Pancreatic ductal adenocarcinoma (PDAC) is the most common malignant tumour of the pancreas, with a 5-year survival rate <6 %(Reference Kamisawa, Wood and Itoi1). Currently, surgery remains the only potential curative therapy for PDAC, though <20 % of patients are eligible for a radical resection when diagnosed. Nevertheless, some patients with resectable PDAC could not tolerate surgery due to compromised nutritional status; thus, they might lose the chance for radical resection.
In Western countries, obesity is associated with cancer development and progression(Reference Kuipers, Grady and Lieberman2), especially in PDAC patients(Reference Jacobs, Newton and Patel3). However, malnutrition is common in diagnosis (hypoalbuminaemia, low BMI, weight loss, etc.)(Reference Gilliland, Villafane-Ferriol and Shah4), especially in China(Reference Xie, Li and Gu5,Reference Xie, Gu and Zhang6) . The status of malnutrition could be attributed to pancreatic exocrine insufficiency, gastrointestinal obstruction and cancerous pain(Reference Kanda, Fujii and Kodera7). Recently, many researches, especially in east Asia(Reference Kanda, Fujii and Kodera7–Reference Yamada, Eguchi and Asaoka9), have focused on the importance of patient’s nutritional status in treating PDAC. Inferior nutritional status can increase the morbidity and mortality in patients followed by pancreatoduodenoectomy(Reference La Torre, Ziparo and Nigri10,Reference Sierzega, Niekowal and Kulig11) . Malnutrition is always accompanied by immunological deterioration, which may contribute to tumour recurrence and metastasis by suppressing tumour-specific immunity, and it had been reported to predict adverse oncological outcomes(Reference Gao, Zhou and Jiang12,Reference Gridley, Stickney and Nutter13) .
Low preoperative Prognostic Nutritional Index (PNI) value was found to be an independent risk factor for poor survival in many malignancies(Reference Sakurai, Tamura and Toyokawa14–Reference Noh, Han and Cho16), including PDAC(Reference Kanda, Fujii and Kodera7). Controlling Nutritional Status (CONUT) is another newly purposed index for objectively assessing patients’ nutritional status(Reference Ignacio de Ulibarri, Gonzalez-Madrono and de Villar17). Compared with PNI, CONUT also took serum cholesterol, which was an important biochemical parameter in most pancreas diseases, into account when accessing patients’ immunonutritional status. The prognostic significance of preoperative CONUT score has been noticed in many malignancies(Reference Ishihara, Kondo and Yoshida18,Reference Iseki, Shibutani and Maeda19) , but few researches evaluated the efficiency in PDAC.
The significant baseline characteristics difference could be found between the two groups, which had been well-known indicators for poor prognosis(Reference Yoshida, Harada and Baba20,Reference Harimoto, Yoshizumi and Sakata21) , thus may produce great bias. Given this dilemma, propensity score matching (PSM), a widely used statistical approach to obtain highly selected patients with similar baseline characteristics and significantly improve the quality of further statistical analysis, is a suitable method to avoid potential bias(Reference Austin22).
In the present study, we balanced the baseline characteristics using PSM to evaluate the significance of preoperative PNI or CONUT score as a predictor of postoperative complications and overall survival (OS) in resectable PDAC patients.
Methods
Ethics statement
This study was approved and was taken in accordance with the Declaration of Helsinki and the Clinical Research Ethics Committee of Huashan Hospital. All patients agreed to voluntarily donate their clinical data and follow-up data for research-related purposes only and signed an informed consent form on admission(Reference Xie, Gu and Zhang6).
Patients
Patients included in this study have to fulfill the following criteria: (1) aged from 18 to 80 years; (2) resectable primary tumour and confirmed by postoperative histopathological examination and (3) Eastern Cooperative Oncology Group score ranged from 0 to 2. Patients with the following criteria were excluded from our study: (1) patients received any preoperative neoadjuvant chemotherapy, chemoradiation therapy or other anti-tumour immunotherapy were excluded from this study and (2) patients with any distant metastasis or arterial involvement, which was confirmed pre- or intra-operatively. From 2012 to 2014, totally, 566 patients were eligible enrolled in this study. After further excluding those with incomplete follow-up data (survival data, postoperative metastasis data, etc.) (n 96), not willing to take part in this study (n 39), patients with perioperative immunotherapy (n 125) and eventually a total of 306 participants were enrolled in the final analyses of the present study. Pathological tumour stage was assessed according to the tumour, node, metastasis (TNM) staging system (AJCC, 8th edition)(Reference Chun, Pawlik and Vauthey23).
Perioperative evaluation and follow-up
Serum samples of all patients were obtained and analysed within 1 week before surgery. Parameters included a complete blood cell count, serum albumin (ALB), alanine transaminase, total bilirubin, total cholesterol, carbohydrate antigen (CA) 125 and CA 19-9. CT or/and MRI was performed to evaluate primary tumour extension and rule out distant metastasis. Positron emission tomography-computed tomography (PET-CT), endoscopic ultrasonography or endoscopic retrograde cholangiopancreatography was also performed to help diagnosis when it was necessary.
All patients were followed every month in the first postoperative 6 months and every 3–6 months since after. Blood tests and CT scans were performed at every visit. OS was the primary outcome of our study. Postoperative pancreatic fistula (POPF), delayed gastric empty, post-pancreatectomy haemorrhage and chylous fistula were defined according to the consensus purposed by the International Study Group of Pancreatic Surgery(Reference Bassi, Marchegiani and Dervenis24–Reference Wente, Veit and Bassi27). Surgical site infection and pleural effusion were based on the clinical manifestations, laboratory results and imaging findings.
Prognostic Nutritional Index score and Controlling Nutritional Status score
Onodera’s PNI was applied in this study, the calculating formula of which was showed in online Supplementary Fig. S1(a). Based on previous studies, we defined 45 as the cut-off value in our study(Reference Noh, Han and Cho16). CONUT score was a sum of three different scores calculated from ALB, total lymphocyte count and total cholesterol (online Supplementary Fig. S1(b))(Reference Ignacio de Ulibarri, Gonzalez-Madrono and de Villar17). In our study, we defined CONUT score <3 as ‘low CONUT’ and CONUT score ≥3 as ‘high CONUT’(Reference Harimoto, Yoshizumi and Inokuchi28).
Propensity score matching
A logistic regression model was used to estimate PSM based on patient’s sex, age, BMI, alanine transaminase, total bilirubin, PNI score (or CONUT score), CA 125, CA 19-9, tumour stage, tumour size, presence of R1 resection and lymph node metastasis. After one-to-one PSM matching without replacement conducted by a 0·1 caliper matching on the estimated propensity score, seventy-two pairs of matched cases were identified when patients were classified by PNI, and seventy-nine pairs were identified when patients were classified by CONUT(Reference Austin, Grootendorst and Anderson29).
Statistical analysis
All statistical analyses were performed with SPSS 23.0 (IBM). Normally distributed data were expressed as mean values and standard deviations, and asymmetrically distributed data were expressed as medians and ranges. The difference of baseline characteristics was analysed by t test for continuous variables and χ 2 test for categorical variables. The Cox regression model was performed to investigate the correlation between patient’s survival and risk factors. The Kaplan–Meier method and log-rank test were used for survival analysis. Outcomes were presented using hazard ratios and associated 95 % CI. P < 0·05 was considered statistically significant.
Results
High or low Prognostic Nutritional Index scores in pancreatic adenocarcinoma patients
Together, seventy-five patients were divided into the low PNI group, while the other 231 patients were in the high PNI group. We found that patients in the low PNI group tended to have more males, lower BMI, lower leucocyte count, lower total cholesterol, higher CA 125 and 19-9 levels, more advanced tumour stages, higher incidence of R1 resection and higher rate of lymph node metastasis (Table 1).
Table 1. Baseline characteristics of patients with high or low Prognostic Nutritional Index (PNI) score
(Numbers and percentages; mean values and standard deviations; medians and ranges)
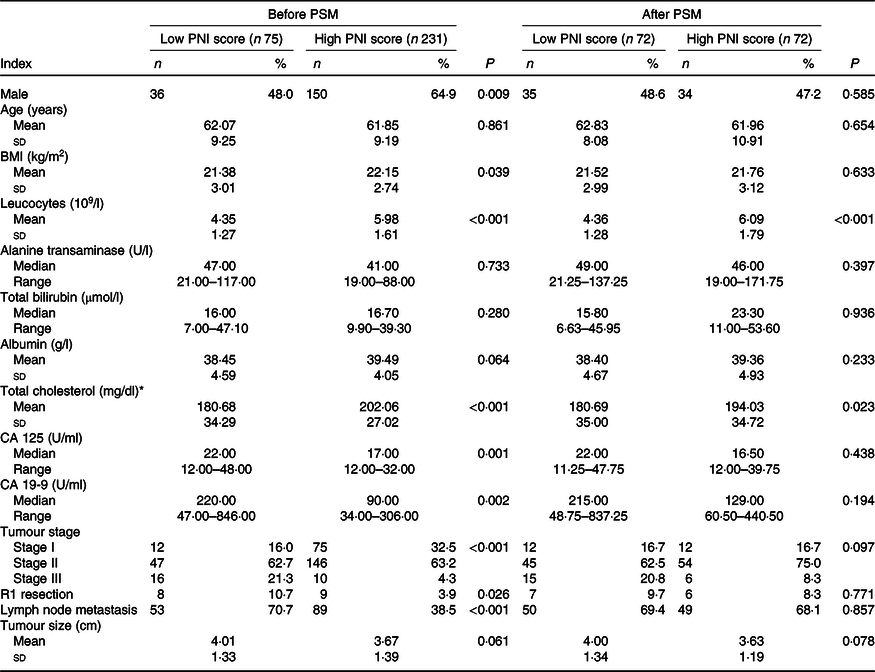
PSM, propensity score matching; CA, carbohydrate antigen.
* To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259.
Low PNI score was proved as an independent prognostic risk factor for OS (hazard ratio 0·31, P < 0·001) (online Supplementary Table S1). Survival in patients with low PNI score was significantly poor than patients with high PNI score (online Supplementary Table S2 and Fig. S2).
High or low Controlling Nutritional Status scores in pancreatic adenocarcinoma patients
After stratified by CONUT score, 217 patients had a low CONUT score, and the remaining eighty-nine patients had a high CONUT score. Patients in the high CONUT group had significantly lower BMI, lower leucocyte count, ALB, total cholesterol, higher CA 125 and CA 19-9 levels, more advanced tumour stage, higher incidence of lymph node metastasis and larger tumour size (all P < 0·05; Table 2).
Table 2. Baseline characteristics of patients with high or low Controlling Nutritional Status (CONUT) score
(Numbers and percentages; mean values and standard deviations; medians and ranges)
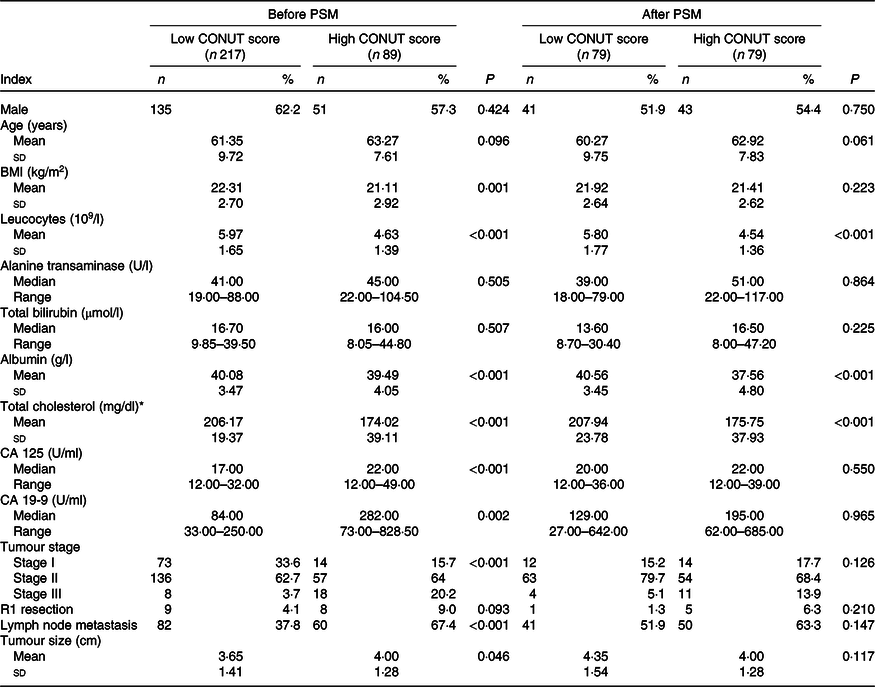
PSM, propensity score matching; CA, carbohydrate antigen.
* To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259.
High CONUT score was proved as an independent prognostic risk factor for poor OS (hazard ratio 3·93, P < 0·001) (online Supplementary Table S3). Furthermore, patients in the high CONUT group had significantly worse survival than patients in the low CONUT group (online Supplementary Table S4 and Fig. S2).
High or low Prognostic Nutritional Index scores in pancreatic adenocarcinoma patients after propensity score matching
After one to one case matching, PSM identified seventy-two pairs of patients. After PSM, low PNI score was still associated with lower leucocyte count and lower total cholesterol in the blood, while other baseline characteristics were not significantly different between the two groups (Table 1). After PSM, the mean survival time in patients with low PNI score was still significantly lower than patients with high PNI score (P < 0·001) (online Supplementary Table S2, Fig. 1).
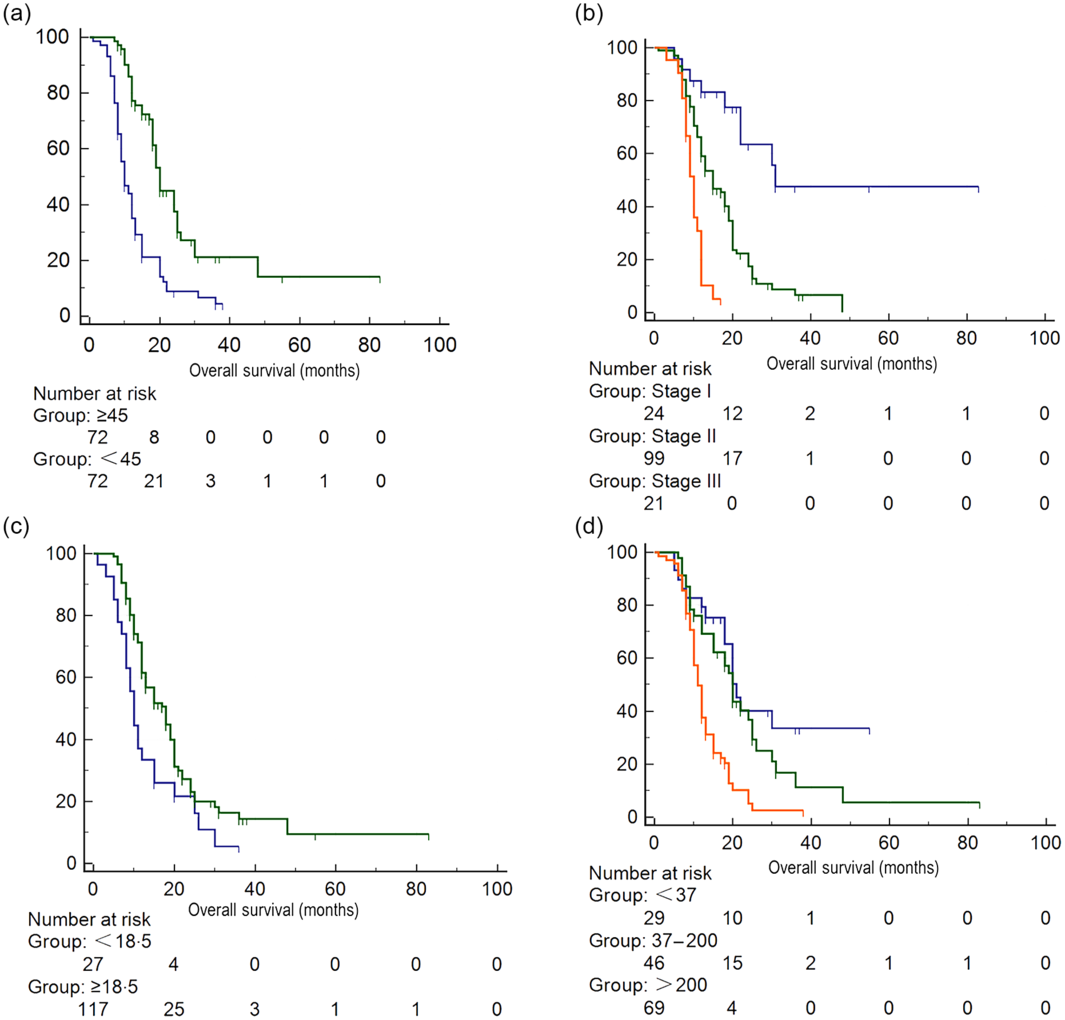
Fig. 1. Survival analysis of patients with different Prognostic Nutritional Index (PNI) scores after propensity score matching. (a) PNI score: ![]() , <45;
, <45; ![]() , ≥45, (b) tumour stage;
, ≥45, (b) tumour stage; ![]() , stage I;
, stage I; ![]() , stage II;
, stage II; ![]() , stage III, (c) BMI:
, stage III, (c) BMI: ![]() , <18·5 kg/m2;
, <18·5 kg/m2; ![]() , ≥18·5 kg/m2, (d) carbohydrate antigen 19-9:
, ≥18·5 kg/m2, (d) carbohydrate antigen 19-9: ![]() , <37;
, <37; ![]() , 37–200;
, 37–200; ![]() , >200.
, >200.
High or low Controlling Nutritional Status scores in pancreatic adenocarcinoma patients after propensity score matching
After matching, PSM identified seventy-nine pairs of patients with similar propensity score from the low and high CONUT group. After PSM, patients in the low and high CONUT score group only showed difference in leucocyte count, ALB and total cholesterol level (Table 2). In fact, these three parameters were just what the CONUT score was calculated from. Preoperative high CONUT score remained an independent prognostic risk factor of poor OS (hazard ratio 3·28, P < 0·001) (online Supplementary Table S3). After PSM, patients in the high CONUT score group had worse mean survival time than patients in the low CONUT score group (online Supplementary Table S4, Fig. 2).
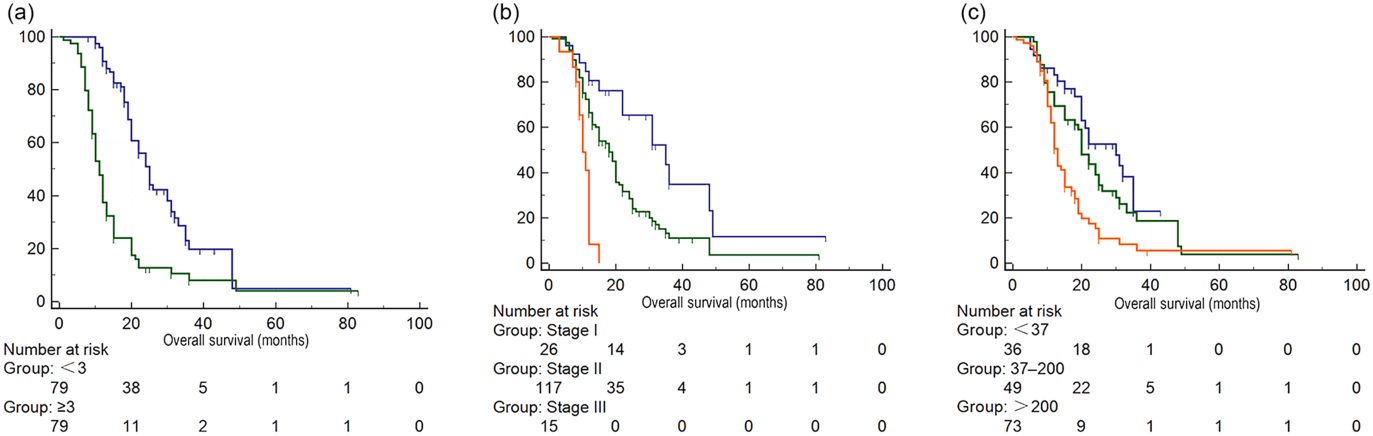
Fig. 2. Survival analysis of patients with different Controlling Nutritional Status (CONUT) scores after propensity score matching. (a) CONUT score: ![]() , <3;
, <3; ![]() , ≥3, (b) tumour stage:
, ≥3, (b) tumour stage: ![]() , stage I;
, stage I; ![]() , stage II;
, stage II; ![]() , stage III, (c) carbohydrate antigen 19-9:
, stage III, (c) carbohydrate antigen 19-9: ![]() , <37;
, <37; ![]() , 37–200;
, 37–200; ![]() , >200.
, >200.
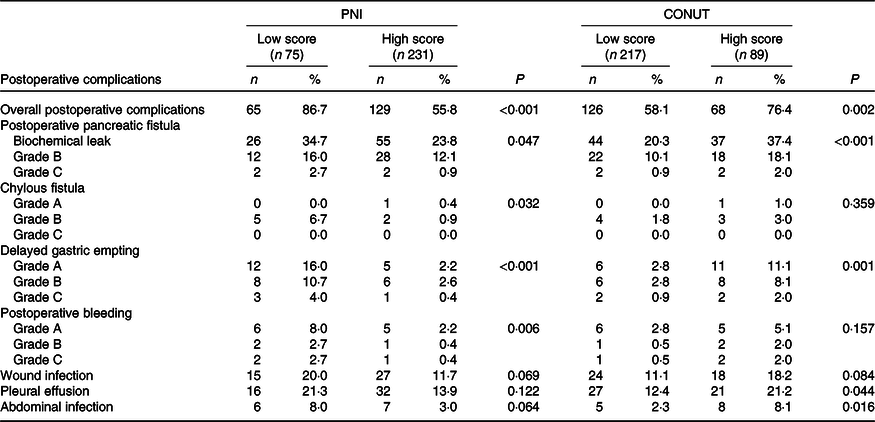
Correlation of Prognostic Nutritional Index/Controlling Nutritional Status score with postoperative complications
The overall postoperative complications in patients with high CONUT score or patients with low PNI score were significantly higher. When patients were stratified by CONUT score, the high CONUT score group tended to have a higher incidence of POPF, DGE, pleural effusion and abdominal infection. Meanwhile, patients with low PNI score were related with the prevalence and severity of POPF, chylous fistula, DGE and postoperative bleeding (all P < 0·05) (Table 3).
Table 3. Postoperative complications in patients with different Prognostic Nutritional Index (PNI)/Controlling Nutritional Status (CONUT) scores
(Numbers and percentages)
Comparison between Prognostic Nutritional Index and Controlling Nutritional Status scores in predicting outcomes
Receiver operating curves were generated for both PNI and CONUT to predict postoperative complications and survival (online Supplementary Fig. S3), and the effectiveness of preoperative PNI or CONUT as a predictor was compared by AUC of each receiver operating curves. According to the result, CONUT tended to have both higher sensitivity and specificity in predicting postoperative complications (0·826 v. 0·740), 1-year survivals (0·783 v. 0·734) and 3-year survivals (0·563 v. 0·540) than PNI.
Discussion
There was increasing evidence suggesting that poor preoperative nutritional status might be a potentially powerful predictor of poor outcomes in cancer patients. PNI and CONUT scores were two objective and easy-use tools to assess patient’s nutritional status in clinical practice and were capable of predicting outcomes in cancer patients. In our study, we demonstrated that both preoperative PNI and CONUT might be reliable predictors of surgical and oncological outcomes in PDAC patients who were eligible for tumour resection. Preoperative low PNI (≤45) or high CONUT score (≥3) was an independent prognostic risk factor for poor OS and higher incidence of postoperative complications.
There were many different clinical scores that had been developed to assess the patient’s nutritional status, including Nutritional Risk Screening Score 2002(Reference Kondrup, Rasmussen and Hamberg30), Subjective Global Assessment(Reference Detsky, McLaughlin and Baker31) and Malnutrition Screening Tool(Reference Stratton, Hackston and Longmore32). Nevertheless, these scores were largely based on some subjective parameters reported by the patients, which might easily cause errors because patient did not usually recall these clearly enough. This kind of error would trigger huge bias, which may contribute to an inaccurate result. Even more, Probst et al. (Reference Probst, Haller and Bruckner33) found none of the above nutritional assessment scores was relevant to outcome in patients with pancreatic surgery. PNI or CONUT score was totally based on objective parameters, which could avoid certain errors and might be more accurate.
In previous studies focusing on the prognostic value of PNI or CONUT in various cancers, patients with different PNI (or CONUT) scores showed the significant difference in several baseline clinicopathological features(Reference Iseki, Shibutani and Maeda19,Reference Harimoto, Yoshizumi and Sakata21,Reference Kuroda, Sawayama and Kurashige34) . Likewise, we also found that patients with compromised nutritional status seemed to suffer more advanced disease. To avoid these potential biases, we utilised PSM method. After PSM, three distinct parameters still showed significant difference between the two groups (ALB, leucocytes and total cholesterol), just because all three factors were what CONUT was calculated from. What is more important, after PSM, patients in the two groups with different PNI or CONUT scores showed no significant difference in all other well-known risk factors (CA 19-9, TMN stage, etc.) for poor prognosis before surgery.
The predictive effect of PNI or CONUT could be attributed to several reasons. First of all, the elements of PNI and CONUT had been reported to have a close relation with the patient’s prognosis. ALB had long been regarded as an important marker of nutritional status, and low ALB was associated with advanced states of cachexia and poor perioperative outcomes in cancer(Reference Delitto, Judge and George35). In PDAC, hypoalbuminaemia had already been noticed being related to the outcome of patients receiving surgery or chemotherapy(Reference Pant, Martin and Geyer36,Reference Hendifar, Osipov and Khanuja37) . Total lymphocyte count was an immunological indicator. The decrease of lymphocytes, primary T lymphocytes, indicated inadequate immune response against tumour. Total lymphocyte count alone might not be sensitive enough in predicting oncological outcomes, as current studies found controversial results on it(Reference Bhatti, Peacock and Lloyd38,Reference Fogar, Sperti and Basso39) . These results suggesting total lymphocyte count was related to the patient’s outcome, but it should be used with other parameters to enhance its predictive power (like PNI and CONUT). Total cholesterol level was a parameter involved in CONUT but not in PNI, and it had been reported to correlate with survival in malignancies(Reference Okuyama, Ichikawa and Sun40). Hypocholesterolaemia-induced compromised membrane integrity might defect the immune function of normal cells and finally lead to cancer progression(Reference Kritchevsky and Kritchevsky41). And hypocholesterolaemia may be the result of cancer growth, as cancer cells tended to overexpress LDL receptor and taken up all the LDL cholesterol into cancer cells(Reference Vitols, Gahrton and Bjorkholm42). In PDAC, tumour cells overexpressed fatty acid synthase and tended to take up cholesterols from circulation as ingredients to build blocks for the cell membrane, which led to hypocholesterolaemia in patients(Reference Swierczynski, Hebanowska and Sledzinski43). All the evidence showed that abnormal cholesterol levels played an important role in PDAC oncogenesis and cancer progression.
As expected, we demonstrated CONUT might be superior to PNI in predicting both survival and complications preoperatively, because CONUT also considered total cholesterol, which played an important role in cancer progression when compared with PNI score(Reference Swierczynski, Hebanowska and Sledzinski43). Interestingly, in another study focused on preoperative CONUT score in colorectal cancer(Reference Iseki, Shibutani and Maeda19), it found that twenty-seven of a total of 204 patients were included in the high CONUT group but not in the low PNI group, while no patient in the low PNI group was excluded from the high CONUT group. This evidence suggested that CONUT might be more sensitive than PNI, which was consistent with our results that CONUT had higher AUC than PNI in predicting survival and complications. In our study, POPF was significantly related to both low PNI and high CONUT score. This result was consistent with several previous studies(Reference Kanda, Fujii and Kodera7,Reference Yang, Tian and Zhuang44) . We assumed that tissue fragility, impaired coagulation caused by compromised nutritional status together with the systematic inflammatory response induced by cancer might delay the healing of the anastomosis and result in POPF(Reference Kanda, Fujii and Kodera7,Reference Sierzega, Niekowal and Kulig11) . Moreover, the high incidence of POPF would also give rise to a higher incidence of post-pancreatectomy haemorrhage, surgical site infection and chylous fistula. Furthermore, high incidence of DGE was probably attributed to increased intra-abdominal complications(Reference Wente, Bassi and Dervenis25) and low BMI in patients with low PNI or high CONUT score.
Interestingly, current clinical guidelines for PDAC did not lay much emphasis on the patient’s immunonutritional status. The guideline only suggested nutrition support in situation of palliative care or for patients presented pancreatic exocrine insufficiency. However, our research demonstrated deteriorating in immunonutritional status before surgery was a common and noteworthy issue in PDAC patients who were eligible for curative tumour resection in China. And the early recognition and objective assessment of nutritional problems is the key point for PDAC management. Preoperative nutrition support could affect both short-term and long-term outcomes independent of other prognostic factors, and similar phenomena have been recognised by other researchers(Reference Kanda, Fujii and Kodera7,Reference Probst, Haller and Bruckner33,Reference Delitto, Judge and George35) . However, few studies reported about the use and the effect of preoperative nutrition support in PDAC patients. Whether PDAC patients would benefit from nutrition support strategies and which patients would benefit from them is still ambiguous. In this study, we have discovered an effective and objective tool to recognise malnutrition PDAC patients. We hope preoperative nutrition supports would improve the survival and reduce postoperative complications of the identified PDAC patients using CONUT index. The results might help us to find an optimal way to manage nutritional status in PDAC patients.
This study had some limitations. The study was performed in a retrospective design, which may have some potential bias. However, we enlarged our sample size and used the PSM method to eliminate the selection bias.
Conclusion
In this study, both preoperative low PNI (≤45) and high CONUT (≥3) scores were reliable predictors of OS and surgical complications in PDAC patients. Compared with PNI, CONUT might be more effective in predicting surgical and oncological outcomes in PDAC.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant number: 81902432) (Z.-B. X.).
Conception and design: Y.-S. M., Z.-B. X., S.-J. H. and D.-L. F.; administrative support: Y.-S. M., Z.-B. X., S.-J. H., C.-F. Z. and D.-L. F.; provision of study materials or patients: Y.-S. M., Z.-B. X. and D.-L. F.; collection and assembly of data: Y.-S. M., S.-J. H. and C.-F. Z.; data analysis and interpretation: Y.-S. M. and C.-F. Z.; manuscript writing: Y.-S. M., Z.-B. X. and D.-L. F.; final approval of manuscript: all authors.
The authors declare that there are no conflicts of interests.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520002299








