Short-chain fructo-oligosaccharides (FOS) are well-known, non-digestible and fermentable sugars that induce marked changes in intestinal bacterial populations(Reference Sakai, Aramaki and Takasaki1). FOS are applied to many functional foods as prebiotics and known to have beneficial influences on host health, such as promoting gut health and mineral absorption, and also ameliorating hyperlipidaemia(Reference Tahiri, Tressol and Arnaud2, Reference Kaufhold, Hammon and Blum3). It has been reported that FOS alter glucose and lipid metabolism in human subjects(Reference Luo, Rizkalla and Alamowitch4) and animals(Reference Kaufhold, Hammon and Blum3, Reference Diez, Hornick and Baldwin5) depending on their colonic fermentation. In addition, recent research has revealed that prebiotic-induced changes of gut microbiota regulate obesity-related inflammation in mice through an improvement of gut permeability(Reference Cani, Possemiers and Van de Wiele6). However, little information is available regarding the effects of FOS on insulin sensitivity.
Increasing type 2 diabetes has become a major social issue globally, and the relationship between childhood obesity and insulin resistance has been considered in recent years. It has been accepted that adipocyte dysfunction, particularly in visceral fat tissue, plays an important role in the development of insulin resistance. Adipocytes synthesise and secrete biologically active molecules called adipocytokines(Reference Funahashi, Nakamura and Shimomura7, Reference Matsuzawa8). Some of these adipocytokines secreted from hypertrophic adipocytes have been shown to directly or indirectly impair insulin sensitivity through the modulation of insulin signalling, and glucose and lipid metabolism(Reference Kershaw and Flier9). Hypertrophic adipocytes produce less adiponectin, which has been reported to lead to an impairment in insulin sensitivity(Reference Kadowaki, Yamauchi and Kubota10). We previously demonstrated that the lipolytic properties of adipocytes differ between the small- and large-intestinal mesenteric fat tissues(Reference Shinoki and Hara11).
The small intestine absorbs proteins, lipids and digestible carbohydrates, whereas the large intestine absorbs bacterial components or metabolites including organic acids, which mainly consist of SCFA produced by the fermentation of non-digestible carbohydrates. These differences might be responsible for the alterations between the properties of mesenteric adipocytes from the small intestine and those from the large intestine. We examined that the change in the mesenteric adipocyte properties is thought to affect body metabolism, for example insulin sensitivity, and FOS may have a large impact on mesenteric adipocytes, especially those from around the large intestine.
We designed the present study to examine the effects of FOS feeding from a young age. The minimum age required to collect enough abdominal adipose tissue to enable the division of the small and large intestines of mesenteric fat cells in rats is 8 weeks. Moreover, after only 2 weeks of a high-sucrose diet from weaning, insulin sensitivity has been found to be impaired (A Shinoki, unpublished results). Therefore, we setup two groups with different terms of FOS feeding as follows: a FOS-free high-sucrose diet from weaning (3 weeks old) for 2 weeks in order to impair insulin sensitivity, followed by FOS feeding for 3 weeks; FOS feeding for 5 weeks from weaning to adjust the final age (8 weeks old). Insulin sensitivity was measured by the homeostatic model assessment for insulin resistance (HOMA-IR). HOMA-IR offers important advantages in estimating insulin sensitivity in rodent studies(Reference Cacho, Sevillano and de Castro12, Reference Muniyappa, Chen and Muzumdar13), even though this index was developed for human studies.
The aims of the present study were to determine the effects of feeding FOS on adipocytokine release from abdominal adipocytes in relation to insulin sensitivity in weaning rats. We also evaluated adipocytokine release from freshly isolated small- and large-intestinal mesenteric fat cells from rats fed a FOS diet.
Materials and methods
Animals and diets
Male Sprague–Dawley rats (Jcl) weighing 50–54 g (3 weeks old; Japan Clea, Tokyo, Japan) were housed in individual stainless-steel cages with wire-mesh bottoms. The cages were placed in a room with a controlled temperature (22 ± 2°C), relative humidity (40–60 %) and lighting (12 h light–12 h dark cycle, 08.00–20.00 hours) throughout the study. The rats had free access to water and a semi-purified sucrose-based diet with the American Institute of Nutrition (AIN)-93 growth formulation(Reference Reeves, Nielsen and Fahey14) (control diet) or the control diet containing a 5 % FOS diet for 3 or 5 weeks (Table 1). The test diets were given every day. Fructo-oligosaccharide (Meioligo-Pt; Meiji Seika Kaisha, Limited, Tokyo, Japan) is a mixture of 42 % 1-kestose, 46 % nystose and 9 % 1F-β-fructofuranosyl nystose, and the average degrees of polymerisation was 3·55.
Table 1 Composition of the experimental diets
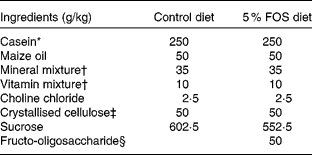
FOS, fructo-oligosaccharides.
* NZMP Acid Casein; Fonterra Limited, Auckland, New Zealand.
† The mineral and vitamin mixtures were prepared according to the American Institute of Nutrition (AIN)-93 growth formulation.
‡ CEOLUS PH102; Asahi Chemical Industry, Tokyo, Japan.
§ Meioligo-Pt; Meiji Seika Kaisha, Limited, Tokyo, Japan.
The present study was approved by the Hokkaido University Animal Committee, and the animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals.
Study design
Rats were divided into three groups of eight or nine animals each and were given the aforementioned control diet, a 5 % FOS diet for 5 weeks, or the control diet for 2 weeks followed by the 5 % FOS diet for 3 weeks (control, FOS-5wk and FOS-3wk, respectively). We used rats with impaired insulin sensitivity induced by feeding a high-sucrose diet for 2 weeks just after weaning. Body weight and food intake were measured every day. Tail blood was collected in heparinised microtubes after fasting for 9 h on day 33. On the last day, rats were killed after the collection of abdominal aortic blood under pentobarbital anaesthesia (Nembutal: sodium pentobarbital, 50 mg/kg body weight; Abbott Laboratories, North Chicago, IL, USA). The caecum and abdominal white adipose tissues (mesenteric, retroperitoneal and epididymal) were immediately excised and weighed. Tail blood plasma and aortic blood serum were collected after centrifugation and stored at − 80°C until subsequent analyses.
Blood analyses
The concentrations of glucose and TAG in tail blood plasma were measured using enzyme assay kits (Glucose CII-test from Wako Pure Chemical Industries Limited, Osaka, Japan; TG-EN from Kainos Laboratories, Tokyo, Japan), and insulin was measured using an ELISA kits (Rat Insulin ELISA kit AKRIN-010T from Shibayagi, Gunma, Japan). The concentrations of adiponectin, leptin, monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor-1 (PAI-1) in the aortic plasma were measured using the ELISA kit (Mouse/Rat Adiponectin ELISA kit from Otsuka Pharmaceutical, Tokyo, Japan; Rat Leptin ELISA kit from LINCO Research, St Charles, MO, USA; Rat MCP-1 ELISA kit from Thermo Fisher Scientific, Inc., Rockford, IL, USA; ZYMUTEST Rat-PAI-1 Antigen Kit from HYPHEN Bio Med, Neuville-Sur-Oise, France). HOMA-IR was used to evaluate insulin sensitivity. It was calculated from fasting insulin and glucose concentrations using the formula of Matthews et al. (Reference Matthews, Hosker and Rudenski15):
where 1 IU insulin = 0·038 mg.
Measurement of adipocytokine release from isolated adipocytes
Excised mesenteric fat tissue was immediately separated into the small- and large-intestinal regions along the portal vein. The isolated adipocytes were prepared from the rat mesenteric fat tissues by the method of Rodbell with minor modifications as described previously(Reference Rodbell16, Reference Shinoki and Hara17). Briefly, mesenteric fat tissues were cut into small pieces with scissors before digestion for 1 h in isolation medium (Krebs–Ringer bicarbonate buffer, pH 7·4; 118 mm-NaCl, 4·7 mm-KCl, 2·7 mm-CaCl2, 1·2 mm-KH2PO4 and 24·9 mm-NaHCO3) containing 4 % (w/v) bovine serum albumin (fatty acid free; Wako Pure Chemical Industries Limited), 0·1 % (w/v) collagenase (type I; Sigma, St Louis, MO, USA), 0·01 % (w/v) trypsin inhibitor (type II-S; Sigma) and 2·5 mm-glucose in a shaking water-bath (70 rpm, 37°C). After incubation, the medium including isolated fat cells was filtered through a 1000 μm nylon mesh. The mature adipocytes floated and formed a layer on the surface of the filtered medium (packed fat cells), and the stromal vascular fraction (capillary, progenitor cells, fibroblastic cells and macrophages) was precipitated. The matured fat cells were washed twice with collagenase-free Krebs–Ringer phosphate buffer containing 3 % (w/v) bovine serum albumin. The isolated mesenteric fat cells (50 μl packed volume) were incubated at 37°C in 950 μl of medium (Krebs–Ringer phosphate buffer, pH 7·4; 122 mm-NaCl, 4·9 mm-KCl, 1·2 mm-MgSO4 and 16·7 mm-phosphate buffer) containing 3 % (w/v) bovine serum albumin and 5 mm-glucose. After incubation for 1 h, the reaction was stopped on ice. The floating fat cells were removed, and the medium was used for adipocytokine measurement.
The rate of adipocytokine release from the isolated small- or large-intestinal mesenteric fat cells is expressed as unit volume of fat cells used(Reference Shinoki and Hara17, Reference Morimoto, Kameda and Tsujita18). The adipocytokine secretion rate from the whole mesenteric fat tissue (small- and large-intestinal regions) was calculated from the secretion rates from the small- and large-intestinal mesenteric fat cells by multiplying the whole mesenteric fat tissue weight.
TNF-α released into the incubation medium by the fat cells was quantified with an ELISA kit (Rat TNF-α Ultra Sensitive ELISA kit from Invitrogen Corporation, Camarillo, CA, USA). Levels of adiponectin and leptin released by the fat cells were measured as described earlier for blood serum.
Measurement of fat cell size
Isolated adipocytes from mesenteric fat tissues were stained with oil red O. Fat cell size was evaluated by the area of each isolated cell, and the mean size of the cell was calculated for more than 100 cells per preparation from each rat. Cell areas were measured using Image J version 1.41 software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
All values are expressed as means with their standard errors of the mean for seven to nine rats. Statistical analyses were performed by one-way ANOVA, and the differences among treatment groups were determined using Tukey–Kramer's test if the difference was found to be significant using one-way ANOVA. A difference with P < 0·05 was considered to be significant. Pearson's correlation coefficients were also calculated.
Results
Body weight and fat tissue weight
There were no differences in initial or final body weight or food intake among the three groups (Table 2), and energy intake was also not different among the three groups (data not shown). However, the relative wet weight of the caecum was higher in the FOS-3wk and FOS-5wk groups than that in the control group (P < 0·001). Relative wet weights of the small-intestinal mesenteric fat (P = 0·024), retroperitoneal fat (P = 0·02) and epididymal fat (P = 0·036) were lower in the FOS-5wk group than those in the control group. The relative weight of the large-intestinal mesenteric fat tissue in both the FOS-3wk and FOS-5wk groups was lower than that in the control group (P = 0·005). There were no differences in the relative weights of the perirenal fat tissue among the three groups.
Table 2 Initial and final body weight, food intake, caecum, and small- and large-intestinal mesenteric, perirenal, retroperitoneal and epididymal fat relative weight in rats fed the experimental diets for 5 weeks
(Mean values with their standard errors, n 7–9)

FOS-3wk, fructo-oligosaccharide feeding for 3 weeks; FOS-5wk, fructo-oligosaccharide feeding for 5 weeks.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Tukey–Kramer's test).
* ANOVA.
Plasma analyses
There were no differences in the plasma concentrations of TAG and glucose after a 9 h fast among the three groups, whereas plasma insulin concentrations decreased by FOS feeding, and the concentration in the FOS-5wk group was much lower than that in the control group (Table 3). Changes in HOMA-IR, an index of insulin sensitivity, were also lower in the FOS-fed groups than in the control group (P = 0·047). The value in the FOS-5wk group was half that in the control group. In the aortic blood plasma, adiponectin concentration was significantly lower in both the FOS-fed groups (P = 0·021), and leptin concentration was lower in the FOS-5wk group compared with the control group (P = 0·005), whereas there were no differences in MCP-1 and PAI-1 among the three groups.
Table 3 Plasma concentrations of TAG, glucose, insulin and homeostatic model assessment for insulin resistance (HOMA-IR) in the tail blood after 9 h fasting, and adiponectin, leptin, monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor-1 (PAI-1) in the aortic blood of rats fed the experimental diets for 5 weeks
(Mean values with their standard errors, n 7–9)
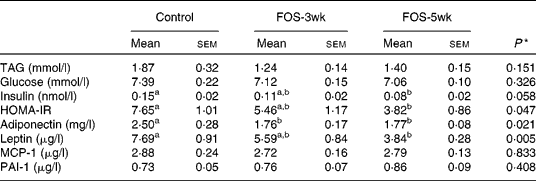
FOS-3wk, fructo-oligosaccharide feeding for 3 weeks; FOS-5wk, fructo-oligosaccharide feeding for 5 weeks.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Tukey–Kramer's test).
* ANOVA.
Secretion rates of adipocytokines from isolated mesenteric fat cells
Leptin release from the small-intestinal mesenteric fat cells was lower in FOS-3wk group than in the control group (P = 0·04; Fig. 1), whereas there were no differences in the secretion rate from the large-intestinal fat cells among the three groups. There were no differences in TNF-α release from the large-intestinal mesenteric fat cells among the three groups; however, the secretion rate from the small intestine was lower, though not significantly, in the FOS-fed groups. Feeding with the FOS diet had no effect on the secretion rate of adiponectin from either type of intestinal fat cell.
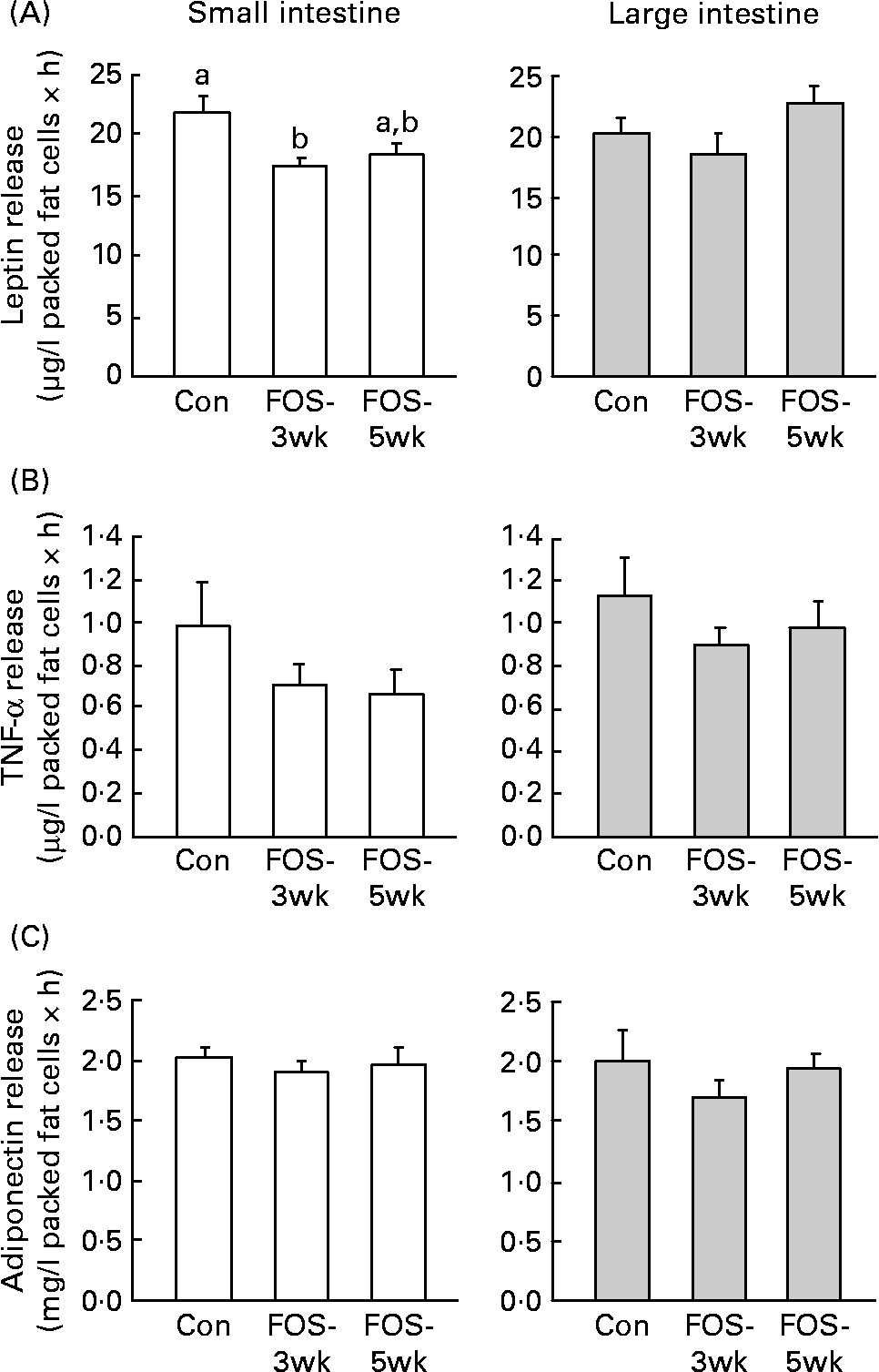
Fig. 1 Secretion rates of (A) leptin, (B) TNF-α and (C) adiponectin in isolated small- and large-intestinal mesenteric fat cells from rats fed the test diets. Values are means, with their standard errors represented by vertical bars (n 7–9). P values (one-way ANOVA) were 0·040 in the small intestine and 0·197 in the large intestine (leptin); 0·303 in the small intestine and 0·548 in the large intestine (TNF-α); and 0·770 in the small intestine and 0·524 in the large intestine (adiponectin). a,b Mean values with unlike letters were significantly different (P < 0·05). Con, control; FOS-3wk, fructo-oligosaccharide feeding for 3 weeks; FOS-5wk, fructo-oligosaccharide feeding for 5 weeks.
A comparable mean size of isolated mesenteric fat cells was measured in rats fed the test diets for 5 weeks. Mean size in the small-intestinal region was 3·53 (sem 0·24) × 103 μm2 in the control group (n 7); 3·66 (sem 0·19) × 103 μm2 in the FOS-3wk group (n 8); 3·49 (sem 0·24) × 103 μm2 in the FOS-5wk group (n 9). Mean size in the large-intestinal region was 3·52 (sem 0·13) × 103 μm2 in the control group (n 8); 3·62 (sem 0·25) × 103 μm2 in the FOS-3wk group (n 8); 3·22 (sem 0·18) × 103 μm2 in the FOS-5wk group (n 9). Mean size of adipocytes in the small- and large-intestinal mesenteric fat cells showed no differences among the three groups.
Secretion rates of adipocytokines from the whole mesenteric fat tissue
The secretion rates of leptin (P = 0·007) and TNF-α (P = 0·047) from the whole mesenteric fat tissue were lower in both the FOS-3wk and FOS-5wk groups than in the control group (Fig. 2). There were no differences in the adiponectin secretion rates from the whole mesenteric fat tissue among the three groups.

Fig. 2 Secretion rates from the whole mesenteric fat tissue of (A) leptin, (B) TNF-α and (C) adiponectin of rats fed the test diets, which were calculated from the secretion rates from the small- and large-intestinal mesenteric fat cells by multiplying the whole mesenteric fat tissue weight based on the weight per unit volume of the fat cells used. Values are means, with their standard errors represented by vertical bars (n 7–8). P values (one-way ANOVA) were 0·007 for leptin, 0·047 for TNF-α and 0·182 for adiponectin. a,b Mean values with unlike letters were significantly different (P < 0·05). Con, Control; FOS-3wk, fructo-oligosaccharide feeding for 3 weeks; FOS-5wk, fructo-oligosaccharide feeding for 5 weeks.
Discussion
In the present study, we evaluated the effects of feeding FOS on mesenteric fat tissue, divided into small- and large-intestinal portions. The results show that FOS feeding led to a clear reduction in abdominal fat weight, which was associated with increases in insulin sensitivity as evaluated by HOMA-IR. We also examined adipocytokine release from the isolated mesenteric fat cells in rats fed the FOS diet. We found that leptin release was significantly reduced by FOS feeding, and TNF-α release showed a similar tendency to leptin release.
HOMA-IR was significantly decreased in the FOS-5wk group, indicating the promotion of insulin sensitivity. The HOMA-IR values in rats fed the FOS diet for 3 weeks were intermediate between those in the FOS-5wk and control groups, though the differences were not significant. These results suggest that it takes a considerable period for FOS feeding to affect insulin sensitivity. Recently, obese dogs were found to show improved insulin resistance after FOS feeding with modulation of the gene transcription of fatty acid or glucose metabolism(Reference Respondek, Swanson and Belsito19), which is in agreement with the present results. We showed caecal hypertrophy by FOS feeding, which indicates that caecal fermentation associated with SCFA production may be increased. It has been reported that FOS feeding largely increased acetic acid production in the colon(Reference Lara-Villoslada, de Haro and Camuesco20). Acetic acid treatment suppresses body fat accumulation by down-regulating lipogenesis and lowering energy intake(Reference Yamashita, Fujisawa and Ito21). It has also been reported that butyrate supplementation at 5 % (w/w) in a high-fat diet prevents the development of dietary obesity and insulin resistance(Reference Gao, Yin and Zhang22). Moreover, the fermentation of dietary fibre including FOS suppresses endotoxaemia and improves metabolic disturbances(Reference Cani, Neyrinck and Fava23). Hence, SCFA may contribute to the reduction of the mass of mesenteric fat tissue in the large intestine shown in the present study. However, the present results revealed a reduction in fat mass also in small-intestinal mesenteric fat tissue. Factors other than SCFA production by FOS might lead to the loss of body fat.
Adiponectin is as an adipocyte-specific protein(Reference Scherer, Williams and Fogliano24–Reference Maeda, Okubo and Shimomura26), and high adiponectin levels are known to enhance insulin sensitivity in general. In contrast, recent studies have shown controversial results, i.e. high adiponectin levels were associated with an increased risk of CVD and/or mortality(Reference Hung, Wang and Lu27, Reference Maiolino, Cesari and Sticchi28), and the authors have speculated that elevations in adiponectin represent acute compensatory mechanisms to counteract metabolic and vascular stress. The present study shows that plasma adiponectin level was decreased in FOS-fed rats though insulin sensitivity was increased, which seems to agree with the previous observations. It has been reported that liver is the main site of adiponectin clearance(Reference Halberg, Schraw and Wang29). This clearance rate is relatively stable with long-term fasting(Reference Combs, Wagner and Berger30). However, both genetically (ob/ob mice) and diet-induced obesity resulted in slower clearance rates of adiponectin(Reference Halberg, Schraw and Wang29). Increased clearance rather than diminished secretion possibly results in lower plasma adiponectin levels as observed in the present study. The present results showed that adiponectin did not contribute, but other adipocytokines may contribute, to the improvement of insulin sensitivity in the FOS feeding groups.
The concentration of plasma leptin was clearly decreased by the feeding of FOS for 5 weeks, and a concomitant decrease in abdominal fat tissue weight was observed. The secretion rate of leptin from the whole mesenteric fat tissue was also markedly decreased by FOS feeding. Plasma leptin concentrations were positively correlated with the leptin secretion rate from mesenteric fat (R 0·492, P = 0·017, n 7–8), which suggests that leptin release from the mesenteric fat contributes to changes in plasma leptin levels. Leptin increases in obesity, type 2 diabetes mellitus, hypertension and the metabolic syndrome. Numerous publications in human studies have suggested leptin as a biomarker for obesity, insulin resistance and the metabolic syndrome(Reference Moran and Phillip31). HOMA-IR was positively correlated with both plasma leptin levels (R 0·568, P = 0·005, n 7–8) and the leptin release rate from mesenteric fat tissue (R 0·427, P = 0·048, n 7–8). These results strongly suggest that suppression of leptin release from mesenteric fat tissue is involved in the FOS-mediated promotion of insulin sensitivity. Leptin is involved in the suppression of appetite(Reference Cioffi, Shafer and Zupancic32–Reference Wang, Chinookoswong and Scully34) and is also known to regulate energy balance, thereby modulating energy expenditure. Until recently, no direct action of leptin outside the brain was anticipated; however, there is some indication that leptin has additional effects in peripheral tissues(Reference Rouru, Cusin and Zakrzewska35–Reference Ceddia, William and Lima37), as leptin receptors have been found in tissues other than the brain. There is some evidence demonstrating that leptin inhibits insulin gene transcription(Reference Seufert, Kieffer and Habener38, Reference Melloul, Marshak and Cerasi39) and insulin secretion(Reference Kieffer and Habener40–Reference Havel42). By activating ATP-dependent K channels or via interactions with the cyclic AMP protein kinase A signalling pathway(Reference Ahrén and Havel43), perhaps by activating phosphodiesterase B3(Reference Zhao, Bornfeldt and Beavo44). In white adipose tissue, leptin reduces insulin-dependent glucose uptake(Reference Frederich, Hamann and Anderson45–Reference Frederich, Löllmann and Hamann47) and impairs insulin-stimulated glucose transport(Reference Barzilai, Wang and Massilon48) and lipogenesis(Reference Funahashi, Shimomura and Hiraoka49). These observations are shown in the physiological range of insulin concentrations (0·1–0·5 nmol/l) and leptin concentrations (0·5–3 nmol/l), which strongly suggests that leptin reduces insulin sensitivity under physiological conditions(Reference Considine, Sinha and Heiman50, Reference Dagogo-Jack, Fanelli and Paramore51). The present results regarding the decreased leptin secretion rate from the whole mesenteric fat tissue and enhanced insulin sensitivity in FOS-fed rats agree with the results from these previous studies, and indicate that the reduction in leptin secretion may contribute to increase insulin sensitivity associated with FOS feeding. The secretion rates of TNF-α from the small- and large-intestinal mesenteric fat cells and from the whole mesenteric fat tissue were reduced by FOS-fed rats without significant differences. However, changes in TNF-α release were very similar to those observed for leptin release. The reduction in TNF-α release may also be associated with an increase in insulin sensitivity. In contrast, there were no changes in plasma concentrations of MCP-1 and PAI-1 by FOS feeding. These results indicate that MCP-1 and PAI-1 are not involved in the improvement of insulin sensitivity by FOS feeding, and also reveal that suppression of leptin and TNF-α release are specific changes with feeding of this oligosaccharide.
We hypothesised that FOS feeding might have larger effects on the secretion rate of adipocytokines from the large-intestinal mesenteric fat cells compared with the small-intestinal region. However, the secretion rate in the small, but not the large, intestinal region was reduced by FOS feeding. Though the mechanism remains unclear, these findings suggest that FOS directly or indirectly acts on the small-intestinal mesenteric fat cells after absorption in intact forms or by fermentation products. A report has shown that raffinose, a non-digestible oligosaccharide in the same category as FOS, was absorbed intact in the intestine(Reference Watanabe, Sonoyama and Watanabe52). FOS are also known to have direct or indirect immunomodulating effects on the intestine(Reference Fukasawa, Murashima and Matsumoto53), which is a possible mechanism to change adipocytokine release by FOS in the small intestine. Even though the absorbed proportion of the orally administered FOS may be quite small, it still seems possible that the FOS absorbed from the small intestine suppressed leptin secretion in the mesenteric adipocytes of the small-intestinal region.
In conclusion, the ingestion of FOS decreased abdominal fat tissue weight and improved insulin sensitivity may be associated with changes in the properties of the small-intestinal mesenteric adipocytes and a reduction in the small- and large-intestinal mesenteric fat mass. The present findings may have implications for the prevention of insulin resistance in human subjects.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. A. S. and H. H. designed the study, analysed the data, wrote the manuscript and had the primary responsibility for the final content; A. S. conducted the study; H. H. provided the essential reagents and materials. There is no conflict of interest in the present study.







