Hyperemesis gravidarum (hyperemesis) is characterised by excessive vomiting during pregnancy, starting before gestational week 23(Reference Fairweather1). Severe forms of the disease often lead to nutritional deficiencies, electrolyte imbalance and weight loss, and are associated with pre-term birth and low birth weight(Reference van Oppenraaij, Jauniaux and Christiansen2). The prevalence of hyperemesis varies from 0·5 to 3·2 %, and the condition is the most common cause of hospitalisation during the first half of pregnancy(Reference Adams, Harlass and Sarno3–Reference Eliakim, Abulafia and Sherer6). Despite extensive research, the aetiology of hyperemesis remains unknown(Reference Verberg, Gillott and Al-Fardan7). Earlier research has suggested various mechanisms as possible triggers for the development of hyperemesis. These include the extreme hormonal fluctuations of early pregnancy and an overactivation of the immune system(Reference Rodien, Jordan and Lefevre8, Reference Minagawa, Narita and Tada9). Although eating disorders and low or high pre-pregnancy BMI have been found to be associated with hyperemesis, pre-pregnancy nutritional status and dietary intake have barely been investigated as possible aetiological factors(Reference Lingam and McCluskey10–Reference Depue, Bernstein and Ross12).
Pregnancy is a physiological state that features enhanced oxidative stress due to high metabolic turnover and elevated tissue oxygen requirements. Hyperaemic patients have been found to have lower total antioxidant activity and higher malondialdehyde concentrations than pregnant women who did not develop hyperemesis(Reference Aksoy, Aksoy and Ozkan13). Small case–control studies have also reported that women with hyperemesis feature higher oxidative stress (including reduced levels of the antioxidant glutathione) and higher reactive oxygen species activity, and have a lower antioxidant status, than pregnant women without hyperemesis(Reference Fait, Sela and Ophir14, Reference Guney, Oral and Mungan15). Low antioxidant status before pregnancy may thus contribute to the development of hyperemesis due to the increased requirement for antioxidants during pregnancy.
In a 1998 study, Signorello et al. (Reference Signorello, Harlow and Wang16) compared the pre-pregnancy dietary intakes of forty-four women who had developed hyperemesis with the dietary intakes of eighty-seven women who had not developed hyperemesis. They found that women who developed hyperemesis had a significantly higher intake of total and saturated fat than women who did not develop hyperemesis. It was speculated that a high intake of saturated fat could increase the concentration of circulating oestrogens, as increased levels of oestrogens have been linked to hyperemesis(Reference Verberg, Gillott and Al-Fardan7, Reference Depue, Bernstein and Ross12). A positive correlation between intakes of PUFA and umbilical cord oestriol concentration was reported in a more recent study, in which long-chain n-3 fatty acids were significantly negatively correlated with oestriol concentration(Reference Nagata, Iwasa and Shiraki17). In a study published in 2009, no association was found between fat intake and oestriol concentration during pregnancy(Reference Lof, Hilakivi-Clarke and Sandin18). However, both studies evaluated dietary intakes during pregnancy, and neither study found any association between dietary intake and hyperemesis progression. Whether dietary intake before pregnancy may play a part in hyperemesis development remains an open question.
In the Norwegian Mother and Child Cohort Study (MoBa), 9000 pregnant Norwegian women answered a FFQ about their dietary intake during the year before pregnancy(Reference Magnus, Irgens and Haug19). Using this information, we wanted to investigate whether pre-pregnancy food and nutrient intake (and the intake of fat in particular) was associated with a risk of developing hyperemesis.
Methods
The present study is a sub-project of MoBa. In brief, MoBa is a nationwide pregnancy cohort study covering 107 000 pregnancies. It also includes follow-up of parents and children for the purpose of aetiological studies. Pregnant women were recruited to the study by postal invitation after signing up for a routine ultrasound examination at their local hospital. The participation rate was approximately 40 %(Reference Magnus, Irgens and Haug19). The mothers-to-be completed three questionnaires during pregnancy. The first questionnaire (Q1), received between weeks 13 and 17 of pregnancy, covered background factors, exposures and health variables. The second questionnaire (Q2) was a FFQ. The version of Q2 used in the present study was sent together with Q1 and asked about the subject's habitual diet during the 12 months before becoming pregnant. The third questionnaire (Q3), received around week 30 of pregnancy, included questions about health during pregnancy. The English translations of the questionnaires can be found at www.fhi.no/morogbarn. The study was carried out in accordance with the Helsinki Declaration (World Medical Association, 2002) and was approved by the Regional Committee for Ethics in Medical Research and the Data Inspectorate. Quality-assured data files (version 4, released in 2008) were used.
Dietary data
The FFQ had questions about 180 food items, grouped together according to a traditional Norwegian dietary pattern. The FFQ was answered around week 17 of pregnancy. Nutrient calculation was performed using Food Calc(Reference Lauritzen20) and the Norwegian Food Composition Table(Reference Rimestad, Borgerjordet and Vesterhus21). The FFQ data file offered 8957 records. Following the exclusion of records deemed to be of poor quality due to missing data or misreporting (energy < 4200 kJ or energy >16 700 kJ)(Reference Schulze, Manson and Ludwig22), 8753 records (98 %) were left for analysis. Daily intakes (g/d) of the 180 listed food items were combined into thirty-two non-overlapping food groups. The FFQ included eighteen questions about commonly used food supplements. Intakes of long-chain n-3 fatty acids, vitamins and minerals through the supplements were calculated separately and included in the calculations of total vitamin and mineral intake.
Outcome variable
The main outcome variable was hyperemesis, defined as prolonged nausea and vomiting during pregnancy that required hospitalisation before week 25 of pregnancy, as reported in Q3. Linking the Q3 data file with the dietary intake information left 7816 records in the dataset. Moreover, pregnancies resulting in multiple births were excluded, leaving 7710 records for analysis.
Other variables
Potentially confounding variables (i.e. variables known to be associated with hyperemesis) were pre-pregnant BMI, as recorded at the first routine examination early in the first trimester of pregnancy (categorised as < 15·5, 15·5–24·9, 25–29·5, 30+ kg/m2 and missing), length of education, as a proxy for socio-economic status (categorised as < 12, 12, 13–16, ≥ 17 years and missing), smoking before pregnancy (categorised as non-smokers, occasional smokers, daily smokers and missing), maternal age (categorised as < 20, 20–29, ≥ 30 years and missing) and parity (categorised as 0, 1+ and missing). Pre-pregnant physical activity during leisure time was checked for and categorised as no exercise, irregular, light, frequent and missing.
Statistics
Continuous and normally distributed variables were analysed using independent-sample t tests. Skewed data were analysed using the Mann–Whitney U test. Nominal data were analysed using χ2 tests. Relative risks were approximated as OR and were adjusted for confounding factors using multiple logistic regression and the tertiles of food and nutrient intake. All analyses were performed using SPSS, version 17 (SPSS, Inc., Chicago, IL, USA).
Results
In the available dataset, ninety-nine of the 7710 women (1·3 %) reported the development of hyperemesis that led to hospitalisation due to the condition. The groups of women who did and did not develop hyperemesis displayed no differences with regard to age (28·8 (sd 5·3) and 29·8 (sd 4·6) years) or pre-pregnancy weight (sd 67·7 (sd 15·0) and 67·4 (sd 12·1) kg). There was a difference in weight change between the two groups at week 17 of gestation: − 1·3 (sd 4·4) kg (hyperemesis) and +3·3 (sd 3·3) kg (non-hyperemesis) (P < 0·001). A higher percentage of women with hyperemesis belonged to the youngest age group, were underweight and reported that they were non-smokers than was the case for women in the non-hyperemesis group. However, there was no difference between the two groups with regard to the listed confounders (χ2 test; Table 1), nor was there any difference in physical leisure activity levels between the two groups (data not shown).
Table 1 Socio-demographic characteristics of women participating in the Norwegian Mother and Child Cohort Study and providing dietary information, categorised into defined groups
(Number of participants and percentages)

Energy intake during the year before becoming pregnant did not differ between the two groups, but the women who developed hyperemesis had a marginally lower mean intake of protein and alcohol (P = 0·06 and 0·07, respectively; Table 2). Estimated intakes of linoleic acid, α-linolenic acid, EPA and DHA were similar for the two groups (Table 2). There were no differences between the two groups with regard to the intake of vitamins and minerals (Table 3). A slightly higher percentage of women in the hyperemesis group did not achieve the recommended intake of various nutrients; however, the differences between the two groups were not statistically significant. The intake of vitamin D, folate and Fe from food was low for both groups, with more than 70 % of the subjects not achieving the recommended intake of 7·5 μg of vitamin D and over 80 % not achieving the recommended intakes of folate (400 μg) and Fe (15 mg) (Table 3).
Table 2 Daily intakes of energy-yielding nutrients from diet, estimated from a FFQ covering the 12 months before pregnancy for women who developed hyperemesis and those without hyperemesis
(Mean values, standard deviations, medians and percentiles)

P5, 5th percentile; P95, 95th percentile.
* Independent-sample t test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables.
Table 3 Daily intakes of vitamins and minerals from diet and food supplements, estimated from a FFQ covering the 12 months before pregnancy in women who developed hyperemesis and those who did not develop hyperemesis
(Mean values, standard deviations and percentages)

RDI, recommended dietary intake.
* The percentage of women within each group who did not achieve the intake recommended by the Nordic Nutritional Recommendations(42).
Of the women who developed hyperemesis, 59 % reported taking a dietary supplement before pregnancy, while 62 % of the women who did not develop hyperemesis took them. The use of folic acid and n-3 fatty acid supplements was similar for the two groups, but among those who reported the use of supplements, the median intake of thiamin (0·4 and 0·1 mg/d), riboflavin (0·5 and 0·1 mg/d), pyridoxine (0·4 and 0·1 mg/d) and niacin (4·2 and 1·1 mg/d) (P < 0·01 for all) was higher in the hyperemesis group than in the non-hyperemesis group.
The intake of fish and seafood, allium vegetables (the onion family), and drinking-water was lower in the hyperemesis group than in the non-hyperemesis group. Fewer subjects in the hyperemesis group had an intake of coffee and non-alcoholic beer (Table 4). The intake of fish and seafood, drinking-water and allium vegetables was categorised into tertiles, which were used in the logistic regression. The intake amounts (in g/d) for the tertiles are given in Table 5.
Table 4 Percentage of users and the daily intake of certain food groups estimated from a FFQ covering the 12 months before pregnancy among women who developed hyperemesis and those who did not
(Mean values and standard deviations)
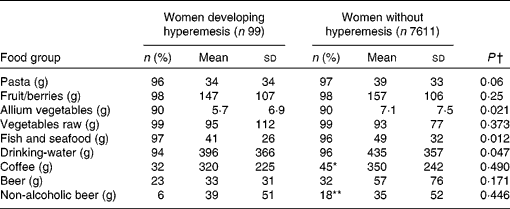
Values were significantly different (χ2 test comparing users and non-users in the two groups): * P < 0·05, ** P < 0·01.
† Mann–Whitney U test.
Table 5 Association between intake of fish and seafood, allium vegetables, and drinking-water and the development of hyperemesis, estimated from a FFQ covering 12 months before pregnancy with the use of logistic regression
(Mean values, standard deviations, odds ratios and 95 % confidence intervals)

* Adjusted for energy intake.
† Additional adjustment for pre-pregnancy BMI, maternal age and smoking.
‡ Additional adjustment for parity and education.
In the unadjusted logistic regression, the highest tertiles of the fish and seafood and allium vegetable groups were associated with a reduced risk of hyperemesis, whereas in the case of water intake, the second tertile was associated with reduced risk (Table 5). In the adjusted models, the intakes of water and fish and seafood remained protective, while the intake of allium vegetables did not. Associations with water intake were not affected by the intakes of fish and seafood and allium vegetables, whereas fish and seafood intake was correlated with allium vegetable intake.
Discussion
In the present study, we investigated food and nutrient intake before pregnancy, reported at gestational week 17. Although no differences in nutrient intakes were observed between women who did and did not develop hyperemesis, women who developed hyperemesis reported a lower intake of fish and seafood, allium vegetables and water. Drinking-water in moderate amounts was associated with the lowest relative risk of hyperemesis development. The intake of coffee and non-alcoholic beer was more frequent in the non-hyperemesis group.
In the present study, 1·3 % of the subjects developed hyperemesis, which is a higher rate than that reported previously for Norway and other Western countries. However, the incidence falls well below the rates reported for other countries(Reference Vikanes, Grjibovski and Vangen5, Reference Trogstad, Stoltenberg and Magnus23, Reference Kallen, Lundberg and Aberg24). Women included in MoBa are not representative of all pregnant Norwegian women, and it has been reported that the selection of women for this cohort study was biased(Reference Magnus, Irgens and Haug19, Reference Nilsen, Vollset and Gjessing25). Women younger than 25 years, smokers and living alone are under-represented, while multivitamin and folic acid users are over-represented in the cohort(Reference Nilsen, Vollset and Gjessing25). These are all factors that could influence the results in different directions(Reference Eliakim, Abulafia and Sherer6, Reference Kallen, Lundberg and Aberg24). Additionally, heavy nausea in early pregnancy may have influenced the subjects' ability and desire to sign up for the study. Such selection bias will influence prevalence estimates but has not been found to change the estimates of the association between exposure and disease(Reference Nilsen, Vollset and Gjessing25). Moreover, women who reported food intake retrospectively and thus nausea at the time of completing the FFQ may have affected the results. However, in the present study, the reported total energy intake before pregnancy did not differ between the hyperemesis and non-hyperemesis groups.
The strengths of the present study include a fairly large sample size and detailed dietary intake data for the year before pregnancy. MoBa included subjects from both urban and rural regions, and covered a wide range of ages and socio-economic groups.
Previous research on hyperemesis has been influenced by the fact that less severe nausea and vomiting, which occurs in up to 80 % of all pregnancies, and hyperemesis have been studied as one and the same condition(Reference Verberg, Gillott and Al-Fardan7). This complicates the comparison of different studies and may explain the divergent results. In the present study, inclusion in the hyperemesis group was conditional upon hospitalisation due to prolonged nausea and vomiting. Inclusion of the severe hyperemesis cases was further supported by a reported average weight loss of 1·2 kg by the hyperemesis group at gestational week 17, whereas the non-hyperemesis group reported an average weight gain of 3·1 kg at week 17.
The aetiology of hyperemesis is poorly understood. Psychological and biochemical explanations have been suggested(Reference Eliakim, Abulafia and Sherer6). Vitamin and antioxidant deficiencies as well as fatty acid peroxidation have been suggested as aetiological factors(Reference Fait, Sela and Ophir14, Reference van Stuijvenberg, Schabort and Labadarios26, Reference Guney, Oral and Mungan27). Vitamin deficiency has been reported in connection with hyperemesis, but not as a cause of the condition(Reference van Stuijvenberg, Schabort and Labadarios26). Intravenous rehydration and multivitamin infusion combined with anti-emetic medication is the treatment of choice for hyperemesis patients (Norwegian Guidelines). By contrast, the use of vitamin supplementation before and during early pregnancy has been found to alleviate the symptoms of women with less severe nausea and vomiting during pregnancy(Reference Eliakim, Abulafia and Sherer6, Reference Kallen, Lundberg and Aberg24). In the present study, no difference in total vitamin intake was observed between the two groups, although a higher intake of some B vitamins from food supplements was observed in the hyperemesis group. This might indicate that dietary intake was lower for these vitamins, however, not showing statistically for the total group. B vitamin supplementation is used in the treatment of hyperemesis, and it is possible that a low B vitamin status may contribute to the development of hyperemesis in some women, although our data do not support this theory.
Antioxidants are ascribed important biological properties such as the prevention of DNA damage(Reference Lu, Lin and Yao28), immunomodulation(Reference Pattison and Winyard29) and reduced lipid peroxidation(Reference Lu, Lin and Yao28, Reference Perron and Brumaghim30). Dietary antioxidants include not only vitamins or minerals, but also hundreds of non-nutrient compounds, such as flavonoids and carotenes. Fruit and vegetables are important sources of antioxidants, but intakes of these did not differ between the two groups, except in the case of allium vegetables. Allium vegetables include onions, shallots, leeks, scallions, chives and garlic, which are rich in flavonoids and organosulphur compounds(Reference Sengupta, Ghosh and Bhattacharjee31). The protective effect of allium vegetables may also reflect other properties of this food group, as allium vegetables have traditionally been known for their antibacterial and fungicidal properties. Garlic, onions and leeks contain the antimicrobial component allicin, which is known to exhibit broad antibiotic properties, effective against Gram-negative bacteria such as Helicobacter pylori (Reference Sivam32). H. pylori infection has been associated with hyperemesis in a dose–response pattern(Reference Golberg, Szilagyi and Graves33, Reference Sandven, Abdelnoor and Wethe34). It has been speculated that certain food items such as garlic and onion may protect against bacterial infections(Reference O'Gara, Maslin and Nevill35, Reference Canizares, Gracia and Gomez36). However, hyperemesis is likely to have a multi-factorial aetiology, and H. pylori infection is probably only one of several risk factors that contribute to the development of the condition.
A higher intake of fat, especially saturated fat, has been reported in women who have developed hyperemesis(Reference Signorello, Harlow and Wang16). In the present study, we found no difference in fat intake between the two groups, although the non-hyperemesis group displayed a slightly higher intake of long-chain n-3 fatty acids that was of borderline significance (P = 0·09). The higher intake of such fatty acids could be explained by a higher consumption of fish and seafood. The consumption of fish and seafood has been found to have a protective effect with regard to pre-eclampsia(Reference Brantsaeter, Haugen and Samuelsen37) and pre-term birth(Reference Haugen, Meltzer and Brantsaeter38). Much of this effect has been attributed to long-chain n-3 fatty acids. However, a high intake of fish may also indicate a healthier general dietary pattern(Reference Brantsaeter, Haugen and Samuelsen37).
The most interesting, and somewhat surprising, result in the present study was that drinking-water intake was associated with a reduced risk of hyperemesis. The effect was not linear, although still protective in the third tertile. Water intake is vital for all life and plays numerous roles in the human body, including acting as a carrier for nutrients and waste products. Sufficient fluid intake facilitates increased diuresis, which may improve the clearance of potentially emetogenic substances from the body(Reference Jequier and Constant39). No clinical study on liquid intake and hyperemesis development provides support for this finding/hypothesis, but the general recommendations for the treatment of hyperemesis include hydration(Reference Goodwin, Poursharif and Korst40, Reference Sheehan41). The strongest protective effect of drinking-water with regard to hyperemesis development was observed in the range of 200–450 g/d, equalling one to two glasses. The mean intake in the third tertile was 800 g/d, corresponding to the generally recommended daily amount, although the highest intake in the third tertile was almost 2 litres/d. This indicates that a higher intake of drinking-water may not be better. Although we did not observe any association between total liquid intake and hyperemesis, further investigation is required to establish whether a low intake of liquid or just a low water intake may influence hyperemesis development.
In the present study, we investigated the nutrient and food intakes of pregnant women during the 12 months before pregnancy. The results indicate that women with a high intake of fish and seafood and a high intake of allium vegetables have a reduced risk of developing hyperemesis. The intake of one to two glasses of water daily seemed also to be protective against hyperemesis development.
Acknowledgements
The present study was supported by the Norwegian Ministry of Health, National Institute of Health/National Institute of Environmental Health Sciences (grant no. N01-ES-85433), National Institute of Health/National Institute of Neurological Disorders and Stroke (grant no. 1 UO1 NS 047537-01), the 6th Research Framework of the European Union (EARNEST) and the Norwegian Research Council/FUGE (Functional Genomics) (grant no. 151918/S10). No conflicts of interests are reported in connection with this paper. M. H., A. V. and P. M. designed the research paper. M. H. analysed the data and wrote the manuscript, while A. L. B., H. M. M. and A. M. G. had the primary responsibility for its final content. All authors read and approved the final manuscript.







