Vitamin D plays an important role in skeletal health, and vitamin D deficiency is known to be a cause of rickets and osteoporosis( Reference Holick 1 ). In addition, a wide range of tissues and cells have been found to possess vitamin D receptors. Observational studies have suggested that low 25-hydroxyvitamin D (25(OH)D) values are associated with an increased risk for several non-skeletal diseases, including cancer( Reference Giovannucci, Liu and Rimm 2 , Reference Lappe, Travers-Gustafson and Davies 3 ), infectious diseases( Reference Bergman, Lindh and Bjorkhem-Bergman 4 – Reference Cannell, Vieth and Umhau 7 ) and CVD( Reference Wang, Pencina and Booth 8 ). Vitamin D inadequacy is being increasingly recognised worldwide, and remains common in children and adults( Reference Holick 1 , Reference Wahl, Cooper and Ebeling 9 , Reference Hilger, Friedel and Herr 10 ).
Human subjects acquire vitamin D from exposure to sunlight, from their diet and from dietary supplements( Reference Holick 1 ). The main natural source of vitamin D is the sun, as vitamin D is synthesised in the skin after exposure to solar UV B radiation (wavelength 290–315 nm)( Reference Holick 1 ). A diet high in oily fish prevents vitamin D deficiency( Reference Chen, Chimeh and Lu 11 ). Vitamin D from the skin and diet converts to 25(OH)D in the liver and subsequently to 1,25-dihydroxyvitamin D (1,25(OH)2D) in the kidney, which is the active form of vitamin D( Reference Holick 1 ). 25(OH)D is the principal form of vitamin D that circulates in the blood stream and can be used as a marker to determine vitamin D status( Reference Holick 12 ).
Hong Kong is a subtropical coastal city in southern China, with sufficient sunshine during the whole year and fish is commonly consumed in the local diet. However, there are few data on vitamin D status by age and sex in residents, and on the effect of dietary and sun exposures on vitamin D status in Hong Kong. Seasonal variation in vitamin D status is thought to play a role in the seasonality of bone mass( Reference Viljakainen, Palssa and Kärkkäinen 13 , Reference Bhattoa, Bettembuk and Ganacharya 14 ). However, there is a paucity of data on the seasonality of vitamin D levels in subtropical Hong Kong, where there is relatively little variation in the hours of sunlight throughout the year.
We conducted a household-based prospective study from September 2009 through December 2010 in Hong Kong( Reference Cowling, Ng and Ma 15 ). The study was primarily designed to study the direct and indirect effectiveness of influenza vaccination among school-age children in preventing influenza virus infections in their households. For the present study, we determined vitamin D status in stored sera to describe the seasonal variation in vitamin D status in children and adults over time, and to investigate the determinants of vitamin D status. Our present study also included an additional questionnaire survey conducted among participating children 6–17 years of age in April and May 2010 to collect information on sun-seeking behaviors, and dietary and supplementary habits that might affect vitamin D status.
Methods
Study participants
Participants included in this study of vitamin D were part of a household-based prospective study of influenza, as describe elsewhere( Reference Cowling, Ng and Ma 15 ). In 2009–2010, we recruited all members of 796 households, and each household included a child 6–17 years of age who was randomly allocated to receive either a single dose of seasonal trivalent inactivated influenza vaccine or placebo in a double-blind manner. Enrollment, collection of serum specimens and vaccinations were performed by trained research staff at a study clinic. Serum specimens were collected at baseline (September 2009 through February 2010) and after 12 months at the end of the follow-up period (‘post-study’, October through December 2010). Serum specimens were also collected 1 month after vaccination from the children who received vaccine or placebo (‘post-vaccination’, October 2009 through February 2010). A subset of participants also provided blood samples half-way through the study (‘mid-study’, April and May 2010).
Using a vitamin D questionnaire designed according to previous studies in the United States( Reference Nucci, Russell and Luo 16 , Reference Bolek-Berquist, Elliott and Gangnon 17 ), we collected data about sun-seeking behaviors, and dietary and vitamin D supplementary habits from these children aged 6–17 years who also provided mid-study serum specimens in April and May 2010. The questionnaires were completed by the children together with their parents.
Ethics
Written consent was obtained from all adult subjects. Proxy written consent from parents or legal guardians was obtained for participants 17 years of age and younger, with additional written assent from those aged 8–17 years. The study protocol was approved by the Institutional Review Board of The University of Hong Kong.
Laboratory analysis
Blood from all household members were collected in tubes containing clot activator and held at 4–8°C from collection until receipt at the laboratory. At the laboratory, each specimen was centrifuged to extract the sera, which was then frozen at − 80°C. The serum specimens were subsequently tested for 25(OH)D using the OCTEIA ELISA 25-Hydroxyvitamin D Immunoassay Kit manufactured by Immunodiagnostic Systems Limited( Reference Roth, Schmidt-Gayk and Weber 18 ). According to the package insert of the assay, the inter-assay CV for the 25(OH)D assay was 4·6–8·7 %, and the intra-assay CV was 5·3–6·7 %. In our own laboratory, we found that the intra-assay CV was 7·4 %.
Statistical analysis
We anticipated that we would have at least 80 % power to detect at least a 9 nmol/l difference in serum 25(OH)D between any two groups (four age groups and male/female) in each season, assuming a standard deviation of 15–18nmol/l based on data available for mean and standard deviation of serum 25(OH)D by sex in a normal population from the literature( Reference Ono, Suzuki and Kotake 19 ). The sample size of sixty-three in each age or sex group would be adequate to test the difference in the mean of serum 25(OH)D by age or sex in a single season. We anticipated that the present overall study sample size of 2694 individuals with repeated measurements would permit reliable comparisons between seasons, by age and sex, and would allow us to identify moderate effects of determinants after accounting for serial correlation in the measurements.
The participants were categorised into four age groups, i.e. 6–17, 18–44, 45–64 and ≥ 65 years. The four seasons were defined as spring (March–May), summer (June–August), autumn (September–November), and winter (December–February), respectively. The 25(OH)D levels were categorised into different seasons based on the data of specimen collection. If two specimens from the same subject were categorised to the same season, we used the average 25(OH)D level of the two specimens. Since no blood specimens were collected in the study during June to August, no data on 25(OH)D levels in the summer of 2010 were available.
We used a generalised linear model to compare the mean of serum 25(OH) by age and sex in each season to estimate age-specific and sex-specific patterns in serum 25(OH)D levels. Since solar radiation can reflect climatic season, we fitted a random-effects linear regression model to obtain quantitative seasonality estimates of serum 25(OH)D based on the repeated measures of serum 25(OH)D, which included daily level of solar radiation as a predictive factor. Daily means of solar radiation were obtained from Hong Kong observatory, and were smoothed using Kernel density smoothing as a proxy measure for seasonal variation in the climate in Hong Kong( 20 ). In a separate secondary analysis, a random-effects sinusoidal linear regression model with annual periodicity was fitted to characterise the seasonal variation of serum 25(OH)D. In the two random-effects linear regression models used to estimate the seasonal variation of serum 25(OH)D, the associations of 25(OH)D with age, sex, educational attainment of the household head, vaccination and chronic conditions were adjusted for. The ratio of serum 25(OH)D levels between the peak season and the trough season in each age group was calculated to estimate the degree of seasonal variation in serum 25(OH)D levels.
Since both vitamin D questionnaires and mid-study sera were collected simultaneously from a subset of participating children aged 6–17 years in April to May 2010, we performed univariable and multivariable analyses to explore the determinants of serum vitamin D levels among children using generalised linear models. A multiple linear model with backward selection was used to exclude variables one by one from an initially complete model. Only the factors with P-values < 0·2 were included in the final model. Statistical analyses were conducted in R version 2.15.1 (R Foundation for Statistical Computing) and SAS version 9.2 (SAS Institute).
Results
Characteristics of participants
In total, 3030 people participated in the previous influenza household study, and fifty-three people from fourteen households withdrew or were lost to follow-up. From 3030 participants, 2694 (89 %) had at least one serum specimen available for 25(OH)D testing (Table 1). Of the 2694 participants, 2459 (91 %) and 1341 (50 %) had two or more and three or more serum specimens available for 25(OH)D testing, respectively (Fig. 1). There was no difference in age, sex, educational attainment of household head, vaccination history and chronic conditions between 3030 participants in the influenza household study and 2694 participants included in the vitamin D analysis (Table 1). The median age of these 2694 participants was 33 years (interquartile range 11–43 years), and 46 % were male. Of these 2694 participants, 21 % reported receipt of 2009–2010 seasonal influenza vaccine, and 16 % had a self-reported chronic condition.
Table 1 Demographic characteristics of participants in this study (Number of participants and percentages)
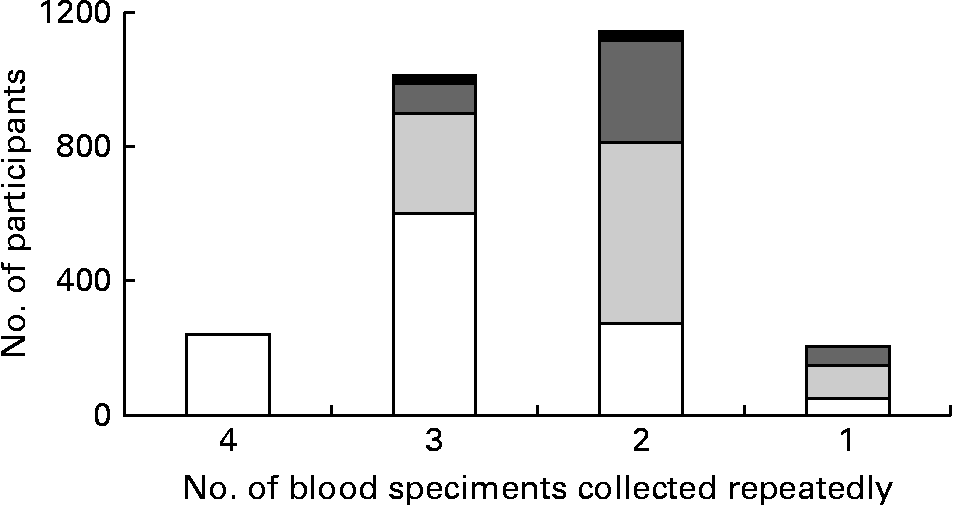
Fig. 1 The number of serum specimens collected repeatedly in four age groups (6–17 years (□), 18–44 years (![]() ), 45–64 years (
), 45–64 years (![]() ) and ≥ 65 years (
) and ≥ 65 years (![]() )).
)).
Mean of serum 25-hydroxyvitamin D by age and sex in different seasons
Table 2 presents the comparative analysis of serum 25(OH)D levels in each season by age and sex. In each season, children aged 6–17 years had significantly lower vitamin D levels (39–53 nmol/l) compared to adults aged 18–44 years (42–57nmol/l) (all P< 0·001). Adults aged 45–64 years (47–63 nmol/l) had significantly higher serum 25(OH)D levels than adults aged 18–44 years in the other three seasons (all P< 0·01) except in the winter of 2009–2010.The mean serum 25(OH)D level in adults aged 65 years or older (41–56 nmol/l) was not significantly different from adults aged 18–44 years in each season. Males had significantly higher serum 25(OH)D levels (3–5 nmol/l) than females in each season.
Table 2 Comparison of serum 25-hydroxyvitamin D (25(OH)D) levels (nmol/l) in each season by age and sex using a generalised linear model (Mean values and 95 % confidence intervals)

Sep, September; Nov, November; Dec, December; Feb, February; Ref., reference group.
* P value for comparing serum 25(OH)D levels in different age/sex groups with referent age/sex group in each season.
Seasonal variation of serum 25-hydroxyvitamin D
The pattern of daily solar radiation showed one peak (August) in Hong Kong (Fig. 2). Using the random-effects linear regression model, we found that the daily level of solar radiation, age and sex were significantly associated with serum 25(OH)D levels after adjusting for other factors (online Supplementary Table S1). For males and females in the age groups of 6–17, 18–44, 45–64 years, the model that included a 5-week lag in solar radiation gave the best fit to time-varying serum 25(OH)D levels (all P< 0·05) (Fig. 3(a)–(c)). We identified significant seasonal fluctuation in serum 25(OH)D levels for males and females in the age groups of 6–17, 18–44 and 45–64 years, which peaked in September (autumn), and dropped to lowest levels in March (Spring). As much as 10·6 % of the variation in vitamin D levels was explained by the inclusion of seasonal variation in solar radiation in the model. In all four age groups, the average of predicted serum 25(OH)D levels in boys/men was 4–9 nmol/l higher than in girls/women (all P< 0·05). In a secondary analysis using the random-effects sinusoidal linear regression model, we found that there was a similar degree of seasonal fluctuation in serum 25(OH)D levels for different age and sex groups to the first random-effects model, while the first random-effects model incorporating solar radiation better explained the seasonal variation in serum 25(OH)D levels. The ratio of serum 25(OH)D levels between the spring and the autumn of 2010 in each age group varied from 1·3 to 1·4.

Fig. 2 Daily levels of solar radiation (MJ/m2) that were obtained based on daily means of solar radiation from Hong Kong observatory using Kernel density smoothing as a proxy measure for meteorological season (![]() , daily means of solar radiation, —, daily level of solar radiation). J, January; F, February; M, March; A, April; M, May; J, June, J, July; A, August; S, September; O, October; N, November; D, December.
, daily means of solar radiation, —, daily level of solar radiation). J, January; F, February; M, March; A, April; M, May; J, June, J, July; A, August; S, September; O, October; N, November; D, December.

Fig. 3 Serum 25-hydroxyvitamin D (25(OH)D) levels (nmol/l) from each individual and a random-effects linear regression model of serum 25(OH)D level fitted to daily level of solar radiation as a covariate, adjusting for age groups and sex. The vitamin D levels in subjects (a) 6–17 years, (b) 18–44 years, (c) 45–64 years and (d) ≥ 65 years, and — in each figure indicate the mean levels of serum vitamin D for men and women in the fitted model (![]() , male and
, male and ![]() , female). J, January; F, February; M, March; A, April; M, May; J, June, J, July; A, August; S, September; O, October; N, November; D, December.
, female). J, January; F, February; M, March; A, April; M, May; J, June, J, July; A, August; S, September; O, October; N, November; D, December.
Factors that influence serum 25-hydroxyvitamin D among children
A total of 321 children completed vitamin D questionnaires and also provided mid-study serum specimens in April and May 2010. The median age of participants in the questionnaire survey was 11 years (interquantile range 9–12 years). As much as 86 % of participants reported a suntan in the past year, and 20 % reported an average of at least 1 h of sun exposure/d in the past week; 21, 30 and 38 % of participants reported having an average of at least 1 daily serving of fish, milk and eggs, respectively; 9, 6 and 60 % reported the use of additional vitamin D supplements, intake of multivitamins, and use of cod liver or fish oil, respectively.
In univariable analyses, younger age, male sex, reporting a suntan, having at least 1 serving of fish/week, having at least 1 serving of milk/d, and taking cod liver oil or fish oil were significantly associated with higher serum 25(OH)D levels (Table 3). In multivariable analysis, younger age, male sex, reporting a suntan, having at least 1 serving of fish/week and having at least 1 serving of eggs/week were independently associated with higher serum 25(OH)D levels (Table 3).
Table 3 The individual characteristics of sun-seeking behaviors, diet and vitamin D supplements, and their associations with serum 25-hydroxyvitamin D (nmol/l) levels among children 6–17 years of age in Hong Kong, in April and May 2010 (Number of participants and percentages; adjusted β coefficients and 95 % confidence intervals)

Ref., reference group.
* A multiple linear model with backward selection was used. Only the factors with P values < 0·2 were included in the final model.
Discussion
In the present study, we characterised seasonal fluctuations in serum 25(OH)D levels in subtropical Hong Kong at 22° latitude, identifying peaks in September and troughs in March, following a lagged pattern relative to climatic seasons. We found that the mean of serum 25(OH)D levels in the peak season for each age group was 1·3 to 1·4 times higher than that in the trough season, while the peak:trough ratios tend to be slightly greater in temperate locations such as the Netherlands( Reference Khoo, Koenen and Chai 21 ), Germany( Reference Zittermann, Scheld and Stehle 22 ), Italy( Reference Carnevale, Modoni and Pileri 23 ) and Japan( Reference Ono, Suzuki and Kotake 19 ). In spring, the means of serum 25(OH)D in each of four age groups were below 50 nmol/l that is recommended by the Institute of Medicine RDA( Reference Ross, Manson and Abrams 24 ), and in the other seasons, these values were below the requirements recommended by the International Osteoporosis Foundation and the US Endocrine Society ( ≥ 75 nmol/l)( Reference Dawson-Hughes, Mithal and Bonjour 25 ). In Hong Kong, the means of serum 25(OH)D in different age groups were also lower than those reports at the similar age groups from Japan, Thailand and Vietnam in Asia and most reports from the countries in North America( Reference Hilger, Friedel and Herr 10 , Reference Nakamura, Nashimoto and Hori 26 – Reference Soontrapa and Chailurkit 31 ). Moreover, the means of serum 25(OH)D the present study reported were lower than that (77 nmol/l) in Taiwan where the latitude (25°) is similar to Hong Kong( Reference Tsai, Hsu and Cheng 32 ). The reasons why living in Hong Kong with lower latitudes does not appear to protect against vitamin D insufficiency is likely due to several factors, potentially including less time spent outdoors, less vitamin D intake from diet or dietary supplements, skin pigmentation of the local Chinese residents( Reference Chen, Chimeh and Lu 11 ), air pollution( Reference Kelishadi, Moeini and Poursafa 33 ) or other racial differences in genetic polymorphism( Reference Powe, Evans and Wenger 34 ).
Similar to the findings from several temperate locations( Reference Ono, Suzuki and Kotake 19 , Reference Davies, Bates and Cole 35 – Reference Kasahara, Singh and Noymer 40 ), the present study estimated that there is substantial seasonal fluctuation in serum 25(OH)D levels in Hong Kong. Previous studies in subtropical Taiwan, Florida and Hong Kong reported the differences in serum 25(OH)D level between summer (or autumn) and winter( Reference Tsai, Hsu and Cheng 32 , Reference MacDonald and Swaminathan 41 , Reference Levis, Gomez and Jimenez 42 ). However, the present study of 15-month study duration was able to predict the year-round seasonal fluctuation by using a cyclic regression model, although there was a lack of data on summer levels of serum 25(OH)D in the present study. Sun exposure and solar radiation are known to be a major determinant of vitamin D status( Reference Holick 1 ) and the seasonal pattern of vitamin D in Hong Kong is consistent with seasonal variation in solar radiation. In Hong Kong, hours of sunlight (136 and 111 h/month, respectively) and solar radiation (10 and 12 MJ/m2, respectively) in winter and spring are lower than those (182 and 182 h/month; 16 and 14 MJ/m2 respectively) in summer and autumn( 20 ). The weather in winter and spring is suitable for outdoor activity in Hong Kong, while in the autumn temperatures are still high (22–27°C), so people also reduce outdoor activity during daytime. A previous study in Hong Kong in the 1980s reported that the means of serum 25(OH)D levels in young healthy people were 26·8 and 23·4 μg/l (equal to 67·0 and 58·5 nmol/l) in September and January, respectively( Reference MacDonald and Swaminathan 41 ), which were higher than the means in the present study for the age group 18–44 years at similar months.
Some previous studies found that ageing is associated with the reduction of vitamin D synthesis; however, the association of age with vitamin D status in children, young adults and middle-aged adults is inconsistent( Reference Ono, Suzuki and Kotake 19 , Reference Sherman, Hollis and Tobin 43 ). The present study found that for adults under 65 years and children aged 6–17 years, serum 25(OH)D levels increased with age. This could be explained by children having the capacity to produce 25(OH)D and 1,25(OH)2D due to healthy renal and liver function, whereas adults may produce less of these metabolites due to declining renal function and decreasing capacity of the skin to produce vitamin D precursors. As in Asian and Western countries, the present study also provided evidence that females had lower 25(OH)D levels than males( Reference Ono, Suzuki and Kotake 19 , Reference Levis, Gomez and Jimenez 42 , Reference Robien, Butler and Wang 44 – Reference Choi, Oh and Choi 46 ). The sex difference in serum 25(OH)D status could be explained by men and boys having more sunlight exposure, and more usage of sunscreen by girls or women because of cosmetic concerns.
We identified five factors associated with higher serum 25(OH)D levels among children 6–17 years of age, namely younger age, male sex, reporting a suntan, having at least 1 serving of fish/week and having at least 1 serving of eggs/week. Only a limited number of foods naturally contain vitamin D. Oily fish and egg yolks are rich in both vitamin D3 and 25(OH)D3, which is consistent with more fish and egg ingestion helping to increase serum 25(OH)D3 levels( Reference Lamberg-Allardt 47 , Reference Schmid and Walther 48 ). A suntan reflects a large amount of cutaneous sun exposure, so children reporting a suntan had higher serum 25(OH)D level( Reference Bolek-Berquist, Elliott and Gangnon 17 ). The higher serum 25(OH)D levels in children aged 6–8 years and boys might be related to more skin synthesis after sun exposure. However, reporting a suntan and the amount of hours of sun exposure in the recent week collected in the questionnaire could not reflect fully the duration of sun exposure in the longer period and the timing of sun exposure related to the zenith angle of the sun.
This present study has several limitations. First, seasonal variation in serum 25(OH)D was assessed using the data collected over 15 months with a lack of data on 25(OH)D in the summer months, and a longer time series of 25(OH)D levels would improve the determination of the seasonal variations of 25(OH)D. Second, the present study had a limited sample size in elderly persons ( ≥ 65 years) and this reduced the precision of estimates in that age group. Third, the factors associated with serum 25(OH)D level among children might not be the same for adults. Finally, we did not select participants at random from the population of Hong Kong, and our estimates of 25(OH)D levels might need adjustment before being used to infer the mean of serum 25(OH)D in the population as a whole.
In conclusion, we identified seasonal variation in serum 25(OH)D in Hong Kong, peaking in early autumn (September) and troughing in early spring (March). Children aged 6–17 years, and girls and women had lower serum 25(OH)D levels than adults, boys and men. For children aged 6–17 years, more sunlight exposure and more intake of fish and eggs could improve vitamin D status.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001683
Acknowledgements
This study was supported by the Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau of the Hong Kong SAR Government (grant nos CHP-CE-03 and 11100862), and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
D. K. M. I. has received research funding from F. Hoffmann-La Roche Limited. J. S. M. P. receives research funding from Crucell NV. G. M. L. has received consulting honoraria from Janssen Pharmaceuticals. B. J. C. has received research funding from MedImmune, Inc. and Sanofi Pasteur, and consults for Crucell NV. The authors report no other potential conflicts of interest.
The authors' contribution are as follows: C. X. and B. J. C. contributed to the study conception and design. V. J. F., S. N., D. K. M. I., A. M.-S. K., G. M. L. and B. J. C. collected data. R. A. P. M. P. and J. S. M. P. conducted laboratory tests. C. X. and V. J. F. analysed data. C. X. wrote the first draft of the paper. All authors contributed to the interpretation of data and approved the final manuscript.
We thank Chan Kit Man, Calvin Cheng, Lai-Ming Ho, Ho Yuk Ling, Nicole Huang, Lam Yiu Pong, Lincoln Lau, Winnie Lim, Tom Lui, Tong Hok Leung, Loretta Mak, Eunice Shiu, Joey Sin, Jessica Wong, Kevin Yau and Eileen Yeung for research support. We thank Susan Chiu for helpful discussions.









