CVD is the main cause of death in the UK, accounting for nearly 200 000 deaths each year( 1 ). Conventionally, the focus of nutrition research has been on single nutrients and individual dietary components( Reference Hu 2 ); however, recently, nutritional epidemiology has also begun investigations into the effect of overall diet quality, using various dietary pattern approaches, on diet–disease relationships( Reference Azadbakht, Mirmiran and Esmaillzadeh 3 ). It has been hypothesised that using a priori and a posteriori methods that encompass the whole diet may aid in the formulation of dietary recommendations, as overall dietary analysis may be easier to translate into public health messages( Reference Fung, Rimm and Spiegelman 4 , Reference Hu, Rimm and Stampfer 5 – Reference Lazarou and Newby 7 ).
There are two fundamentally different analytical approaches for measuring dietary patterns in epidemiological studies: ‘a priori’ and ‘a posteriori’. A priori techniques evaluate dietary intake data using predefined indices or scoring systems( Reference Hearty and Gibney 8 ) that have been constructed according to existing nutritional guidelines or are based on existing knowledge about diet–disease relationships( Reference Nettleton, Schulze and Jiang 9 ). Application of these a priori scores allows comparability across cohorts as the scoring is not driven by the specific population or dataset in hand( Reference Reedy, Wirfalt and Flood 10 ). There are now numerous a priori dietary scores, but one that is closely related to CVD is the Mediterranean Diet Score (MDS)( Reference Trichopoulou, Costacou and Bamia 11 ). The Mediterranean diet has long been reported to be associated with the attenuation of CVD risk factors, such as decreased total cholesterol, TAG, blood pressure, homeostasis model assessment-estimated insulin resistance (HOMA-IR), BMI and C-reactive protein levels and increased HDL levels, in randomised intervention studies( Reference Esposito, Marfella and Ciotola 12 , Reference Estruch, Martinez-Gonzalez and Corella 13 ), prospective studies (7-year follow-up in the Framingham Heart Study Offspring Cohort)( Reference Rumawas, Meigs and Dwyer 14 ) and in case–control studies( Reference Martinez-Gonzalez, Fernandez-Jarne and Martinez-Losa 15 , Reference Panagiotakos, Pitsavos and Chrysohoou 16 ). Furthermore, adherence to a Mediterranean-style dietary pattern has been reported to be associated with lower CVD mortality in prospective studies with follow-up periods ranging from 3·78 to 20 years( Reference Knoops, de Groot and Kromhout 17 – Reference Fung, Rexrode and Mantzoros 21 ), which has been confirmed in a meta-analysis of prospective studies( Reference Sofi, Cesari and Abbate 22 ).
A posteriori methods are dependent on multivariate statistical techniques such as principal component analysis (PCA). PCA is frequently used in nutritional epidemiology to gain a better understanding of the relationships between the foods consumed in the diet and health-related outcomes. It identifies foods that are frequently consumed together, reducing the data in hand into groups or patterns on the basis of the degree to which they are correlated with one another( Reference Trichopoulos and Lagiou 23 ). For each derived pattern, a score that defines the position of each individual along a gradient is obtained; hence, individuals receive a ‘score’ for each derived pattern( Reference Reedy, Wirfalt and Flood 10 ). PCA is, therefore, a data-driven approach; the preparation of data can be a time-consuming process, and as it relies on clear statistical guidance, it is limited to a research setting. Although PCA-derived dietary patterns are specific to the population from which they are derived, there is some commonality in the nature of the patterns observed between studies; for example, ‘Western-type’ and ‘prudent-type’ dietary patterns are often identified. In terms of CVD, prospective studies within adult populations have generally shown that a prudent/healthy dietary pattern is associated with a lower risk of cardiovascular morbidity and mortality( Reference Centritto, Iacoviello and di Giuseppe 24 – Reference Heidemann, Schulze and Franco 26 ), whereas a Western-type dietary pattern is associated with an increased risk of cardiovascular mortality and morbidity( Reference Hu, Rimm and Stampfer 5 , Reference Centritto, Iacoviello and di Giuseppe 24 , Reference Eilat-Adar, Mete and Nobmann 27 ). Data on the relationship between dietary patterns and cardiovascular risk in younger age groups are lacking. As cardiovascular risk factor status in young adulthood may be associated with coronary atherosclerosis later in life, it is important to determine whether a relationship between dietary patterns and cardiovascular health is apparent in younger age groups to inform public health strategies for CVD( Reference Pletcher, Bibbins-Domingo and Liu 28 ).
Some scientific research has stated that dietary intake patterns are established at an early age and remain relatively unchanged during pubertal development( Reference Clavien, Theintz and Rizzoli 29 , Reference Lake, Mathers and Rugg-Gunn 30 ). On the other hand, it has been proposed by several investigators that significant changes in an individual's eating habits occur during the transition from childhood to adulthood( Reference Cusatis, Chinchilli and Johnson-Rollings 31 – Reference Gallagher, Robson and Livingstone 35 ), and such changes have been attributed to physiological changes in relation to growth and maturation as well as the increasing independence and interaction of adolescents within their social environment( Reference Wang, Bentley and Zhai 36 ), resulting in less desirable changes occurring between adolescence and young adulthood such as increased fast food consumption and binge drinking( Reference Harris, Gordon-Larsen and Chantala 37 ). There is little information available regarding the tracking of dietary patterns during this time frame and whether tracking, or change in, dietary habits during this time has any impact on health status.
Therefore, the present study aimed to investigate the cross-sectional relationship between a posteriori dietary patterns and an a priori dietary score (MDS) and CVD risk biomarkers in a young adult population (Young Hearts (YH)3). Furthermore, it also aimed to examine whether there was a relationship between the change in the MDS and the change in CVD risk biomarkers between YH1 and YH3.
Methods
Study sample
The YH Project was designed as an ongoing study investigating biological and behavioural risk factors for CVD in a representative sample of young people in Northern Ireland. Between 1989 and 1990, a 2 % representative sample (n 1015) of school-aged (12–15 years) children from Northern Ireland was invited to take part in a cross-sectional analysis of lifestyle and health, YH1( Reference Boreham, Savage and Primrose 38 ). Between 1997 and 1999, these participants were invited back to take part in another screening phase (n 487, aged between 20 and 25 years), YH3. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Research Ethics Committee of Queen's University Belfast. Written informed consent was obtained from all participants at both time points. Detailed methodology has been described previously( Reference Gallagher, Robson and Livingstone 35 ), and it is explained in brief below.
Anthropometry
Each participant's height (m) and weight (kg) (and subsequent calculation of BMI (kg/m2)) were measured at both time points. Standing height was measured to the nearest mm using a Harpenden portable stadiometer (Holtain) and body weight was measured to the nearest 0·1 kg using an electronic balance (Seca; 200 kg × 0·1 kg). Waist circumference was measured to the nearest 0·1 cm using a non-stretch tape measure( Reference Gallagher, Robson and Livingstone 35 ).
Blood collection and assessment of biomarkers
At YH1, non-fasting blood samples were drawn from the antecubital vein and separated into serum and plasma within 4 h. Total cholesterol concentration was estimated using an enzymatic technique( Reference Twisk, Boreham and Cran 39 ). HDL concentration was determined using phosphotungstic Mg reagents. No other CVD risk biomarkers were assessed at YH1. At YH3, fasting blood samples (40 ml) were again drawn from the antecubital vein. The samples were immediately separated into plasma, serum and buffy coat before storage in aliquots at − 70°C until analysis. Sample analysis included quantification of plasma glucose and insulin (subsequent calculation of HOMA-IR), cholesterol fractions (total, HDL and LDL), TAG and homocysteine. Further laboratory techniques used in the YH protocol are explained in detail elsewhere( Reference Gallagher, Savage and Murray 40 , Reference Woodside, McMahon and Gallagher 41 ).
Dietary intake assessment
At both study time points, dietary intake information was obtained using the diet history method, consisting of a detailed open-ended one-to-one interview, to determine the habitual weekly (7 d) food intake of each participant. Reported energy and macronutrient intakes were calculated using computerised databases based on UK food composition tables as described previously( Reference Strain, Robson and Livingstone 42 ). There was an indication of under- or over-reporting in 288 participants when the energy intake:BMR ratio was calculated with cut-offs for under-reporting set at < 1·14 and those for over-reporting set at >2·5. To maximise power and for comparability with previous work( Reference Boreham, Savage and Primrose 38 , Reference Strain, Robson and Livingstone 42 ), all participants were included in the analysis. Although data were collected on vitamin supplementation in the YH3 cohort, these data were not included in the final analyses in the present study owing to the fact that use of vitamins and nutritional supplements was erratic in many of the volunteers.
Other assessments
Blood pressure was measured twice from the right arm using a Hawksley random-zero sphygmomanometer (taken to the nearest 2 mmHg; Hawksley & Sons Ltd) in both YH1 and YH3 cohorts. At YH1, smoking status was assessed by means of a recall questionnaire, and at YH3, the participants classified themselves as current cigarette smokers, non-smokers or former smokers. Those who smoked provided information on the number of cigarettes smoked per d. Social class was identified at YH1 using father's occupation and was divided into eight groups: group 1, ‘Professional’; group 2, ‘Management’; group 3, ‘Skilled non-manual’; group 4, ‘Skilled manual’; group 5, ‘Semi-skilled’; group 6, ‘Unskilled’; group 7, ‘Other’; group 8, ‘Student’. Physical activity (PA) was assessed in the YH3 cohort using a modification of the Baecke questionnaire, aimed at assessing habitual work activity, sports activity and non-sports leisure activities. For each of these three categories, a score based on a five-point Likert scale was calculated and summed, giving the total possible score ranging from 3 to 15( Reference Boreham, Robson and Gallagher 33 ). Arterial stiffness was measured in the YH3 cohort using the non-invasive optical method to determine pulse wave velocity (PWV), measured in ms, explained in detail elsewhere( Reference Gallagher, Savage and Murray 40 ).
A posteriori dietary patterns – principal component analysis
The factor analysis procedure in SPSS version 17.0 for Windows (SPSS, Inc.) was used to perform the PCA. Over 1000 individual foods were identified from the diet histories and these foods were manually reduced to thirty-one food groups. Foods were categorised on the basis of food type and macronutrient content. PCA reduced the food groups into a smaller number of underlying factors or dietary patterns that could explain variations in dietary intake; the number of factors, or dietary patterns, retained was based on a combination of food group components with an eigenvalue >1, examination of the break point in the scree plot and interpretability of the factors themselves( Reference Hu, Rimm and Stampfer 5 ). Males and females were analysed together to maximise statistical power. Orthogonal rotation (varimax) was used to produce factors that were uncorrelated and more interpretable( Reference Conway 43 ). When presented, small coefficients were suppressed to absolute values ≥ 0·2. PCA produced uncorrelated factors and a resulting ‘factor score’ for each participant for each of the four factors. Scores were categorised into fifths for each dietary pattern, with the fifth dietary pattern indicating greatest conformity with that particular dietary pattern.
A priori Mediterranean Diet Score
The MDS was originally constructed by Trichopoulou et al. ( Reference Trichopoulou, Kouris-Blazos and Wahlqvist 44 ) and later revised to include fish( Reference Trichopoulou, Costacou and Bamia 11 ). The components that make up the MDS were split into beneficial (high intakes of vegetables, legumes, fruits and nuts, cereals and fish) and less desirable/beneficial (high intakes of meat and meat products and dairy products and ratio of MUFA:SFA) components. Alcohol consumption was also included (a value of 1 was assigned to men if their consumption was between 10 and 50 g/d and to women if their consumption was between 5 and 25 g/d). For all characteristics within the MDS, a cut-off point that corresponded to the median values specific to each sex was applied (except for alcohol as explained above), with a value of 1 being assigned to those above the median value for the beneficial components (0 assigned to those below) and a value of 1 being assigned to those below the median value for the less desirable/beneficial components (0 assigned to those above). A score for each of the 7 d was calculated and an average taken; a score of 9 indicated the strongest adherence to the Mediterranean diet, whereas a score of 0 indicated no adherence to the Mediterranean diet.
Statistical analyses
All statistical analyses were carried out using Statistical Package for Social Sciences (SPSS version 17.0; SPSS for Windows, SPSS, Inc.). For the cross-sectional analysis, fifths were used to categorise individuals for PCA-derived dietary patterns and thirds for the MDS. Continuous variables are presented as means and standard deviations and categorical variables as absolute frequencies and percentages. When examining the general participant characteristics, continuous variables were compared using ANOVA in fifths or thirds groupings. Categorical data were examined using χ2 tests for trends. Multivariable linear regression analysis was used to examine the relationship between dietary patterns (in fifths or thirds) and CVD risk factors while adjusting for other potentially confounding variables. Data are presented for unadjusted and adjusted models controlling for common confounding factors identified from similar studies in the literature including the following: age; BMI; sex; PA; social class; smoking status; energy. As indicated by Northstone et al. ( Reference Northstone, Ness and Emmett 45 ), energy was adjusted at as late a stage as possible to allow assessment of the true effects of energy on dietary patterns. Data for adjusted models (adjusted means and 95 % CI) are given in tables only where adjustment has influenced the corresponding P value.
For the longitudinal analysis, paired-samples t tests were used to summarise the sample characteristics. The MDS was split into ‘most adherent’ and ‘least adherent’ on the basis of the median and was then further categorised into four groups depending on scores at YH1 and YH3: group 1, ‘least adherent MDS at YH1 and least adherent MDS at YH3’; group 2, ‘least adherent MDS at YH1 and most adherent MDS at YH3’; group 3, ‘most adherent MDS at YH1 and least adherent MDS at YH3’; group 4, ‘most adherent MDS at YH1 and most adherent MDS at YH3’. Multivariable regression analysis was then performed to examine the relationship between these four groups and CVD risk biomarkers at YH3; different models of adjustments were applied: model 1 adjusted for age, sex, BMI, social class, PA, and smoking status and model 2 further adjusted for energy as per the method of Northstone et al. ( Reference Northstone, Ness and Emmett 45 ). It has been reported that all measures of adiposity are highly correlated( Reference Retnakaran, Hanley and Connelly 46 ); therefore, in the present study, BMI was chosen as the sole measure of adiposity within the adjusted model because BMI has been indicated as the most commonly used measure of adiposity in national and international obesity prevalence statistics and is therefore useful for analysing historical trends and comparing international research results( 47 ). The change in CVD risk biomarkers (total cholesterol, HDL, SBP and DBP) between YH1 and YH3 was calculated and the four groups of change in the MDS were again assessed using multivariable regression analysis. At YH3, values for TAG and homocysteine concentrations were negatively skewed, and natural log transformation of the data did not affect the end interpretation; therefore, skewed data are presented to maintain continuity.
Finally, the proportion of tracking for the MDS was assessed for males and females separately and together. The proportion of volunteers who remained in the same third for the a priori dietary score suggests tracking; however, 33 % of the volunteers would remain in the same third over time if one assumes that he or she could move randomly into any of the thirds at follow-up( Reference Li and Wang 48 ). Weighted k values were then calculated to measure the agreement between an individual's relative position at YH1 and YH3 by considering disagreement close to the diagonal less heavily than disagreement further away from the diagonal( Reference Wang, Ge and Popkin 49 ). It should be noted that κ = 0 when the observed agreement equals that expected by chance and κ = 1 when tracking is perfect( Reference Landis and Koch 50 ).
Results
Mean dietary, anthropometric and cardiovascular-related variables at YH1 and YH3 are given in Table 1. As expected, height, weight and BMI of both males and females (and the cohort as a whole) increased significantly (P< 0·001) from YH1 to YH3. The number of smokers also increased significantly within this time period, i.e. between the mean ages of 14 and 23 years (P< 0·001, males; P= 0·002, females; and P< 0·001, all). Total cholesterol concentration did not change significantly from YH1 to YH3, but HDL concentration decreased between the two time points in males (P< 0·001) and increased in females (P< 0·001); however, when assessed together, no significant difference was apparent for HDL concentration (P= 0·774). SBP and DBP increased significantly from YH1 to YH3 (P< 0·001 for males and females and when assessed together for both SBP and DBP). Dietary components listed in Table 1 are expressed per 4·184 kJ to adjust for differences in energy intake( Reference Nicklas, Farris and Smoak 51 ). Total energy intake decreased significantly between adolescence and young adulthood in females (P= 0·024) but not in males or when the cohort was examined as a whole. Energy-adjusted total fat intake (expressed per 4·184 kJ) decreased significantly in males and in the cohort as a whole (P= 0·009 and P= 0·010, respectively), but did not change significantly in females. Energy-adjusted fibre intake (expressed per 4·184 kJ) decreased significantly in males, females and the cohort as a whole (P< 0·001) between YH1 and YH3. Both males and females and the cohort as a whole displayed a significantly lower MDS at YH3 than at YH1 (P= 0·019, males; P= 0·004, females; and P< 0·001, all).
Table 1 General health and lifestyle characteristics and dietary intake of males and females who participated in Young Hearts (YH)1 and YH3 (Mean values and standard deviations)

SBP, systolic blood pressure; DBP, diastolic blood pressure; MDS, Mediterranean Diet Score.
* For each sex, differences between variables measured at YH1 and those measured at YH3 were assessed using paired-samples t tests and categorical data were assessed using McNemar's test.
Young Hearts 3 dietary patterns
Factor loadings, which represent correlation coefficients between food groups and dietary patterns, for the derived dietary patterns are given in Table 2. A total of four dietary patterns were identified: ‘drinker/social’; ‘healthy’; ‘Western’; ‘sweet tooth’, and, together, they explained 29·5 % of the variance. Dietary patterns were named according to the food groups loading highest in each of the four dietary patterns. Factor 1 – drinker/social was characterised by the highest factor loadings for white bread, alcohol, fats and meat dishes. Factor 2 – healthy was characterised by high factor loadings for fruits, vegetables and brown bread. Factor 3 – Western was characterised by high factor loadings for soft drinks, crisps and chips. Lastly, factor 4 – sweet tooth was characterised by the highest factor loadings for puddings, chocolates and confectionery.
Table 2 Factor loading* matrix for Young Hearts 3 (n 487) participants
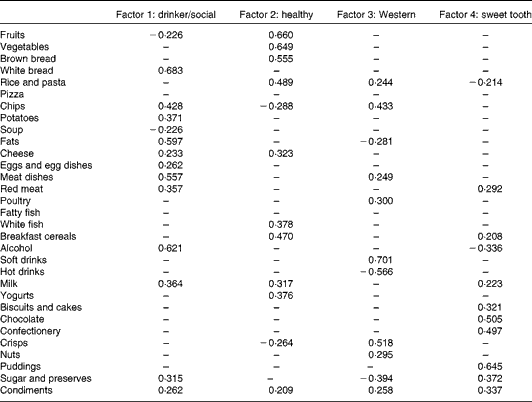
* Only food groups with factors loadings >0·2 were included.
More males than females were in the highest fifth for the drinker/social dietary pattern (P< 0·001) and the Western dietary pattern (P= 0·003). Those with the highest drinker/social score had greater weight (P< 0·001), height (P< 0·001) and waist circumference (P< 0·001) and tended to be smokers (P= 0·002). Participants with a higher healthy score had a higher PA score (P< 0·001), a higher socio-economic status (P< 0·001) and were less likely to be current smokers or to have ever smoked (P< 0·001). Higher Western scores were characterised by increased weight (P= 0·029) and waist circumference (P= 0·009). Higher sweet tooth scores were characterised by a lower BMI (P= 0·037) and also a trend of fewer current smokers and more people who had never smoked (P= 0·002). Participants with a high MDS had higher PA scores (P= 0·002). P values for categorical variables were calculated using unadjusted χ2 test, whereas P values for continuous variables were calculated using linear regression analysis.
Cross-sectional relationships between a posteriori dietary patterns and CVD risk factors at Young Hearts 3
The associations between the various CVD risk biomarkers and the PCA-derived dietary patterns are summarised in Table 3. In general, cardiovascular risk factors were significantly related to the four dietary patterns in similar ways when partially adjusted (for age, sex, BMI, socio-economic status, PA and smoking status; data not shown) than when fully adjusted (i.e. further adjusted for energy intake); thus, only the fully adjusted data are given in the table. After full adjustment, differences in CVD risk factors across dietary patterns were as follows: participants with a higher drinker/social score had lower LDL and homocysteine concentrations and exhibited a trend for higher TAG concentrations of borderline significance. A high healthy dietary pattern score was significantly inversely associated with PWV and homocysteine concentrations. A high Western dietary pattern score was positively associated with HDL-cholesterol concentrations, SBP, DBP and homocysteine concentrations. The fourth and final PCA-derived dietary pattern, sweet tooth, was positively associated with LDL concentrations, SBP, and DBP and inversely associated with HDL concentrations.
Table 3 CVD risk factors for the five groups of four dietary patterns determined by a posteriori principal component analysis in men and women who participated in Young Hearts 3 (Mean values and standard deviations; adjusted mean values and 95 % confidence intervals)

SBP, systolic blood pressure; DBP, diastolic blood pressure; PWV, pulse wave velocity; HOMA, homeostasis model assessment.
* Data were analysed using linear regression analysis (unadjusted, adjusted and further adjusted as described with CVD risk factors as the outcome and dietary patterns in quintiles as a continuous variable).
† Fully adjusted for age (years), sex, BMI (kg/m2), social status, physical activity, smoking status and energy (kJ).
Cross-sectional relationships between a priori Mediterranean Diet Score and CVD risk factors at Young Hearts 3
The means and standard deviations (and adjusted difference in the mean and 95 % CI) of the same CVD risk biomarkers at YH3 across tertiles of the MDS calculated at YH3 are given in Table 4. In the partially adjusted model, the MDS displayed a significant inverse relationship with PWV (data not shown for partially adjusted model).
Table 4 CVD risk factors for the three groups using the Mediterranean Diet Score (MDS) in men and women who participated in Young Hearts 3 (Mean values and standard deviations; adjusted mean values and 95 % confidence intervals)
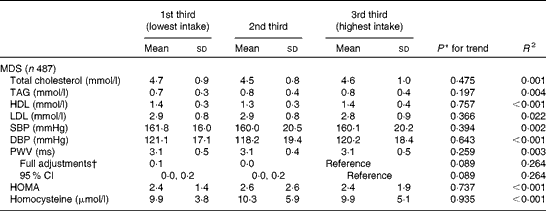
SBP, systolic blood pressure; DBP, diastolic blood pressure; PWV, pulse wave velocity; HOMA, homeostasis model assessment.
* Data were analysed using linear regression analysis (unadjusted, adjusted and further adjusted as described with CVD risk factors as the outcome and dietary patterns in tertiles as a continuous variable).
† Fully adjusted for age (years), sex, BMI (kg/m2), social status, physical activity, smoking status and energy (kJ).
Longitudinal change in the Mediterranean Diet Score and CVD risk factors between Young Hearts 1 and Young Hearts 3
Means for the MDS were as follows: YH1, 4·1 (sd 1·5, range 0·0–9·0); YH3, 3·7 (sd 0·9, range 0·8–6·1). As has been discussed previously, each participant was categorised as having a ‘most adherent’ or ‘least adherent’ MDS at YH1 and YH3, and four categories were then constructed to summarise the change in the MDS between YH1 and YH3. The majority of individuals were in group 1 – ‘least adherent MDS at YH1 and least adherent MDS at YH3’ (56·7 %), followed by those in group 2 – ‘least adherent MDS at YH1 and most adherent MDS at YH3’ (25·5 %), group 3 – ‘most adherent MDS at YH1 and most adherent MDS at YH3’ (9·0 %), and finally group 4 – ‘most adherent MDS at YH1 and least adherent MDS at YH3’ (8·8 %). Table 5 summarises the association between the change in the MDS from YH1 to YH3 and the change in CVD risk biomarkers at YH3. A significant association was observed for serum homocysteine concentrations after adjustment for age, sex, BMI, social class, PA and smoking status (data not shown), and this result was maintained when further adjusted for energy (data given in Table 5 when a significant result was indicated). Serum homocysteine concentration was highest in the group with ‘a most adherent MDS at YH1 and a least adherent MDS at YH3’ and lowest in the group with ‘a least adherent MDS at YH1 and a most adherent MDS at YH3’. No other significant results were obtained.
Table 5 Change in the Mediterranean Diet Score (MDS) from Young Hearts (YH)1 to YH3 and CVD risk biomarkers at young adulthood (YH3) (Mean values and standard deviations; adjusted mean values and 95 % confidence intervals)

SBP, systolic blood pressure; DBP, diastolic blood pressure; PWV, pulse wave velocity; HOMA, homeostasis model assessment.
* Data were analysed using multivariable regression analysis (unadjusted, adjusted and further adjusted as described).
† Fully adjusted for age (years), sex, BMI (kg/m2), social status, physical activity, smoking status and energy (kJ).
Table 6 summarises the association between the change in the MDS from YH1 to YH3 and the change in CVD risk biomarkers across the same period. No statistically significant association between the change in the MDS and the change in CVD risk biomarkers was found in unadjusted or adjusted analyses (data not shown).
Table 6 Change in the Mediterranean Diet Score (MDS) from Young Hearts (YH)1 to YH3 and change in CVD risk biomarkers from YH1 to YH3 (Mean values and standard deviations)
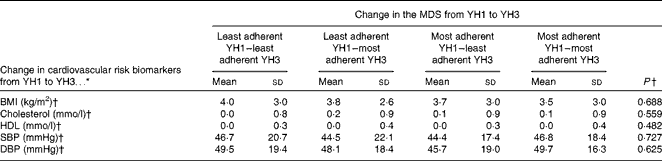
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Change calculated as YH3 values minus YH1 values.
† Data were analysed using multivariable regression analysis (unadjusted, adjusted and further adjusted as described).
Table 7 summarises the tracking patterns of the MDS from YH1 to YH3. No tracking was identified for the MDS from YH1 to YH3.
Table 7 Tracking patterns of the Mediterranean Diet Score between Young Hearts (YH)1 and YH3* (Proportions and 95 % confidence intervals)
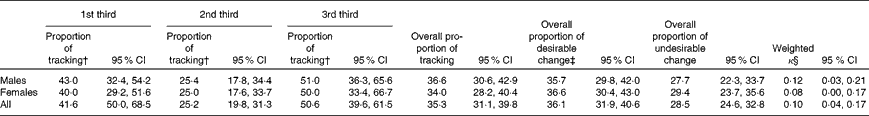
* n 487 (males n 249 and females n 238).
† Tracking by chance (random was 33 %).
‡ Desirable change was defined as a positive change in the a priori dietary score from YH1 to YH3, i.e. 1st third at YH1 to 2nd third or 3rd third at YH3. Undesirable change was defined as a change in the opposite direction, i.e. 2nd third at YH1 to 1st third at YH3.
§ Weighted κ measured the agreement based on the third positions; k>0·1 indicates no tracking.
The analyses summarised in Tables 3–7 were repeated following removal of the bottom 10 % of under-reporters (based on the ratio of energy intake:BMR) in the sample. This did not affect the results (data not shown).
Discussion
Cross-sectional relationships between a posteriori dietary patterns, Mediterranean Diet Score and CVD
The inverse association observed between a healthy dietary pattern (a similar relationship was also observed for the MDS) and PWV in the present study is a new and important finding within this age group. Long-term elevated PWV is directly related to the risk of developing CVD( Reference Keogh, Grieger and Noakes 52 ). Little is known about the relationship between dietary intake as assessed by PCA-derived dietary patterns and arterial structure and function, as assessed by PWV, but some evidence is emerging relating dietary intake and arterial stiffness( Reference Kesse-Guyot, Vergnaud and Fezeu 53 ). A systematic review supports the evidence that strong adherence to a Mediterranean-style dietary pattern in dietary interventions is associated with significant improvements in endothelial function( Reference Serra-Majem, Roman and Estruch 54 ). The application of dietary indices such as the MDS to studies in younger populations is increasing, but has mostly been limited to examining relationships with anthropometric measures. However, in a recent study carried out in Greece( Reference Lydakis, Stefanaki and Stefanaki 55 ), adherence to the Mediterranean diet was found to be negatively correlated with arterial stiffness, independent of obesity, in 12-year-old children.
The role of homocysteine as an independent risk factor for CVD remains controversial; however, it has been proposed that it may be involved in both inflammatory and endothelial function pathways( Reference Nettleton, Steffen and Mayer-Davis 56 ) and has been shown, in a preliminary study, to be an independent risk factor for cardiovascular mortality in older people( Reference de Ruijter, Westendorp and Assendelft 57 ). The inverse relationship between homocysteine and vitamin B intake and status is well established( 58 ), but its relationship with dietary patterns in general is less explored( Reference Fung, Rimm and Spiegelman 4 , Reference Appel, Miller and Jee 59 ). In the present study, homocysteine was the biomarker that was found to be most often associated with the a posteriori dietary patterns (being associated with three out of the four PCA patterns), and these associations were generally in the expected directions. Similar relationships have been reported in studies in adult populations in the literature for broadly ‘healthy’ and ‘Western/refined’ dietary patterns( Reference Fung, Rimm and Spiegelman 4 , Reference Nettleton, Steffen and Mayer-Davis 56 , Reference Gao, Yao and McCrory 60 – Reference Yakub, Iqbal and Iqbal 62 ). The present study, however, is one of the first studies to report that such relationships between dietary patterns and homocysteine are already evident in younger adults.
In relation to other prominent associations between CVD risk biomarkers and dietary patterns, lipids were also found to display some notable associations in the present study. The ‘sweet tooth’ dietary pattern was found to be associated with an adverse lipid profile (increased LDL concentrations and decreased HDL concentrations), and this has also been reported by Newby et al. ( Reference Newby, Muller and Tucker 63 ) in the Baltimore Longitudinal Study of Aging and Eilat-Adar et al. ( Reference Eilat-Adar, Mete and Nobmann 27 ) in Alaskan Eskimos (mean age 41·7 years). This has not been reported previously in younger populations. This may be because the foods that loaded most highly within the ‘sweet tooth’ dietary pattern (namely puddings and chocolate) are high in fat, particularly saturated fat, as well as sugar, and therefore have the potential to alter plasma lipid concentrations. Again, similar to the findings reported herein, another study has reported that a Western dietary pattern is positively associated with HDL( Reference Fung, Rimm and Spiegelman 4 ). It has been proposed that the high total fat content associated with a Western dietary pattern may be responsible for increased HDL concentrations. Recent research has indicated that adverse lipid concentrations in young adulthood are associated with coronary atherosclerosis 20 years later and, therefore, Pletcher et al. ( Reference Pletcher, Bibbins-Domingo and Liu 28 ) highlighted that more emphasis should be placed on the reduction of LDL concentrations and the elevation of HDL concentrations in younger age populations.
Comparison of dietary pattern approaches
There is limited scientific evidence directly comparing a priori and a posteriori approaches in terms of their association with health parameters or ability to predict chronic disease development. To our knowledge, nine studies( Reference Reedy, Wirfalt and Flood 10 , Reference Osler, Heitmann and Gerdes 64 – Reference Chan, Chan and Woo 71 ) to date, all conducted in adult populations, have examined both a priori and a posteriori dietary patterns. Among the cross-sectional and case–control studies( Reference Fung, Hu and Barbieri 67 , Reference Miller, Lazarus and Lesko 69 , Reference Kastorini, Papadakis and Milionis 70 ), one cross-sectional study reported that the a priori method was superior at assessing sex hormone concentrations( Reference Fung, Hu and Barbieri 67 ) compared with PCA-derived dietary patterns and both case–control studies reported similar results for both methods in relation to colorectal cancer risk( Reference Miller, Lazarus and Lesko 69 ) and classification of acute coronary syndrome and stroke outcomes( Reference Kastorini, Papadakis and Milionis 70 ). Of the six prospective studies comparing a priori dietary scores with a posteriori dietary patterns, three indicated that both methods had a similar ability to predict disease development or mortality( Reference Reedy, Wirfalt and Flood 10 , Reference Kant, Graubard and Schatzkin 66 , Reference Panagiotakos, Pitsavos and Stefanadis 68 ), one study indicated that the a posteriori method was superior at assessing all-cause and CV mortality( Reference Osler, Heitmann and Gerdes 64 ), one study found that the a priori MDS was associated with stroke risk, but no associations were evident for a posteriori dietary patterns( Reference Chan, Chan and Woo 71 ), and one study found that neither a priori MDS nor a posteriori dietary patterns were associated with the risk of hypertension( Reference Schulze, Hoffmann and Kroke 65 ). In the present study, more relationships with CVD risk biomarkers were evident for the PCA-derived dietary patterns than for the MDS. However, the examination of the relationship between the MDS and cardiovascular risk factors was limited by the range of MDS achieved in this Northern European cohort (only reaching a maximum of 6 points out of 9 points on the MDS scale). Thus, a posteriori methods may be advantageous in some situations. A priori and a posteriori approaches are, by their definitions, designed to answer different questions: a posteriori analysis investigates what is accounting for the variation in intakes and how well these variances are related to risk, whereas a priori index-based dietary analysis investigates whether or not variation from a predefined diet is related to risk( Reference Reedy, Wirfalt and Flood 10 ). Thus, the value of using both approaches in tandem in epidemiological research warrants further consideration as application of both may yield a more complete picture of the complex relationship between dietary exposure and disease status.
Longitudinal relationship between the Mediterranean Diet Score and CVD
It has been proposed that diet and lifestyle in childhood and adolescence may have a lifelong impact on the risk of chronic diseases, such as hypertension, diabetes, certain cancers and CVD( Reference Williams, Chairman and Hayman 72 ). Longitudinal studies investigating the stability of whole dietary intake and subsequent effect, if any, on CVD risk biomarkers are well placed to enhance understanding of this issue and, so, to aid public health planning and priority setting in relation to diet and health. There is controversy within the current literature about the extent to which dietary intake tracks from childhood to adolescence and into adulthood. There are a number of studies that have reported that tracking of dietary intake exists. However, it must be noted that methods of tracking, including the follow-up time and analysis, have been inconsistent within the studies. The majority of the positive literature has focused on the assessment of dietary intake tracking between adolescence and adulthood over an average of 20·6 years( Reference Lake, Mathers and Rugg-Gunn 30 , Reference Bertheke Post, de Vente and Kemper 73 – Reference Welten, Kemper and Post 76 ) and between childhood and adolescence, with an average follow-up period of 6·5 years( Reference Clavien, Theintz and Rizzoli 29 , Reference Wang, Bentley and Zhai 36 , Reference Patterson, Warnberg and Kearney 77 , Reference Kelder, Perry and Klepp 78 ).
Research has also been conducted between adolescence and young adulthood, similar to the age groups in the present study. A study conducted by Patterson et al. ( Reference Patterson, Warnberg and Kearney 77 ) reported evidence of slight dietary intake tracking for food groups and micro- and macronutrients over a 6-year period. The data used were obtained from the European Youth Hearts Study (EYHS I and EYHS II), which was similar to the present study in terms of participants’ age and follow-up period. However, it was clear that many of the participants of the EYHS I and EYHS II did not remain in the same third of dietary intake, and therefore their behaviours in both childhood and adolescence were potentially modifiable( Reference Patterson, Warnberg and Kearney 77 ); this is in agreement with the observation made in the present study that the YH participants did not remain within the same third of dietary intake from YH1 to YH3 for the MDS. The longitudinal analysis focused on the MDS at YH1 and YH3 and the longitudinal relationship between diet and CVD risk biomarkers. The MDS decreased significantly between the two time points (for males and females and the cohort as a whole); that is, the participants did not maintain their ranking. Longitudinal assessment of dietary intake between the two time points did not, in general, indicate an association between the change in a priori dietary score and the change in CVD risk biomarkers at YH3 or the change in risk biomarkers between adolescence and young adulthood.
Not all such longitudinal studies have, however, provided evidence of tracking. Studies reporting considerable changes in eating behaviour between childhood and adolescence have attributed these to physiological changes as well as increasing independence and peer influence( Reference Wang, Bentley and Zhai 36 ). There are a number of studies that support the findings from the present study that dietary intake is not stable. Previous research on the YH cohorts has indicated changes in micro- and macronutrient intakes between adolescence and young adulthood( Reference Boreham, Robson and Gallagher 33 – Reference Gallagher, Robson and Livingstone 35 ). Bertheke Post et al. ( Reference Bertheke Post, de Vente and Kemper 73 ) also indicated that dietary intake was not stable during adolescence as defined by groups of foods and nutrients even though their long-term investigations from childhood to adulthood and their reports on micro- and macronutrient intakes indicated low-to-moderate tracking.
In summary, the present study does not, in general, indicate tracking of the MDS or a longitudinal relationship between the MDS and CVD risk factors. Although it would have been encouraging in some respects to have found tracking of dietary intake between adolescence and young adulthood, it is perhaps not surprising that this was not the case. Many significant life changes that could affect dietary intake take place in the decade between adolescence and young adulthood. Most notably, alongside the major physical changes that occur, increased independence and autonomy will have a significant impact on food choice( Reference Fitzgerald, Heary and Nixon 79 ). The idea that dietary patterns may track from childhood/adolescence to adulthood, omitting the transitional period when dietary patterns may be particularly erratic, has not been fully investigated. It is possible that although adolescence and young adulthood are periods of substantial change, individuals may eventually revert back, later in adulthood, to dietary patterns learnt at a younger age. This idea has been insinuated by Bertheke Post et al. ( Reference Bertheke Post, de Vente and Kemper 73 ), who reported that although there was moderate stability over time (between 13 and 33 years), dietary intake was changeable and open to influence during the late adolescence/young adulthood phase. It was somewhat unexpected that changes in diet, i.e. those whose diet effectively worsened between the two time points, were not correlated with changes in cardiovascular risk biomarkers over time. There are some potential explanations for this: the typical CVD risk biomarkers assessed may not have developed into clinically significant values by such a young age in life and the stability of total cholesterol and HDL-cholesterol from adolescence to adulthood may be low( Reference Twisk, Kemper and Mellenbergh 80 , Reference Twisk, Kemper and van Mechelen 81 ). Also a period of 10 years, commencing at the early life stages, may well be too short to examine changes in cardiovascular risk biomarkers given the slow evolution of atherosclerosis.
Strengths/limitations
As with all studies, there are a number of limitations that must be recognised. The PCA has limitations associated with its execution, which have been discussed by others( Reference Martinez, Marshall and Sechrest 82 – Reference Schulze, Hoffmann and Kroke 85 ). What is encouraging is that the dietary patterns that have been identified in the present study (drinker/social, healthy, Western and sweet tooth) have also been identified by others and appear to be reasonably reproducible among different populations( Reference Newby, Muller and Hallfrisch 86 ). Another factor to consider is that knowledge regarding the validity of dietary assessment techniques in producing PCA-derived dietary patterns is limited. Dietary pattern analysis has mainly relied on FFQ as the original data source, and Hu et al. ( Reference Hu, Rimm and Smith-Warner 83 ) have reported that FFQ are valid tools for dietary intake assessment in terms of the reproducibility of dietary patterns. In the present study, diet history information was used for the dietary pattern analysis, as done previously by Slattery et al. ( Reference Slattery, Boucher and Caan 87 ). Although the diet history method is robust and well respected in nutritional epidemiology, it has been infrequently used in dietary pattern analysis. This method is also subjective and, therefore, may be particularly vulnerable to exaggeration of perceived ‘healthy’ foods and underestimation of perceived ‘unhealthy’ foods. The inclusion of over- and under-reports may have had an impact on the results presented, for example, precluding the detection of significant associations if some foods were systematically under-reported and therefore not represented within the dietary patterns. However, it must be noted that the reproducibility of the diet history method has been investigated and confirmed by van Staveren et al. ( Reference van Staveren, de Boer and Burema 88 ). Longitudinal studies of dietary intake are also subject to changes in food availability, food consumption patterns and food promotion( Reference Sempos, Flegal and Johnson 89 ); such changes are impossible to predict or calculate. The assessment of dietary intake using the MDS may also introduce error into these investigations. Median cut-offs were applied in the assessment of dietary intake using the MDS and therefore an element of a posteriori assessment was introduced. The study population was from Northern Europe, which may explain why the full spread of the MDS was not obtained for the young adult participants at YH3; however, the full spread was achieved for the adolescent participants at YH1 possibly due to parental influence. The YH1 cohort was a 2 % representative sample of the whole of Northern Ireland (age specific), and although the follow-up at YH3 was only about a 50 % response rate, the characteristics of those who responded at YH3 did not differ markedly from those who did not respond. The strengths of the present study include the wealth of information collected at both time points, which enabled the adjustment for a large number of confounding variables, giving even more strength to the results. However, it must be noted that non-fasting blood samples were obtained at YH1 and fasting blood samples were obtained at YH3. However, previous research has indicated that there are no clinically significant differences between fasting and non-fasting lipid measurements( Reference Craig, McNeill and Macdiarmid 90 ). All other data collection methodologies were standardised between the two time points to allow for accurate comparison and conclusions to be drawn. It must also be recognised that investigations that involve two time points are by default linear, and it is therefore impossible to determine the form of potential change over time( Reference Rogosa and Gottman 91 ).
Conclusion
Dietary pattern analysis, using a posteriori and a priori techniques, revealed that some associations with CVD risk biomarkers observed in older populations were already evident in the young adult population. One new observation in the young adult population was an inverse association between a PCA-derived healthy dietary pattern and PWV. A longitudinal association between a priori dietary score and CVD risk biomarkers was not evident in this sample, and nor was there evidence of tracking of the MDS between adolescence and young adulthood. Given the natural evolution of atherosclerosis, longer-term follow-up may be required to fully explore and reveal any such associations. Even though the MDS did not track strongly between these two life stages in this sample, the associations between dietary patterns and CVD risk biomarkers that were evident in young adulthood indicate that it is still prudent to try to encourage good dietary habits early in life to maximise chances of maintaining as healthy a diet as possible throughout life.
Acknowledgements
The authors thank all the study participants for their time, interest, co-operation and contribution.
The Northern Ireland YH1 Project was supported by the Northern Ireland Chest, Heart and Stroke Association and the Department of Health and Social Services (Northern Ireland). The YH3 Project was funded by the Wellcome Trust and the British Heart Foundation.
The authors’ contributions are as follows: L. J. M., A. M. G. and C. A. B. were primarily responsible for the conception of the project, development of the overall research plan and overseeing the study; A. M. G. and C. E. N. conducted the dietary intake assessment and collected the data; H. J. M., C. R. D. and M. C. M. prepared the dataset for dietary pattern assessment, carried out the statistical analyses and interpreted the resultant data; C. R. C. provided advice on statistical analyses and assisted with the interpretation of data; H. J. M. and M. C. M. drafted the manuscript and all the other authors contributed to the editing and proof reading of the final version of the article; M. C. M. had the primary responsibility for the final content.
The members of the YH Study Group are as follows: Liam J. Murray; Maurice J. Savage; Ian S. Young; J. J. Strain; Alison M. Gallagher; Gordon Cran; Paula J. Robson; Jayne V. Woodside.
None of the authors has any conflicts of interest to declare.









