeneous group of tissues with different metabolic rates(Reference Elia62,Reference Müller, Wang and Heymsfield63) . This means that changes in the composition of FFM (losses of high-metabolic rate organs v. skeletal muscle v. body water) may dramatically influence the prediction of REE. Therefore, using two-compartment models to assess FM and FFM presents some limitations for the prediction of REE when comparing individuals before and after WL(Reference Bosy-Westphal, Braun and Schautz64). Interestingly, studies that assessed AT using MRI reported lower or non-significant values for AT(Reference Müller, Enderle and Pourhassan22,Reference Pourhassan, Bosy-Westphal and Schautz26,Reference Bosy-Westphal, Schautz and Lagerpusch28,Reference Bosy-Westphal, Kossel and Goele31) . This could be due to the ability to accurately assess tissue-organ components without relying on assumptions, also allowing to account for the specific metabolic rates associated with each tissue(Reference Elia62,Reference Müller, Wang and Heymsfield63) . Therefore, the most accurate method to examine AT may be the estimation of REE based on the data collected from the MRI and the organ’s specific metabolic rates(Reference Müller and Bosy-Westphal61). However, MRI is not common in clinical practice due to the high time and cost investment(Reference Bosy-Westphal, Braun and Schautz64), being used only in a limited number of studies(Reference Bosy-Westphal, Braun and Schautz64). Overall, the observed variability in AT between studies may be also due to the method used to assess it, as well its assumptions.
Also, it is important to state that AT in REE is generally considered as a greater than predicted decrease in REE after accounting for changes in body composition. However, when it comes to TDEE, AT is usually calculated using a similar method, which could lead to inaccurate calculations as this approach does not account for changes in PA behaviours that could influence EE independently of the presence of AT.
Lastly, comparing weight reduction surgeries, gastric banding seems to be the one associated with the lowest (or non-existent) AT. Although it remains unknown why different surgeries may lead to different magnitudes of AT, its technical procedure could be a potential explanation. This stomach removal in sleeve or gastric bypass surgeries (v. gastric banding procedures) may alter the concentration of hormones related to energy balance regulation or lead to different changes in body composition (different contributions of FM and FFM), and therefore influence AT. Moreover, after these types of surgeries, the digestibility and absorption after a meal are altered(Reference Quercia, Dutia and Kotler65). In fact, nutritional deficits are one of the major long-term complications of bariatric surgery(Reference Damms-Machado, Friedrich and Kramer66,Reference Lefebvre, Letois and Sultan67) . Since the stomach undergoes a short cut, the gut receives less processed food, which may decrease absorption and stimulate defecation(Reference Gregory, Twells and Lester68). Therefore, the metabolisable energy of the food should also be taken into account.
Limitations
There are important limitations that need to be addressed. As expected, a large heterogeneity in the methods used to assess AT was found between studies, which could in part explain the equivocal results. Considering the quality assessment tool, it is important to state that the data included in this review ranged from weak to moderate study designs. Therefore, the need to establish a universal definition and assessment protocol of AT is warranted. Defining how AT is assessed will decrease the risk of bias and strengthen the comparisons between studies.
Recommendations for future studies
Due to the aforementioned limitations, the standardisation of the methods to assess AT is crucial in order to fully understand whether this compensatory response occurs during and/or after WL.
Firstly, a regression equation to predict REE should be created based on the population’s baseline information and it should provide a good fit for the observations. The use of general predictive equations already published should be avoided since they were made using other population’s characteristics. Moreover, apart from precise measurements of FM and FFM, variables such as age and sex may be included as they have been shown to influence REE(Reference Johnstone, Murison and Duncan69). Furthermore, residuals should be calculated before and after WL. If residuals are statistically different from zero at baseline, it means that participants already have a predicted REE different from the measured value. Therefore, residuals at baseline should be taken into account when assessing AT.
Previous research has demonstrated that AT may be associated with the state of energy balance(Reference Drummen, Tischmann and Gatta-Cherifi57). Therefore, measurements of EE should be conducted in a similar state of energy balance. Furthermore, assessing AT in a neutral energy balance condition not only will assure a similar condition to baseline but will also eliminate the potential influence of an acute state of energy deficit. However, it is important to note that neutral energy balance and weight stabilisation are not synonyms. Since an energy deficit will inevitably lead to glycogen depletion, a neutral energy balance post-WL may lead to a short-term weight gain due to increases in water stores. Therefore, a neutral energy balance should be confirmed by not having FM changes during a period of time, although a small increase in FFM may occur. An alternative method to estimate the state of energy balance is to use the ‘intake-balance’ method. Based on changes in energy stores (i.e. changes in body weight(Reference Hall and Chow70) or composition(Reference Racette, Das and Bhapkar71,Reference Shook, Hand and O’Connor72) ), it is possible to estimate the state of energy balance.
Despite AT being reported in twenty-seven out of thirty-three studies, the methodological quality of each study needs to be taken into consideration, since well-designed studies (online Supplementary File 2) reported lower or non-statistically significant values for AT. Furthermore, studies that assessed AT during a period of WL maintenance suggested that its magnitude cannot be a primary driver of weight regain(Reference Martins, Gower and Hill16). In fact, when AT was measured under conditions of weight maintenance, values for AT were found to be reduced or statistically non-significant, comparing to when assessed during conditions of negative energy balance (Table 1). Also, studies comprising bariatric surgeries reported that AT tended to disappear throughout time. On the other hand, studies with poorer methodological designs that measured AT immediately after WL (under conditions of negative energy balance) must be interpreted carefully. Although it remains unknown how much time would be needed to reverse the potential occurrence of AT under conditions of energy deficit, a period of several weeks in a true state of neutral energy balance could be necessary.
Conclusions
AT was found in (at least) one of the EE components in twenty-seven out of thirty-three studies, suggesting that WL may lead to a greater than predicted decrease in EE. Overall, these findings suggest that although WL may lead to AT in some of the energy expenditure components despite a high inter-individual variability, these values may be small or non-significant when higher-quality methodological designs are used. Furthermore, AT seems to be attenuated, or non-existent, after periods of weight stabilisation or neutral energy balance. Therefore, more high-quality studies are warranted not only to disclose the existence of AT in each energy expenditure component but to understand its clinical implications on weight management outcomes.
Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (C. L. N. PhD Scholarship SFRH/BD/143725/2019).
C. L. N. and A. M. S. contributed to the conception and design. C. L. N., N. C. and R. F. contributed to data acquisition, analysis and interpretation. C. L. N. and N. C. wrote the systematic review. A. B., M. H., L. B. S. and A. M. S. revised it critically for important intellectual content. All the authors approved the final version of the manuscript.
The authors reported no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521001094