Dry beans (Phaseolus vulgaris L.) are a widely available and affordable staple food crop. Worldwide consumption varies considerably, ranging from 2 to 3·5 kg/capita per year in Europe and the United States to as high as 40 kg/capita per year in Burundi(1, Reference Mitchell, Lawrence and Hartman2). Numerous health benefits have been associated with the regular consumption of dry bean, and emerging evidence supports a relationship between dry bean consumption and chronic disease prevention(Reference Geil and Anderson3, Reference Bennink and Rondini4). Given the dramatic and continued rise in chronic disease incidence and prevalence globally, there is potential for increased bean consumption as a component of an international effort to reverse the impact of chronic diseases in all sectors of the population(Reference Van Cleave, Gortmaker and Perrin5).
Dry bean consumption has been linked to reductions in the occurrence of a number of chronic diseases including type 2 diabetes, CVD and cancer. Elevated total cholesterol and LDL-cholesterol are associated with CHD risk, and multiple studies have reported that dietary dry bean interventions involving consumption of approximately 92 g cooked dry bean/d for at least 3 weeks resulted in significantly reduced serum cholesterol levels(Reference Anderson and Gustafson6–Reference Winham, Hutchins and Johnston8). In a Latin American population, consumption of approximately 79–92 g of cooked dry beans/d was associated with a 38 % lower risk of myocardial infarction compared with non-consumption(Reference Kabagambe, Baylin and Ruiz-Narvarez9). Prospective cohort studies have also found a significant correlation between dry bean consumption and reduced risk for the development of colon and breast cancer(Reference Adebamowo, Cho and Sampson10–Reference Aune, De Stefani and Ronco12). The Polyp Prevention Trial examined the effect of a high fruit and vegetable diet intervention on the recurrence of adenomatous polyps in the large bowel, and found that individuals who increased their consumption of legumes (including dry bean and lentils) experienced a significantly reduced risk for advanced adenoma recurrence (OR = 0·35)(Reference Lanza, Hartman and Albert11). The Nurses Health Study II revealed an effect of pulse consumption on breast cancer risk, with a significant inverse association between consumption of at least two servings of dry beans per week (approximately 160–180 g/week) and breast cancer (relative risk = 0·76)(Reference Adebamowo, Cho and Sampson10).
Edible dry beans are an excellent source of dietary protein, resistant starch and fibre, are low in fat and are a good source of B vitamins and numerous mineral nutrients(Reference Geil and Anderson3, Reference Bennink and Rondini4). In addition to being a rich source of nutrients, beans contain a broad array of non-nutrient bioactive substances many of which have been reported to exert either beneficial or potentially harmful effects on human health(Reference Southon13–Reference Thompson, Brick and McGinley16). Of particular interest to the work reported herein, some of the phytochemicals found in bean seeds are synthesised as natural defence mechanisms against insects or bacterial and mould infestations, and have the potential to elicit toxic effects when consumed ‘uncooked’ by mammals(Reference Southon13, Reference Lajolo and Genovese15). Multiple anti-nutritive molecules occur in dry beans, including amylase inhibitors, trypsin inhibitors and the lectin protein phytohaemagglutinin(Reference Pusztai, Clarke and King17, Reference Nasi, Picariello and Ferranti18). Phytohaemagglutinin is a major concern for uncooked bean intake, as it has demonstrated toxic effects such as gastroenteritis, nausea and diarrhoea in mammals and humans(Reference Rodhouse, Haugh and Roberts19). There is also some question as to whether phytohaemagglutinin plays a role in the aetiology of immune-based chronic diseases such as type 2 diabetes and rheumatoid arthritis(Reference Nasi, Picariello and Ferranti18). However, these anti-nutritive factors are rendered harmless by proper cooking(Reference Jaffe and Lette20).
Rodent models are commonly used in the evaluation of the safety and efficacy of dietary factors. In the present study, rats were fed a range of dietary concentrations of cooked bean that bracketed amounts relevant to human consumption globally (Table 1 ). The hypothesis evaluated was that increasing bean intake does not elicit adaptive changes in gene expression that are indicative of hepatic stress and toxicity. Based on a recent report that the magnitude of altered gene expression and the number of genes affected in the liver were greater using a short- rather than long-term feeding design, the study hypothesis was tested using a 7 d dietary exposure model(Reference Narasaka, Endo and Fu21).
Table 1 Rat bean diet comparison to human consumption
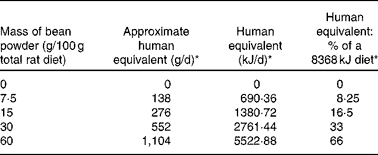
* Based on assumption of 920·48 kJ per average serving of beans.
Experimental methods
Animals
Weanling female Sprague–Dawley rats (n 42) were obtained from Taconic Farms and housed in the institutional vivarium. Room temperature was maintained at 25 °C with 30 % relative humidity and a 12 h light–12 h dark cycle. Animals were fed AIN-93-G powdered diet(Reference Reeves, Nielsen and Fahey22) until 27 d of age, followed by feeding of the experimental diets from 27 to 34 d of age. Rats were randomly assigned to five groups that contained: 0 % (n 18), 7·5 % (n 6), 15 % (n 6), 30 % (n 6) or 60 % (n 6) w/w red bean incorporated into a modification of AIN93G diet as previously described(Reference Thompson, Thompson and Brick23). Animals were housed three per cage and had ad libitum access to food and water at all times during the study. The animals were weighed at the time of randomisation and at study termination. The Colorado State University Animal Care and Use Committee-approved protocols were used for animal research.
Experimental diets
The experimental diets were a modification of AIN-93-G diet, and were identical in composition to those previously described for inhibition of mammary carcinogenesis(Reference Thompson, Thompson and Brick23). Dry red bean was provided by Archer Daniels Midland Company and sent to Bush Brothers & Company for canning. Raw beans were packed in standard brine without additives and then cooked and canned according to commercial standard. The fully processed, canned beans were sent to Van Drunen Farms where they were drained, freeze-dried and milled into a homogeneous powder. The bean powder was stored at − 20°C until incorporated into rodent diets. Diets were formulated using specific guidelines(Reference Reeves, Nielsen and Fahey22) and adjusted to have the same percentage of crude protein, fat and carbohydrate using the proximate analysis of the red bean powder (Warren Analytical). Ground cooked red bean powder was added to AIN93G for a final 7·5, 15, 30 or 60 % w/w concentration. Differences in macronutrient composition were balanced with purified diet components. Casein and maizestarch were adjusted to maintain similar macronutrient content across red bean dosage groups. The control diet contained 7·5 % crude fibre to be consistent with experimental dry bean diets. The percentage of dry beans incorporated into the diets is expressed as mass of bean powder in g/100 g of total diet(Reference Thompson, Thompson and Brick23).
Study design
Young, rapidly growing rats were used because of sensitivity to detecting adverse effects of dietary agents. Adaptive changes in gene expression were assessed after short-term feeding for 7 d. Commercially cooked and canned bean was used to eliminate potential confounding of results by dry bean components that may be toxic in raw beans or when dry beans are not properly cooked. The absence of phytohaemagglutinin activity was confirmed in the red bean powder that was evaluated (data not shown). The small red bean market class was assessed since this market class has been reported to have a very high concentration of bioactive phenolic compounds with antioxidant activity(Reference Wu, Gu and Prior24). The dose range of beans studied brackets the range of reported dry bean intake in various populations worldwide(1).
Necropsy
Rats were stratified across control and experimental groups for necropsy. Necropsy occurred when the rats were 34 d of age and had received the experimental diets for 7 d. Non-fasted animals were euthanised via inhalation of gaseous CO2 followed by cervical dislocation. The livers were removed immediately after plasma collection, freeze-clamped, snap-frozen in liquid N2 and stored at − 80°C.
RNA isolation
Total RNA was isolated from the liver using the RNeasy Mini RNA isolation kit (Qiagen) according to the manufacturer's protocol. RNA yield was determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific) and integrity was assessed using the Experion automated microfluidic capillary electrophoresis system (Bio-Rad).
Gene expression analysis
Gene expression analysis was performed using the RT2 Profiler PCR Array: Rat Stress and Toxicity Pathway Finder (SuperArray) (a complete list of included primer sets can be found at: http://www.sabiosciences.com/rt_pcr_product/HTML/PARN-003A.html). For the analysis, 500 ng of purified RNA sample was used according to the manufacturer's protocol. The following quantitative PCR conditions were applied on the iCycler thermal cycler (Bio-Rad): initial denaturation step at 95°C for 10 min followed by forty cycles of 95°C for 15 s, and then of 60°C for 1 min. Cycle threshold (C t) values were obtained using the iCycler software (Bio-Rad). The C t values were averaged from five housekeeping genes (acidic ribosomal protein P1 (Rplp1), hypoxanthine-guanine phosphoribosyltransferase (Hprt), ribosomal protein L13a (Rpl13A), lactate dehydrogenase A (Ldha) and beta-actin (Actb)) for normalisation purposes. Fold change values for gene expression were determined using the SuperArray online RT2 Profiler PCR data analysis software comparing all treatments to control (0 % red bean) using the ![]() method(Reference Livak and Schmittgen25).
method(Reference Livak and Schmittgen25).
Statistical analysis
Data were analysed using SAS 9.1 (SAS Institute, Inc.); and two-way ANOVA was performed on adjusted C t values and weight gain to determine the statistical significance of increasing red bean dose. Because eighty-four genes were simultaneously investigated, the Benjamini-Hochberg multiple testing adjustment for false discovery rate was performed(Reference Benjamini and Hochberg26). Gene expression changes with a P-value < 0·05 were determined to be significantly affected by beans consumption. Subsequently, Dunnett's test for post hoc analysis was performed for all genes that were found to be significantly affected by bean dose in order to determine which bean doses (7·5, 15, 30, 60 %) were significantly different from the control (0 %).
Results
Body weight was measured at the time of randomisation and at the end of the study. As shown in Fig. 1, no differences in growth rate were observed among diet groups. Controlling the false discovery rate at 5 % for multiple testing across genes, the expression of eight genes of the eighty-four examined was significantly different after consumption of red bean diet compared to control (0 % w/w beans). The eighty-four genes in the array that were investigated for bean consumption effects are listed in Table 2 with their associated P-value. The expressions of the following genes: cyclin G1 (Ccng1), cytochrome p450 3a11 (Cyp3a11), cytochrome p450 7a1 (Cyp7a1), flavin containing mono-oxygenase (Fmo1), glutathione-S-transferase (Gstm1), heat shock protein 8 (Hspa8), macrophage migration inhibitory factor (Mif) and UDP glucuronosyltransferease 1 family, polypeptide A6 (Ugt1a6) were significantly changed by bean in the diet (Table 2). Increased expression of Cyp3a11, Fmo1, Gstm1 and Mif was detected at the highest dietary concentration of dry bean, whereas Ccng1 and Hspa8 expressions were down-regulated compared to the control group (Fig. 2). The results presented herein are consistent with the absence of acute liver stress or toxicity responses even in rats fed as much as 60 % w/w beans in the diet. Expression of Cyp7a1 and Ugt1a6 increased progressively with increasing dietary concentration of bean (Fig. 3). These genes are recognised for induction following exposure to xenobiotics; additionally, activity of these genes has been associated with health benefits as will be discussed next.

Fig. 1 Body weight gain over time among dietary treatment groups. Animals were weighed on 20 and 34 d of age. No significant differences in body weight were observed among dietary treatment groups. ![]() , 0 %;
, 0 %; ![]() , 7·5 %;
, 7·5 %; ![]() , 15 %;
, 15 %; ![]() , 30 %;
, 30 %; ![]() , 60 %.
, 60 %.
Table 2 Genes analysed for changes by bean diets using the RT2 Profiler PCR Array: Rat Stress and Toxicity Pathway Finder

FDR, false discovery rate; Entrez ID, Entrez gene identifier.
* Genes considered to be significantly affected by bean dose.

Fig. 2 Dietary bean dose-dependent changes in hepatic gene expression. Fold change refers to gene expression after 7 d of feeding a bean diet containing 7·5, 15, 30 or 60 % w/w compared with expression from consumption of 0 % bean as measured by quantitative real-time PCR (w/w is equal to the mass of bean powder in g/100 g of total diet). Fold changes for each of the six transcripts significantly affected by bean diet are provided for each bean dose group. (a) Cyclin G1 (Ccng1); (b) cytochrome p450 3a1 (Cyp3a11); (c) flavin containing mono-oxygenase 1 (Fmo1); (d) glutathione S-transferase mu1 (Gstm1); (e) heat shock protein 8 (Hspa8); (f) macrophage migration inhibitory factor (Mif). Dunnett's post hoc analysis was performed to individually compare each dosage group to the control (0 %), and those that were found to be significantly different are marked with: *P < 0·05, **P < 0·01, ***P < 0·001.

Fig. 3 Dietary bean intake induced gene expression of hepatic cytochrome p450 7a1 (Cyp7a1) and UDP glycosyltransferase 1 (Ugt1a6) family polypeptide A6. (a) Cyp7a1; (b) Ugt1a6. Fold change refers to gene expression after 7 d of feeding a bean diet containing 7·5, 15, 30 or 60 % w/w compared with expression from consumption of 0 % bean as measured by quantitative real-time PCR (w/w is equal to the mass of bean powder in g/100 g of total diet). Dunnett's post hoc analysis was performed to individually compare each dosage group to the control (0 %), and those that were found to be significantly different are marked with: *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
A classical first-step approach to assessing the safety of foods in humans and animals has centred on calculating an apparent digestibility following dietary intake, with limited attention on dose-dependent differences(Reference Crampton, Farmer and Mc27–Reference Kelsay, Goering and Behall30). The digestibility and nutritional properties of whole cooked beans (Phaseolus vulgaris L.)(Reference Bressani and Elias31–Reference Admassu Shimelis and Kumar Rakshit35) have been reported and illustrates the complex interactions that can occur among protein digestibility, bean phytochemicals and potential anti-nutrients. The effect of bean consumption on increased nitrogen excretion in rats, however, did not seem to affect the biological value of the protein when compared to casein or uncooked beans(Reference Costa de Oliveira and Sgarbieri36). A more recent study employed a short- and long-term experimental feeding study design in rats with dietary wheat flour and assessed the potential for adverse health effects via changes in liver gene expression using a DNA microarray(Reference Narasaka, Endo and Fu21). This study reported that short-term feeding significantly modulated more genes and the magnitude of liver gene expression when compared to longer-term feeding. Another notable difference of the short-term feeding response was the alteration of genes relating to components of the insulin-like growth factor signalling pathway that is implicated in a number of chronic diseases(Reference Narasaka, Endo and Fu21). This report provides strong rationale for assessing the response to 7 d of bean consumption and for evaluating rat hepatic stress and toxicity genes with increasing bean dose intake in rats. To our knowledge, no studies have examined dose-dependent acute effects of increased cooked bean consumption, such that findings may inform the safety profile of acute hepatic responses to increased bean consumption in humans.
Dry bean intake was designed to encompass levels of consumption occurring in human populations worldwide(1). As recently reported(Reference Mitchell, Lawrence and Hartman2), intake in the United States tends to be very low and current information indicates that only 17 % of individuals in the United States consume dry bean on any given day; whereas in parts of Africa, consumption is estimated to be as high as 40 kg/capita per year. The inclusion of 60 % w/w dry bean in the experimental design, which represents an amount equivalent to approximately 1104 g cooked bean/d in the human diet, greatly exceeds the amount of bean consumed by any known population and created a robust opportunity to inspect the animals' response for evidence of stress induction or toxicity.
Genes significantly affected by bean dose
Ccng1, also referred to as Cyclin G1, is a protein involved in the G2/M checkpoint as a downstream mediator of the p53 pathway(Reference Kimura, Ikawa and Ito37). The gene expression of Ccng1 did not increase in a linear fashion in response to increased red bean in the diet, but instead, the expression peaked at 7·5 % bean and was significantly decreased compared to control at 60 % bean, consistent with a nonlinear dose–response (Fig. 2(a)). However, if the p53 pathway was meaningfully affected by the dry bean diet, it would be expected that other proteins in the p53 pathway proteins would have been affected as well(Reference Levine, Hu and Feng38). Here, four other genes in the p53 pathway, Bcl2-associated X protein (Bax), caspase 8 (Casp8), growth arrest and DNA-damage-inducible, 45α (Gadd45a) and p53 binding protein homologue (Mdm2), were included in this analysis; however, they did not display significant expression changes in response to dry bean dose.
Cyp3a11 is a member of the CYP3 family of cytochrome p450 proteins. This phase I enzyme metabolises many exogenous compounds including prescription drugs, as well as endogenous substrates such as steroids and bile acids(Reference Nebert and Russell39). A marked, significant increase in Cyp3a11 expression was only detected in the 60 % red bean diet when compared to lower doses and control (Fig. 2(b)). Further examination of red bean consumption effects would be of interest in order to determine which bean components were responsible for an effect on this phase I drug metabolising enzyme.
Fmo1 is a member of the flavin mono-oxygenase family that catalyses the oxygenation of a broad range of substrates including nucleophilic nitrogen, sulphur, phosphorus and other heteroatom-containing xenobiotics, chemicals and drugs(Reference Cashman40, Reference Krueger and Williams41). Fmo1 is primarily expressed by the liver; however, species-specific differences have been noted, such that less Fmo1 is expressed by adult human liver compared to rat(Reference Cashman40). Fmo1 expression was significantly increased in response to the 60 % red bean diet (Fig. 2(c)). It is possible that bean components in the red bean diet are responsible for directly or indirectly inducing expression of this gene family as part of a normal metabolic process.
Gstm1 is an isoform of glutathione S-transferase. These transferases are involved in the detoxification of a wide variety of chemicals. Gstm1 is a hepatic glutathione S-transferase, and has been found to have an antioxidant response element within the promoter region(Reference Hayes, Flanagan and Jowsey42). Slight induction was detected in animals fed the lower bean doses with significantly increased Gstm1 expression observed at 60 % w/w red bean consumption (Fig. 2(d)). The antioxidant content of small red bean used in this study was recently reported(Reference Thompson, Brick and McGinley16); and taken together, these findings suggest that the role of this chemical class of compounds from beans be further examined as possible triggers for rat liver Gstm1 induction.
Hspa8 encodes for HSC70, a member of the 70 kDa heat shock protein family. Heat shock proteins, also known as chaperones, assist with protein synthesis, repair and degradation. They are induced in response to improperly folded proteins, and thus their up-regulation is sometimes measured as an indicator of cellular stress(Reference Rokutan, Hirakawa and Teshima43). HSC70 is constitutively expressed and involved in many housekeeping chaperone functions(Reference Daugaard, Rohde and Jaattela44). Overall, eleven genes were analysed that are involved in heat shock response pathways, and Hspa8 was the only affected gene. Expression of Hspa8 was decreased at the 60 % w/w bean dose (Fig. 2(e)). Given that Hspa8 expression was reduced in response to increased bean consumption, contrary to what would be expected for cellular stress, these findings suggest that dry bean consumption did not alter heat shock protein expression in a manner indicative of a stress response in the liver.
Mif is a macrophage migration inhibitory factor, and is a pro-inflammatory cytokine mainly produced by macrophages and monocytes(Reference Paralkar and Wistow45). Mif expression can be altered by exposure to pathogens(Reference Bacher, Meinhardt and Lan46) but may also be affected by inflammation, neuroendocrine mechanisms and glucocorticoids(Reference Donn and Ray47). While changes in Mif expression have not been thoroughly investigated in response to diet or bioactive food components, Mif expression was significantly up-regulated in liver at the 60 % w/w bean dose compared to control (Fig. 2(f)). A total of thirteen genes involved in inflammatory pathways were included in this array, and Mif was the only one in this group that demonstrated a significant change following bean consumption. Dry bean modulation of Mif expression alone did not reveal a role for beans in the initiation of a pro-inflammatory response.
Candidate genes for disease prevention by beans
Ugt1a6 is a UDP-glucuronosyltransferease (UGT) protein-encoding gene. These conjugative enzymes mediate phase II detoxification reactions as they are responsible for conjugation of the glucuronic acid group of uridine diphosphoglucuronic acid to the functional group of a wide range of substrates(Reference Iyanagi48). Fig. 3(a) shows that Ugt1a6 expression was up-regulated in a dose-dependent manner by dry bean consumption, with noteworthy induction at 7·5 % (1·5-fold), 15 % (2-fold), 30 % (2-fold) and 60 % (3-fold) w/w dry bean when compared to control. Increased Ugt1a6 expression by beans may exert cancer preventive effects in the liver of humans by facilitating the metabolism of environmental carcinogens.
Conjugation with a glucuronic acid moiety increases the solubility of its substrate, and facilitates excretion into bile or urine. Substrates for UGT enzymes include endogenous compounds such as biliary acids, steroid hormones, bilirubin, retinoic acids and fatty acids, as well as exogenous compounds including carcinogens, drugs, environmental toxicants and dietary constituents(Reference Guillemette49). Expression of the UGT1 class of enzymes is regulated via a positive feedback mechanism, such that concentrations of a particular substrate can elicit expression of the specific UGT1 isoenzyme responsible for that substrate's modification/detoxification. Thus, an increase in the concentration of a single substrate for Ugt1a6 from dietary bean intake may be the reason for further up-regulation of Ugt1a6 observed at higher doses of bean consumption. As a result of up-regulating the Ugt1a6 enzyme, glucuronidation reactions increase, facilitating removal of carcinogens or environmental toxins from the body. Induction of phase II enzymes such as UGT are considered a major mechanism by which phytochemicals become active for cancer prevention(Reference Iyanagi48, Reference Nagar and Remmel50), and is identified from this study as a target pathway that merits further investigation following increased consumption of beans in humans.
Cyp7a1 encodes for cytochrome p450 7A1, which has also been reported as cholesterol 7α hydoxylase or cholesterol 7α-mono-oxygenase. Increased expression of Cyp7a1 may promote cholesterol saturation of bile and increased excretion of cholesterol from the body(Reference Lehmann, Kliewer and Moore51, Reference Lu, Repa and Mangelsdorf52) and thus can result in lowered serum levels of cholesterol. Fig. 3(b) shows that Cyp7a1 expression increased with dietary dry bean consumption in a dose-dependent manner, with the greatest induction detected at 60 % w/w. Multiple diet intervention studies have demonstrated plasma cholesterol-lowering effects of a bean-containing diet(Reference Anderson and Gustafson6–Reference Winham, Hutchins and Johnston8, Reference Nervi, Covarrubias and Bravo53–Reference Birketvedt, Travis and Langbakk56). The mechanisms responsible for decreased serum cholesterol levels are not fully elucidated, even though this is the most consistently observed health benefit correlated with dry bean consumption. To our knowledge, these data are the first to demonstrate that induction of liver Cyp7a1 expression by dietary bean intake may be a mechanism by which increased bean consumption is protective against heart disease. These findings merit further investigation of inducible Cyp7a1 expression as a mechanism for cholesterol-reducing effects in humans.
Concluding remarks
The absence of major changes in growth rate or hepatic expression of stress and toxicity-related genes following increased dietary intake of cooked dry beans indicated that adverse effects are unlikely to occur when consumed at elevated daily doses that promote chronic disease prevention. This conclusion is based on patterns of expression for eighty-four genes involved in stress and toxicity pathways. Overall, six genes were identified from the present study that were up- or down-regulated relative to the control group, and only when the concentration of bean greatly exceeded typical consumption (e.g. 30 % or 60 % w/w).
In this study, two of the eighty-four genes, Cyp7a1 and Ugt1a6, were up-regulated with a linear response to increasing bean dose. The Cyp7a1 and Ugt1a6 gene pathways are novel target pathways that merit further investigation following bean consumption in humans due to their association with disease prevention. These data imply that high doses of dry bean consumption can be safely tested in human intervention trials to evaluate therapeutic and chronic disease preventive properties. While epidemiological studies have shown positive associations between dry bean consumption and reduced risk for cancer and heart attack; prospective, randomised and placebo-controlled clinical intervention trials are required to critically evaluate these relationships, and such studies need to consider the full range of dietary intakes that typify the world's populations, albeit within safe and achievable limits. In order to fully realise the potential for beans' health benefits, an experimental design that will examine amounts up to 600 g of cooked dry bean intake/d may be necessary for humans. Studies of increased bean consumption in human subjects should still include standard toxicity profiling of blood components and serum analytes, particularly for participants with chronic disease. There may be a number of barriers to increased bean consumption, e.g. perceived gastrointestinal discomfort, lack of knowledge about preparation methods, and perceptions that dry bean is ‘poor man's food’, that may limit the amount of dry bean that people will consume on a daily basis. However, this study demonstrates that the potential for stress, inflammatory or toxic responses at doses that may exhibit disease prevention activity is unlikely to be a major concern.
Acknowledgements
The present study was supported in part by USAID Grant No. REE-A-00-03-00094-00, the Bean Health Alliance, and United States Department of Agriculture National Institute of Food and Agricultural Agriculture and Food Research Initiative Project No.: 2009-01929. The authors thank John McGinley and Elizabeth Neil for their excellent technical assistance. E. L. D. conducted the rodent feeding, assessment of gene expression and data analyses. E. P. R. and M. A. B. participated in various aspects of study implementation and data evaluation and interpretation. H. J. T. designed the feeding study and provided oversight on all aspects of the experiment. All authors participated in the writing of the manuscript. The authors declare that there are no conflicts of interest in relation to this study.







