Neurodegenerative diseases including Parkinson's disease (PD) are the seventh leading cause of death and a major cause of disability in high-income countries(Reference Lopez, Mathers and Ezzati1). With the ageing of populations, the importance of PD as a public health issue is expected to increase. The exact pathogenetic mechanisms underlying the selective dopaminergic cell death in PD remain largely unknown, but evidently both genetic and environmental factors are contributing to the risk(Reference de Lau and Breteler2). Several biological mechanisms for the role of diet in the development of PD have been proposed, including suggestions that antioxidants could protect neurons from oxidative injury, and PUFA could have neuroprotective properties(Reference Gaenslen, Gasser and Berg3). As curative treatment for PD remains a challenge, it is essential to identify potential modifiable risk factors.
While dietary factors have been systematically explored as predictive factors for major chronic illnesses, there have been few such studies with regard to PD. In previous studies, the results on the associations between the risk of PD and the intake of individual nutrients and food groups have been inconsistent, and evidence from prospective studies is rather scarce, according to recent reviews(Reference Gaenslen, Gasser and Berg3, Reference Ishihara and Brayne4). Furthermore, little is known about the combined effect of multiple dietary factors on the risk of developing the disease. Only one study has examined the prediction of the overall quality of diet or dietary patterns on the risk of PD, concluding that a plant-based dietary pattern which includes fish may protect against PD(Reference Gao, Chen and Fung5).
We investigated whether individual food groups or the overall quality of diet, as defined by a modification of the Alternate Healthy Eating Index (mAHEI)(Reference McCullough, Feskanich and Stampfer6), predict PD incidence in a Finnish population.
Methods
Study population
A total of 62 440 individuals aged 15 years or over participated (82·5 % of those invited) in the Finnish Mobile Clinic Health Examination Survey (FMC) conducted in different regions of Finland in 1966–72(Reference Aromaa7). As part of the main study, detailed data about food consumption were collected from 10 054 randomly selected individuals using a dietary history interview(Reference Järvinen, Seppänen and Knekt8, Reference Järvinen9). Of these, participants aged 40–79 years were included in this cohort study on PD (n 4738). However, subjects identified as PD cases or who reported the use of antipsychotic medication due to psychotic disorders (International Classification of Diseases (ICD-10) codes F20–F39) at baseline were excluded. Thus, the final study population comprised 4524 subjects (2388 men and 2136 women). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki.
Data on population samples held at the National Institute for Health and Welfare can be used on the basis of the law on Personal Data and the legislation applying to the National Institute for Health and Welfare.
Assessment of exposure
The total habitual food consumption during the previous year was estimated using a dietary history interview(Reference Järvinen9). Trained interviewers used a questionnaire which included over 100 food items and mixed dishes common in the diet of Finns at that time. The consumption of food items was estimated per d, week, month or year while also considering portion sizes and the methods of food preparation. Individual consumption of food items and mixed dishes was converted to g/d. The nutrient intakes of food items were computed using a food composition database compiled at the Social Insurance Institution, which was based on the Finnish food composition tables(Reference Rastas, Seppänen and Knuts10).
All subjects completed a mailed, self-administered, health questionnaire that was checked by a trained nurse at baseline examinations. The health questionnaire provided information on, for example, sociodemographic background, previous and current illnesses, use of medication, smoking and leisure-time physical exercise(Reference Knekt11).
At baseline examinations, height (without shoes) and weight (in light indoor clothing) were measured and BMI (kg/m2) was calculated. Casual blood pressure was measured using the auscultatory method(Reference Aromaa7). Venous blood samples were collected and serum total cholesterol concentrations were determined via an autoanalyser modification of the Liebermann–Burchard reaction(Reference Huang, Chen and Wefler12).
PD cases (ICD-10 code G20) were identified through linkage with the nationwide Drug Imbursement Register of the Social Insurance Institution. All individuals in Finland with PD are eligible for medication free of charge. In order to obtain this allowance, the patient must apply for it and attach a certificate from the treating neurologist stating that all the diagnostic criteria are met, including symptom history and reports of clinical examinations (stating the presence of resting tremor, bradykinesia and/or muscle rigidity along with other findings). The allowance is granted after approval by a neurologist on behalf of the Social Insurance Institution. In an ongoing validation of the register, the certificates for PD drug reimbursement and selected hospital records were re-evaluated retrospectively by our study neurologist (J. Lyytinen) according to the National Institute of Neurological Disorders and Stroke diagnostic criteria for PD(Reference Gelb, Oliver and Gilman13, Reference Jankovic14). Of the originally identified PD cases reviewed, 80 % met the criteria for PD, consistent with other estimates of the percentage of people clinically diagnosed with Parkinsonism in a general population who meet strict PD criteria(Reference Schrag, Ben-Shlomo and Quinn15). During the 41 years of follow-up (1966–2007), eighty-five PD cases were identified. The individual follow-up refers to the period of time from the baseline examination to the date of PD diagnosis, death or the end of the observation period, whichever came first.
Dietary factors and dietary index
Dietary factors were assessed systematically by including a comprehensive set of individual food groups and nutrients in the study. The dietary index was formed based on the definition of the Alternate Healthy Eating Index (AHEI) developed by McCullough et al. (Reference McCullough, Feskanich and Stampfer6). We aimed for a similar definition whenever possible, but a comparison with AHEI information on multivitamin use and alcohol consumption could not be included, since these variables did not exist in our data. Thus, the current dietary index mAHEI included the intake of seven components (vegetables, fresh fruits, nuts and legumes, the ratio of white:red meat, whole grain, the ratio of polyunsaturated:saturated fat and trans fats), which were divided into quintiles. The first six components received scores in an ascending order, so that one point was assigned for intakes in the lowest quintile and five points for intakes in the highest quintile. The trans fats component received scores in the descending order, with the lowest quintile gaining a value of five points and the highest quintile a value of one point. The total score ranged from 7 (worst) to 35 (best). A higher score suggested a higher dietary quality.
Statistical analyses
The confounding factors were chosen based on the literature and inclusion of them in the models was based on analyses in our own population. The general linear model was used to study the association between potential confounding factors and the dietary index and PD. The Cox proportional hazards model(Reference Cox16) was used to estimate the relative risks (RR) and 95 % CI for PD according to tertiles for the consumption of food groups and quartiles for the mAHEI. The significance of trend was tested by including continuous variables in the models. In the case of categorical variables, heterogeneity was tested.
Definitions of three main models were included. The first model included age (continuous) and sex. The second model also included marital status (unmarried, married, widow/er or divorced), urbanisation (rural, urban or industrial), geographical area (South-West, South, Central, West, East or North), smoking (never, has quit ≥ 10 years ago, has quit 1–9 years ago, has quit < 1 year ago, smokes only pipe or cigars, smokes < 15 cigarettes/d or smokes ≥ 15 cigarettes/d), BMI ( < 23, 23–24·9, 25–27·4, 27·5–29·9 or ≥ 30 kg/m2), leisure-time physical activity (not at all, less than weekly, weekly or daily), and intake of energy (kJ/d). The third model further included hypertension (no or yes), serum total cholesterol (mmol/l), diabetes mellitus (no or yes) and, in addition, in women, parity (0, 1–3 or ≥ 4). Since the results in these three models were essentially similar, only the results for the third model are presented in the text and tables. There were differences between men and women in the associations between diet and PD, so most of the analyses were stratified by sex (P for interaction = 0·08). Analyses with exclusion of the first 5 years of the follow-up were performed to examine the effect of the preclinical disease phase, which resulted in the exclusion of seven PD cases. All analyses were carried out using SAS software version 9 (SAS Institute Inc.)(17).
Results
At baseline, PD cases were more often non-smokers than were subjects who were free from the disease (Table 1). PD was not notably associated with any other background variable. Subjects who had high diet quality scores were younger and more often married, and they had a higher BMI and serum total cholesterol level (Table 2). They were also less likely to live in rural areas and to smoke, and more likely to take part in leisure-time physical activities, to have diabetes and to have a higher energy intake.
Table 1 Baseline characteristics*
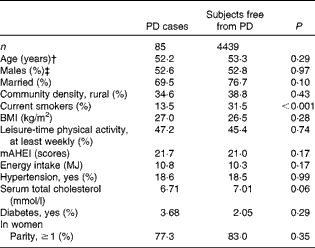
PD, Parkinson's disease; mAHEI, modified Alternate Healthy Eating Index.
* Adjusted for age and sex.
† Adjusted for sex.
‡ Adjusted for age.
Table 2 Selected characteristics* of the study population by quartiles (Q) of the modified Alternate Healthy Eating Index
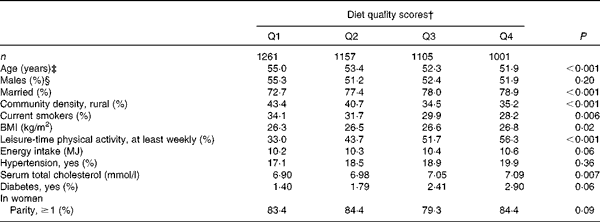
* Adjusted for age and sex.
† Cut-off points of Q1–Q4 for men are 7–18, 19–21, 22–24 and 25–34, and for women 8–18, 19–21, 22–24 and 25–34.
‡ Adjusted for sex.
§ Adjusted for age.
In the third multivariate model, no statistically significant associations were found in men or in women between the risk of PD and most of the individual food groups examined (Table 3). However, in men, the RR of PD between the highest and the lowest tertile due to the intake of fruits and berries was 3·67 (95 % CI 1·30, 10·36; P for trend = 0·60) and the intake of fresh fruits 2·41 (95 % CI 1·01, 5·77; P for trend = 0·48). In contrast, among women, there were signs of an inverse association between the consumption of berries and the risk of PD (the highest v. the lowest tertile, 0·54, 95 % CI 0·23, 1·27; P for trend = 0·02). In women, subjects in the highest tertile of the consumption of milk had a higher risk of PD compared with subjects in the lowest tertile (RR 3·31, 95 % CI 1·10, 9·93; P for trend = 0·09) (Table 3). A non-significant increase in the risk of PD was also seen in men (Table 3). When men and women were combined, subjects in the highest tertile of the consumption of milk had a higher risk of PD compared with subjects in the lowest tertile (RR 2·16, 95 % CI 1·09, 4·28). In addition, a statistically significant inverse association was observed between the consumption of processed meat and sausages and the incidence of PD in women, with the RR of developing the disease between the highest and lowest tertiles being 0·39 (95 % CI 0·16, 0·95; P for trend = 0·008). A similar decrease in RR was seen in men, but the association between processed meat and sausages and the incidence of PD was not statistically significant. However, the association was strengthened when the analysis was made in the total population (RR 0·49, 95 % CI 0·27, 0·89). The results for men and women analysed together were not further presented due to the strong interaction of sex on the association between diet and PD, and the above-mentioned results were the only statistically significant associations regarding analyses in the total population.
Table 3 Multivariate-adjusted* relative risks (RR) and 95 % CI of Parkinson's disease by individual food groups†

het., Heterogeneity; M, male; F, female.
* Adjusted for age (continuous), sex, marital status (unmarried, married, widow/er or divorced), community density (rural, urban or industrial), geographical area (South-West, South, Central, West, East or North), smoking (never, has quit ≥ 10 years ago, has quit 1–9 years ago, has quit < 1 year ago, smokes only pipe or cigars, smokes < 15 cigarettes/d or smokes ≥ 15 cigarettes/d), BMI ( < 23, 23–24·9, 25–27·4, 27·5–29·9 or ≥ 30 kg/m2), leisure-time physical activity (not at all, less than weekly, weekly or daily), energy (continuous), hypertension (no or yes), serum total cholesterol (continuous), diabetes (no or yes) and, in addition, in women, parity (0, 1–3 or ≥ 4).
† The main food groups are indicated as ‘total’, including the intake of subgroups of corresponding foods.
‡ Sex-specific cut-off values for tertiles.
§ Parkinson's disease cases/total population.
∥ Median value 0 g/d, median classes were used when distribution did not allow classification into tertiles.
¶ Consists of mainly whole milk in this study population.
Diet quality defined by the mAHEI did not predict PD incidence (Table 4). The RR between the highest and lowest quartiles of the mAHEI was 1·83 (95 % CI 0·65, 5·18; P for trend = 0·11) in men and 0·97 (95 % CI 0·38, 2·48; P for trend = 0·91) in women in the third multivariate model.
Table 4 Multivariate-adjusted* relative risks (RR) and 95 % CI of Parkinson's disease by quartiles (Q) of the modified Alternate Healthy Eating Index
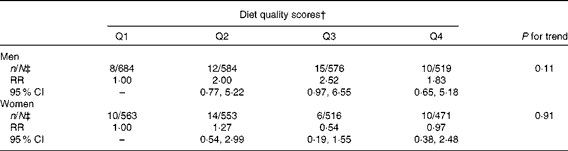
* Adjusted for age (continuous), sex, marital status (unmarried, married, widow/er or divorced), community density (rural, urban or industrial), geographical area (South-West, South, Central, West, East or North), smoking (never, has quit ≥ 10 years ago, has quit 1–9 years ago, has quit < 1 year ago, smokes only pipe or cigars, smokes < 15 cigarettes/d or smokes ≥ 15 cigarettes/d), BMI ( < 23, 23–24·9, 25–27·4, 27·5–29·9 or ≥ 30 kg/m2), leisure-time physical activity (not at all, less than weekly, weekly or daily), energy (continuous), hypertension (no or yes), serum total cholesterol (continuous), diabetes (no or yes) and, in addition, in women, parity (0, 1–3 or ≥ 4).
† Cut-off values for Q1–Q4: ≤ 18, ≤ 21, ≤ 24 and >24.
‡ Parkinson's disease cases/total population.
Excluding the first 5 years of the follow-up did not notably alter the results concerning the individual food groups or the diet quality index (data not shown).
Discussion
Only a few associations were found between PD risk and the individual food groups examined. For women, the main findings in Table 3 were a positive association between milk consumption and PD incidence, and an inverse association between the intake of processed meat and sausages and PD occurrence. Women also had a weak inverse association between the consumption of berries and the risk of PD. For men, positive associations between the consumption of fruits and berries, and fresh fruits, and PD incidence were found (Table 3). The results for men and women combined were mostly statistically non-significant (data not shown), with two exceptions showing an increased PD risk for milk consumption, and a decreased PD risk for the consumption of processed meat and sausages.
In women, higher milk consumption was significantly associated with an increased risk of PD, which is in line with previous findings(Reference Chen, O'Reilly and McCullough18–Reference Park, Ross and Petrovitch20). It is not clear whether nutrients or other constituents in milk would mediate this inverse association. However, it is unlikely to be due to Ca, vitamin D or fat, as they had no association with the disease risk when derived from sources other than dairy products(Reference Chen, O'Reilly and McCullough18). Regarding milk fat, this suggestion is supported in the present study, as reduced-fat dairy was also associated with PD risk (data not shown). One possible explanation for this is that dairy products lower the circulating levels of uric acid(Reference Garrel, Verdy and PetitClerc21), which has been inversely associated with PD risk(Reference Davis, Grandinetti and Waslien22–Reference Weisskopf, O'Reilly and Chen24). Another possibility is that dairy products are contaminated with neurotoxins due to their exposure to pesticides, which may increase the risk of PD(Reference Priyadarshi, Khuder and Schaub25, Reference Weisskopf, Knekt and O'Reilly26).
In women, an inverse association was observed between the intake of processed meat and sausages and PD incidence. Previous studies have presented inconsistent results for the association between PD risk and the consumption of different meats, the intake of proteins or the intake of Fe(Reference Ishihara and Brayne4). A large cohort study conducted in the USA found no associations for the consumption of total meat, red meat, poultry or fish(Reference Chen, Zhang and Hernan19). However, meat is a source of niacin, a vitamin reported to be inversely associated with PD and hypothesised to act via mechanisms related to nicotinamide metabolism(Reference Hellenbrand, Boeing and Robra27).
In men, we found that a high consumption of fruits and berries and fresh fruits was associated with increased PD risk. In contrast, among women, there was a weak inverse association between the consumption of berries and the risk of PD. Furthermore, the consumption of vegetables was not associated with the risk of developing the disease in either men or women. The present results do not support the prevailing hypothesis that the consumption of fruits and vegetables containing antioxidant vitamins will protect against the presumed oxidative stress in the pathogenesis of PD. Correspondingly, evidence from previous epidemiological studies is inconsistent, showing mainly no association between the consumption of fruits and vegetables or intakes of antioxidant vitamins and PD risk(Reference Ishihara and Brayne4). One alternative is that the use of pesticides in farming may increase the risk of PD, thus masking the potential benefit of consuming fruits and vegetables. Furthermore, a reason why berries but not fruits were inversely associated with PD incidence could be that the use of pesticides does not have an effect on the berries gathered from nature. However, a nested case–control study within the Finnish Mobile Clinic Health Examination Survey did not find strong evidence for an increased risk of PD due to being exposed to pesticides(Reference Weisskopf, Knekt and O'Reilly26).
In the present study, the mAHEI, a proxy measure of diet quality, did not predict PD incidence. In contrast, the only previous study on diet quality and PD risk showed that a plant-based dietary pattern which included fish, indicating a higher diet quality, was inversely associated with PD incidence(Reference Gao, Chen and Fung5). The present finding that there was no association between the two is, however, in agreement with the fact that most of the food items examined in the present study were not associated with PD risk. The mAHEI is, apparently, a good tool for assessing diet quality since it is inversely associated with the incidence of CHD in the present study population (K Sääksjärvi and P Knekt, unpublished results). As studies based on the dietary data of the FMC have given consistent findings on the associations with several chronic diseases, such as CHD(Reference Knekt, Järvinen and Reunanen28), type 2 diabetes(Reference Montonen, Järvinen and Heliövaara29) and stroke(Reference Mizrahi, Knekt and Montonen30), it cannot be excluded that the lack of an association is merely due to changes in dietary habits during the extremely long follow-up period.
Interestingly, certain lifestyle factors that are commonly considered to increase the risk of CVD(Reference Srinath Reddy and Katan31–Reference van Dam33) seem to be protective factors for PD in the present study, for example consumption of foods that are major sources of saturated fat (processed meat and sausages), and, as found in our previous study(Reference Sääksjärvi, Knekt and Rissanen34), smoking and the consumption of unfiltered boiled coffee. Furthermore, the consumption of foods containing antioxidant vitamins (fruits and berries) was associated with an increased risk of PD in men, even though they are commonly considered to lower the risk of CVD(Reference Srinath Reddy and Katan31). This suggests a hypothesis that these two diseases do not share similar pathogenic processes. Another explanation could be that in the 1960s and 1970s, people died at a relatively young age in Finland due to CVD, men about their mid-60s and women about their mid-70s(Reference Martelin, Koskinen, Valkonen, Koskinen, Aromaa, Huttunen and Teperi35), whereas the peak incidence of PD is generally between 70 and 79 years of age(Reference Twelves, Perkins and Counsell36). Therefore, selection of the population is possible.
In the present study, an effect modification of sex was observed. One explanation is that a biological mechanism contributes to sex differences, as, for example, reproductive hormones have been associated with the risk of PD(Reference Bourque, Dluzen and Di Paolo37). Furthermore, women consumed considerably less alcohol than men, which may explain the difference if alcohol is an important confounding factor. Further studies should examine sex and other potential effect-modifying factors, since the conflicting results in the epidemiological literature on PD risk factors could be due to undetected interactions between them.
The main strengths of the present study lay in its prospective design and in the comprehensive dietary assessment of habitual food consumption. Furthermore, as information on several probable risk factors for PD was collected, we were able to account for potential confounding factors. However, information on some potential confounding factors, such as alcohol intake(Reference Ragonese, Salemi and Morgante38), coffee consumption(Reference Sääksjärvi, Knekt and Rissanen34) and vitamin D status(Reference Knekt, Kilkkinen and Rissanen39), was not available. It is possible that the low consumption of alcohol is associated with a diet containing an ample amount of milk, with both contributing to low plasma urate, which in turn predicts an elevated risk of PD(Reference Weisskopf, O'Reilly and Chen24). There are some methodological limitations to consider. First, since the follow-up time was extremely long, and no repeated measurement data were available, possible changes in diet during the follow-up period may have weakened the strength of the association. On the other hand, a benefit of the long follow-up period was that it exceeded the long preclinical phase of PD, which was demonstrated by the fact that the exclusion of individuals who had developed the disease during the first 5 years of the follow-up did not notably alter the results. Second, it cannot be excluded that the small number of PD cases or many comparisons may have caused chance findings. Finally, the estimated RR could be attenuated due to a misclassification of cases (despite confirmation from a neurologist) and unidentified PD cases, though this is not of great importance since the prevalence of PD is low.
Summary
In summary, we did not find the expected link between the consumption of several food groups and PD incidence. However, the present study supported the suggestion that the consumption of milk in large quantities increases the risk of PD in women. In addition, diet quality was not associated with PD incidence in the present study. These findings suggest that the role of diet in the development of PD is probably rather modest. Additional large prospective studies are needed to provide more information on important effect-modifying and confounding factors.
Acknowledgements
K. S. was supported by a grant from Doctoral Programs in Public Health, a network for doctoral training in the field of public health funded by the Ministry of Education, and by Juho Vainion Säätiö, a foundation supporting scientific research and publication. P. K., A. L., S. M., M. H., H. R. and R. J. have no financial disclosures to declare, as they were salaried by their institutes. P. K., K. S. and S. M. designed the study and wrote the protocol. K. S. and R. J. managed the literature searches. P. K. designed and K. S. and H. R. undertook the statistical analysis, and K. S. wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript. The authors declare that there are no conflicts of interest.






