The term ‘benign breast diseases’ (BBD) is applied to describe a wide range of non-cancerous breast diseases. These diseases are classified into three groups based on their influence on breast cancer risk: (1) non-proliferative diseases, which are not associated with breast cancer risk; (2) proliferative diseases without atypia, which stimulate the growth of lobular and ductal cells in breast tissue( Reference Hunt, Green and Buchholz 1 ) and which increase the breast cancer risk by 1·3–1·9 times( Reference Hartmann, Sellers and Frost 2 ); and (3) proliferative diseases with atypia such as atypical ductal and lobular hyperplasia( Reference Hunt, Green and Buchholz 1 (. The risk of breast cancer is 3·7–4·2 times higher in the third group( Reference Hartmann, Sellers and Frost 2 ). Although BBD cases constitute >90 % of reasons for attending breast clinics( Reference Foulkes and Gateley 3 ), these diseases are usually disregarded in comparison with breast cancer( Reference Courtillot, Plu-Bureau and Binart 4 ). In autopsy studies, it is estimated that one in two women will be diagnosed with some grades of cystic fibrosis, and one in five will suffer from fibroadenoma in their lifetime. The role of endogenous hormones, oral contraceptive and oestrogen replacement therapy in the development of BBD entails further investigation. Obesity is established as the sole protective factor, although it is not recommended because of adverse effects. No relationship has been established between the age at menarche, smoking, alcoholic drinks and caffeine consumption with BBD( Reference Goehring and Morabia 5 ). Several studies have been undertaken to assess the association between food, macronutrients and micronutrients with the risk of BBD( Reference Goehring and Morabia 5 – Reference Frazier and Rosenberg 17 ). Regarding the association of food intake and BBD, some controversial results are reported and no specific dietary pattern has been yet proposed( Reference Goehring and Morabia 5 ). Ingram et al.( Reference Ingram, Nottage and Roberts 10 ) found the consumption of red meats, savoury dishes (pizza, pie and stew) and starches to be harmful, whereas eating poultry, fish and fruits is beneficial. Fruits and vegetables are suggested to protect against BBD( Reference Galvan-Portillo, Anchez and Lopez-Carrillo 8 , Reference Hislop, Band and Deschamps 9 , Reference Wu, Ray and Lin 16 ). In the Women’s Health Initiative (WHI) trial, a low-fat dietary pattern with reduced fat intake and increased fruit and vegetable consumption did not alter the progression of BBD( Reference Rohan, Negassa and Caan 15 ). The dietary patterns have grabbed the attention of researchers during the past decade( Reference Wu, Mimi and Tseng 18 , Reference Buck, Vrieling and Flesch-Janys 19 ), because of the involvement of the interactions of dietary components and synergic effects of food ingredients( Reference Edefonti, Randi and La Vecchia 20 – Reference Männistö, Dixon and Balder 23 ). The use of dietary patterns could better reflect the nutritional behaviour in individuals( Reference Sieri, Krogh and Pala 24 , Reference Cottet, Touvier and Fournier 25 ). Moreover, dietary patterns are more appropriate tools for public health nutrition recommendations( Reference Edefonti, Randi and La Vecchia 20 , Reference Fung, Hu and Holmes 22 , Reference Männistö, Dixon and Balder 23 , Reference Cottet, Touvier and Fournier 25 ). So far, no study has evaluated the relationship between the dietary patterns and BBD morbidity. Because of the high prevalence of these disorders in women( Reference Goehring and Morabia 5 ) and the risk of cancer development among them( Reference Hartmann, Sellers and Frost 2 ), we aimed to investigate the relationship between dietary patterns and BBD.
Methods
Study population
The present study is part of a case–control research project on women diagnosed with breast cancer and BBD, as well as healthy individuals attending the Iranian Center for Breast Cancer affiliated with Academic Center for Education, Culture and Research (ACECR) in Tehran, from February 2014 to April 2015. The aim of the project was to compare the dietary patterns of patients with breast cancer and BBD with healthy women.
The sample size was calculated using an α of 0·05 and a power of 80 %, detecting a minimum significant outcome measure OR of 2·5. About eighty subjects were estimated for each of the three groups, and in practice eighty patients with breast cancer, ninety-six patients with BBD and seventy healthy women were recruited. As healthy subjects were not easily available, for maintaining the power of study, we increased the number of BBD patients to ninety-six. In the present study, we compared the dietary patterns in two groups of BBD patients and healthy women.
The cases were older than 20 years, diagnosed with BBD (including fibrocystic diseases, ductal ectasia, fat necrosis, papillomatosis, adenosis with and without sclerosing, fibroadenoma, ductal hyperplasia, atypical lobular hyperplasia and atypical ductal hyperplasia) by the physicians of ACECR within the past 1 month. The controls were selected from women who accompanied patients or those who attended ACECR for check-up, and were diagnosed as healthy by the physicians, without any symptoms or complaints for BBD. We excluded individuals with BMI≥40 kg/m2, history of diagnosis with CVD, diabetes, hypertension, dyslipidaemia, renal diseases, liver or gastrointestinal diseases, food allergy, stroke, fibromyalgia, multiple sclerosis, Parkinson’s disease, current alcoholism or drug addiction, as well as self-reported withdrawal in the past 3 months, current pregnancy or lactation in the past 12 months. In total, ninety-six patients with BBD, aged 20–70 years, were selected as the case group and seventy non-diseased healthy individuals were selected as the control group using convenience sampling. The controls were healthy subjects who were visiting the centre for routine screening. By using frequency matching, the two groups were matched for age (age groups of 10 years) and menopause status.
Socio-demographic and lifestyle characteristics
Individual-specific information was collected via face-to-face interviews. Individual characteristics including age, residence status, educational level, job status, marital status, age at menarche, age at first full-term pregnancy, number of pregnancies, history of lactation, menopause status, history of oral contraceptive use and oestrogen therapy, family history of breast diseases, cigarette, hubble-bubble and tobacco-pipe smoking, vitamin and mineral supplement intake were recorded.
Anthropometric and physical activity measures
Weight was measured to the nearest 100 g with light clothing and without shoes using a weighing scale (Seca:813; Seca United Kingdom), and height was measured using a non-elastic measuring tape to the nearest 1 mm without shoes, heels together touching the wall and looking straight forward. BMI was calculated by dividing weight in kilograms by height in metres squared. Waist circumference (WC) was measured at a level midway between the lower rib margin and the iliac crest at the end of a gentle expiration and at standing position using a tape to the nearest 1 mm. In order to eliminate individual errors, the height, weight and WC were measured only by the first researcher.
International Physical Activity Questionnaire-short form was used to assess the level of physical activity of the participants( Reference Moghaddam, Aghdam and Jafarabadi 26 ). Individuals were asked about the frequency (d) and duration (min) of severe, moderate, jogging and sedentary physical activity during the past 7 d. To gain basic scoring, individuals must have at least 10 min of continuous physical activity. Physical activity score was calculated as metabolic equivalent task per minute per week (MET-min/week) for severe, moderate and jogging activities. The score of engaging in different levels of activity over the past week is calculated by multiplying the frequency of days by duration of time (min), as well as by constants of 8, 4 and 3·3, respectively. Finally, the sum of three scores was calculated as total exercise per week (total MET-min/week).
Dietary intake assessment
Usual dietary intake of individuals during the past year was assessed using a valid and reliable semi-quantitative FFQ from Tehran Lipid and Glucose Study( Reference Mirmiran, Hosseini Esfahani and Mehrabi 27 ). This 168-food-item questionnaire was completed via face-to-face interview by a trained dietitian. Dietary intake of six food groups including breads and cereals, meats, dairy products, vegetables, fruits and miscellaneous foods was assessed. Participants were asked to report the frequency of consumption for each food item in terms of daily (e.g. bread), weekly (e.g. rice and meat), monthly and yearly intakes. Reported food frequency was converted to daily intake (per gram) with respect to serving sizes( Reference Ghafarpour, Houshiar-Rad and Kianfar 28 ). Kitchen measuring cups were used to convert food servings. Food items were analysed for energy and nutrient contents using food composition tables (United States Department of Agriculture; USDA). Mixed foods were calculated according to their component ingredients.
Food groups and identifying dietary patterns
Food items were divided into twenty-five groups according to their composition and content (Table 1). The dietary patterns were extracted from questionnaires, based on the food intake data of the cases and controls. Extracted food groups were entered into the factor analysis, and dietary patterns were found according to principal component analysis. A change point in the scree plot was used to identify the number of patterns. With respect to inter-correlations among variables, factor loadings of >0·2 were considered to determine the number of food groups in each dietary pattern. Dietary patterns were labelled based on their food items (Table 2).
Table 1 Food grouping used in factor analysis

Table 2 Factor loadings of food groups for Healthy and Unhealthy dietary patternsFootnote *
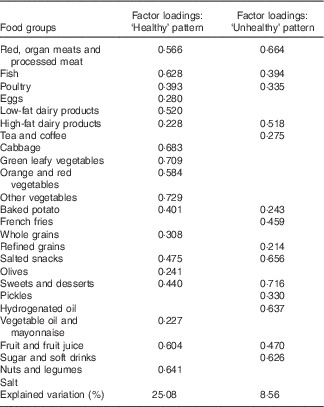
* Factor loadings >0·2 are presented. Kaiser–Meyer–Olkin measure of sampling adequacy=0·83. Bartlett’s test of sphericity=<0·001
Statistical analysis
Kolmogorov–Smirnov test was applied to evaluate the normality of the data. Data analysis was performed using SPSS (version 16). Normally distributed quantitative variables, physical activity and qualitative variables were compared by independent t test, Mann–Whitney test and χ 2 test, respectively. As each participant received a factor score for each explored dietary pattern, we categorised the participants into tertiles of dietary pattern scores, so that each tertile contained approximately thirty individuals. Eventually, using logistic regression model with and without adjustment for different variables, the association of dietary patterns scores with BBD risk was assessed. The OR and 95 % CI were calculated for each dietary pattern tertile by logistic regression analysis. The relationship between the dietary patterns and BBD risk was adjusted for age, BMI, energy intake and confounders, with a P value<0·05 in logistic regression. P values<0·05 were considered significant.
Results
Table 3 provides general data for patients and healthy women. Mean age of cases and controls was 42·5 and 43·4 years, respectively, which was not significantly different. Mean age at first full-term pregnancy was significantly higher in cases compared with healthy controls, whereas the percentage of employed women and those who had taken oestrogen was significantly higher in controls than in cases (P<0·05) (Table 3). No other significant differences were observed. One of the patients was recognised with the history of tobacco use.
Table 3 Characteristics of participants with benign breast disease and the controls (Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)

MET, metabolic equivalent task.
* Obtained by χ 2 test for categorical variables and by t test and Mann–Whitney U test for continuous variables with normal and skewed distribution, respectively.
Two dietary patterns, entitled Healthy and Unhealthy, were obtained by factor analysis. Table 2 shows the factor loadings of food groups for the two dietary patterns, wherein the factor loadings >0·2 were reported. Higher factor loading of a given food group indicates the greater contribution of that specific food group in the dietary pattern. Two dominant dietary patterns explained 33 % of total variance. Healthy dietary pattern, with the variance of 25 %, consisted of fish, poultry, eggs, low-fat dairy products, cabbage, green leafy vegetables, yellow-orange vegetables, other types of vegetable, baked potato, legumes, nuts and seeds, whole grains, oil and mayonnaise, olives, fruits, dried fruits and fruit juices. Unhealthy dietary pattern, with the variance of 8 %, comprised red meats, organ and processed meats, high-fat dairy products, French fries, pickles (in vinegar or brine), refined grains, sweets and desserts, sugars and soda, tea and coffee, animal and solid fats and salted snacks. No significant differences were revealed when comparing frequency and tertile percentages of Healthy and Unhealthy dietary patterns in both case and control groups (P>0·05). The percentages of patients in the first, second and third tertiles of Healthy dietary pattern were 36·5, 33·3 and 30·2 %, respectively, whereas the percentages of healthy women were 24·3, 30·0 and 45·7 %, respectively. Regarding Unhealthy dietary pattern, the percentages of patients in the first, second and third tertiles were 33·3, 36·5 and 30·2 %, respectively, whereas the percentages of healthy women were 37·1, 28·6 and 34·3 %, respectively (Table 3).
The OR of BBD morbidity across tertiles of dietary pattern, before and after adjustment for confounding factors, is presented in four different models in Table 4. Before adjusting the confounders, subjects in the highest tertile had lower OR for BBD compared with those in the lowest tertile (model 1: OR 0·44; 95 % CI 0·2, 0·95). This relationship remained significant after adjusting for age, BMI and energy intake (model 2: OR 0·44; 95 % CI 0·20, 0·99). After adjusting for age at first pregnancy, job status and history of oestrogen therapy, the association remained close to significance level (model 3: OR 0·42; 95 % CI 0·17, 1·06). However, after addition of age, BMI and energy intake to the previous model, the association did not remain significant (model 4: OR 0·65; 95 % CI 0·24, 1·74).
Table 4 Benign breast diseases morbidity according to dietary patterns tertile (T) (Crude and adjusted odds ratios and 95 % confidence intervals)
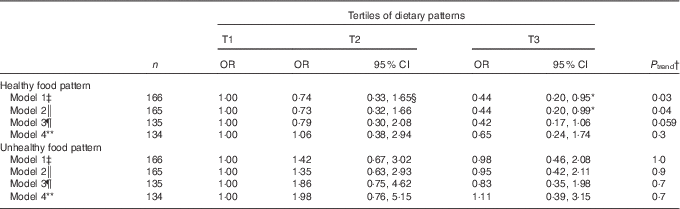
* Statistically significant.
† Numbers represented the P valuetrend of OR in a row.
‡ Model 1 was not adjusted.
§ All OR and their corresponding 95 % CI were calculated by performing logistic regression.
|| Model 2 was adjusted for age (years), BMI (kg/m2) and energy intake (kcal).
¶ Model 3 was adjusted for age at first pregnancy (years), job status (employed/housekeeper) and history of oestrogen therapy (yes/no).
** Model 4 was adjusted for the variables included in the models 2 and 3.
No significant relationship was found between Unhealthy dietary pattern and BBD risk even after adjusting for potential confounders (P trend>0·05) (Table 4).
Discussion
In the current study, the association between dietary patterns and BBD morbidity was investigated for the first time. Our results showed that the Healthy dietary pattern might be inversely associated with BBD, although after adjusting for some confounding variables a clear relationship was not observed. Besides, no significant relationship was found between the Unhealthy dietary pattern and BBD.
In line with the present findings, a study in Australia showed that higher consumption of poultry, sea foods, leafy and red-brown vegetables and fruits is associated with a lower risk of BBD( Reference Ingram, Nottage and Roberts 10 ). In addition, Galvan-Portillo et al.( Reference Galvan-Portillo, Anchez and Lopez-Carrillo 8 ) reported that a higher fruit and dairy product intake could be related to a lower risk of BBD. Hislop et al.( Reference Hislop, Band and Deschamps 9 ) demonstrated a protective role against proliferative breast diseases with the intake of green vegetables. Consistent with these findings, in a case–control study, it was suggested that fruit and vegetable intake could be associated with a lower risk of fibrocystic breast disease, and the association was shown to be stronger in case of proliferative and atypical diseases, compared with non-proliferative conditions( Reference Wu, Ray and Lin 16 ). However, in a clinical trial for 7·7 years, low-fat dietary pattern with decreased fat intake to 20 % of total energy intake (decreased SFA intake to <7 % of total energy intake), increased fruit and vegetable intake to equal or more than five servings per d and increased cereal intake to six or more servings per d resulted in no-risk reduction of proliferative breast diseases( Reference Rohan, Negassa and Caan 15 ). The Healthy dietary pattern in our study consisted of food groups that might justify the reduction in BBD morbidity. Fruits and vegetables are rich in vitamins, antioxidants (e.g. vitamin C, vitamin E and β-carotene), minerals, fibre and other bioactive components. Antioxidants prevent DNA mutations and damage by eliminating free radicals( Reference Weisburger 29 ). Moreover, fruits and vegetables seem to contain ingredients with anti-proliferative effects on the breast epithelium( Reference Wu, Ray and Lin 16 ). Fibres reduce circulatory levels of oestrogen and thus prevent breast cell proliferation( Reference Baer, Schnitt and Connolly 6 ). These ingredients impede the reabsorption of oestrogen from the gastrointestinal tract( Reference Cohen 30 ), leading to increased levels of oestrogen-binding globulin and lower bioavailability of these molecules( Reference Goldin, Woods and Spiegelman 31 ). Nuts are rich sources of unsaturated fatty acids and bioactive ingredients (such as high-quality plant proteins, fibre, minerals, tocopherols, phytosterols and phenolic compounds) with a huge impact on health( Reference Ros 32 ), and they can reduce benign and progressive breast diseases( Reference Baer, Schnitt and Connolly 6 ). For instance, walnut contains bioactive compounds (α-linolenic acid and phytosterols) with anti-proliferative effects on breast epithelial cells( Reference Heuvel, Belda and Hannon 33 ).
According to our data, a higher intake of Unhealthy dietary pattern in crude and adjusted models was not associated with BBD risk. In Shanghai cohort study, intake of red meats, eggs, fried foods, dairy products (except for non-proliferative diseases), rice and other cereals (except for proliferative diseases) was not related to breast fibrocystic conditions( Reference Wu, Ray and Lin 16 ). Ingram et al.( Reference Ingram, Nottage and Roberts 10 ) showed that red meats, savoury foods (pizza, pie, stews, etc.) and starch are harmful for BBD. The consumption of fat from red meats was shown to be associated with proliferative forms of BBD in Canadian women( Reference Hislop, Band and Deschamps 9 ). We found no association between Unhealthy dietary pattern and BBD. This might stem from the recent transition in Iranian dietary patterns from traditional habits, which was similar to our Healthy dietary pattern, to new patterns resembling Western style or Unhealthy dietary pattern( Reference Ghassemi, Harrison and Mohammad 34 , Reference Galal 35 ). Therefore, it is possible that our participants consumed healthy foods in their childhood and adolescence and their habits changed to unhealthy diet in adulthood( Reference Ghassemi, Harrison and Mohammad 34 ). However, risky time period for the development of BBD is unknown, but childhood and adolescent diet may have a role in BBD incidence in adulthood( Reference Baer, Schnitt and Connolly 6 , Reference Ruder, Dorgan and Kranz 36 ). Moreover, some food ingredients from Unhealthy dietary pattern might reduce the BBD risk. For example, although red meats and high-fat dairy products contain SFA, which are associated with increased risk of BBD( Reference Hislop, Band and Deschamps 9 , Reference Lubin, Wax and Ron 13 ), retinol found in these foods reduces the risk( Reference Hislop, Band and Deschamps 9 , Reference Rohan, Cook and Potter 14 ). Furthermore, organ meats contain nutrients such as folate and methionine functioning in DNA methylation and thus prevent carcinogenesis( Reference Rohan, Jain and Howe 37 ). Therefore, there could be a relation between folate intake and proliferative BBD, as a precursor of breast cancer( Reference Lakhani 38 ).
The strength of the current study lies in selecting the patients who were diagnosed within the past month. This minimised the possible effect of the diagnosis on dietary patterns. In addition, it was a new research field on not only Iranian women but also in the globe. Nevertheless, our study had some limitations. First, by applying factor analysis in identifying the dietary patterns, food groups are categorised based on researcher’s decision and previous literature, and thus changing the type of categorisation could lead to different results. Second, response errors in FFQ are possible. Under- or over-reporting of food consumption may result in errors in accurate estimation of consumed food( Reference Kerver, Yang and Bianchi 39 ). However, in order to lower the bias, short-time interval between diagnosis and interview was used. Third, like all case–control studies, we could not determine the cause and effect relationship between the dietary patterns and BBD. Fourth, in spite of the high participation rate, samples may not be representative of the target population and selection bias is plausible. Regardless of these limitations, this study was the first attempt to examine the relationship between major dietary patterns and BBD.
For future studies, prospective designs are recommended to assess the relations between dietary patterns and BBD. In addition, larger sample size could be useful in studying dietary patterns with three subgroups of BBD separately. Also, the larger sample size may increase the chance to include more food groups to define dietary patterns in more detail.
According to the present findings, Healthy dietary pattern might be associated with a lower BBD risk; nevertheless, Unhealthy dietary pattern was not related to these diseases. However, this result should be interpreted with caution because of its limitations. Further studies are needed to confirm this finding.
Acknowledgements
The authors sincerely express their appreciation to the participants of this study.
This research project was supported by Tehran University of Medical Sciences, grant no. 93-454-111.
The authors’ contributions are as follows: Z. S. M. and G. S. contributed to the study design and revised the manuscript. Z. T., F. K., S. K. and F. D. contributed to data collection and wrote the manuscript. M. Q. and F. K. contributed to data analysis and interpretation of the data. All authors listed approved the content of the submitted manuscript.
The authors have no conflicts of interest to declare.







