Although diet is thought to affect the natural history of heart failure (HF)(Reference Ghali, Kadakia and Cooper1), only a few studies have sought to determine the effect of nutritional intake in patients with HF(2–Reference Lennie, Chung and Habash7). Many of these studies were small and examined the effect of dietary supplements, rather than nutrient intake from food. The intake of Ca, Mg and K could influence the course of HF through effects on blood pressure, inflammation, endothelial function and other pathways(Reference Weaver, Bowman and Russell8–Reference Bolland, Grey and Avenell16). In one small study, high-dose supplementation with multiple micronutrients, including Ca and Mg, improved left ventricular function and quality of life among HF patients with reduced ejection fraction(Reference Witte, Nikitin and Parker6). However, the effect of Ca, Mg and K intake on survival in patients with HF is not known. We therefore examined the associations of Ca, Mg and K intake from foods, as well as total intake from diet and supplements, with all-cause mortality among Women's Health Initiative (WHI) participants who had a HF hospitalisation. We hypothesised that higher intake of Ca, Mg and K would be associated with lower rates of all-cause mortality in women with HF.
Methods
Study population
The WHI has been previously described in depth(Reference Hays, Hunt and Hubbell17, Reference Langer, White and Lewis18). Briefly, the WHI consisted of a clinical trial component with 68 132 post-menopausal women aged 50–79 years at the study entry and an observational study component with 93 676 post-menopausal women aged 50–79 years at the study entry, recruited between 1993 and 1998 through forty clinical centres. The WHI clinical trial included trials of hormone therapy, dietary modification (DM) and Ca plus vitamin D. The clinical trial and observational study were closed in 2004–05, and participants were invited to continue in the WHI extension study, which started in 2005. For the present analysis, we focused on the 4043 participants who had an adjudicated hospitalisation for HF between study entry and 2005. HF hospitalisations were locally adjudicated with central adjudication for quality control and were defined as hospitalisations with a final diagnosis of HF accompanied by medical treatment for HF, including diuretics, digitalis, vasodilators or angiotensin-converting enzyme inhibitors. Supporting evidence of HF from imaging was also noted. We excluded twenty-nine participants who did not survive at least 1 d past HF hospitalisation, 480 participants missing information on Ca, Mg, K or covariates and 194 participants with implausible energy intake ( < 2510 or >20 920 kJ/d) producing a final sample size of 3340 women. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the institutional review boards at all the participating centres. Written informed consent was obtained from all the participants.
Nutrient intake
A modified block FFQ(Reference Patterson, Kristal and Tinker19) was administered during a screening visit for all the participants and additionally at year 1 for all participants in the DM trial, yearly thereafter for a proportion of the DM participants and at year 3 for the observational study participants. The most recent diet assessment prior to HF hospitalisation was selected for each participant. The FFQ contained 122 questions about the frequency of consumption of foods and beverages over the previous 3 months, nineteen adjustment items and four summary questions(Reference Patterson, Kristal and Tinker19). The frequency questions included predefined responses up to ‘2+ times per d’ for foods and ‘6+ times per d’ for beverages. There were also questions on portion sizes (small, medium or large), as compared with the specified medium-size portion. Pictures were used to help with portion size estimations.
Dietary nutrient intake was calculated by multiplying the frequency of consumption of each food by the nutrient content for the specified portion size and then summing over foods. Dietary nutrient intake was adjusted for energy intake using the residuals method(Reference Willett20). To calculate total nutrient intake, the nutrient intake from supplements was added to the energy-adjusted dietary nutrient intake. In a study of the measurement characteristics of the FFQ, deattenuated correlation coefficients comparing 8 d of dietary intake (four 24-h recalls and 4 d of diet records) with the FFQ were 0·73 for dietary Ca, 0·78 for total Ca, 0·72 for dietary Mg, 0·69 for total Mg, 0·67 for total K and 0·67 for total K(Reference Patterson, Kristal and Tinker19). The test–retest intraclass correlation coefficient was 0·84 for Mg, and all correlations were ≥ 0·67(Reference Patterson, Kristal and Tinker19).
Covariates
Covariates were assessed by questionnaires and physical measurement (blood pressure and height and weight used to calculate BMI). As with the dietary assessment, the most recent assessment of covariates prior to HF hospitalisation was selected for each individual. For co-morbidities, such as myocardial infarction and revascularisation, self-reported conditions existing at the WHI baseline and occurring during the WHI follow-up prior to HF hospitalisation were combined.
Mortality follow-up
The outcome of interest was all-cause mortality. Follow-up for these analyses started on the date of HF hospitalisation and continued through to the date of death or the last contact with the participant prior to August 2009. Deaths were ascertained by family, friends or health-care providers directly reporting the death to the WHI; family, friends or the US Postal Service responding to mailings; Internet searches (e.g. obituary search engines); the social security death index; or the National Death Index searches(21).
Statistical analysis
We summarised covariates using means for continuous variables and percentages for categorical variables by mortality status. We calculated Pearson's correlations among Ca, Mg and K intakes. In the subset of women with FFQ completed both before and after HF hospitalisation, we compared intakes from pre- and post-HF questionnaires to assess diet stability.
Cox proportional hazards models were used to estimate the hazard ratios and 95 % CI of mortality associated with quartiles of the dietary intake of Ca, Mg and K as well as total intake from diet and supplements. Model 1 adjusted for age at HF hospitalisation (linear) and total energy intake (linear). Model 2 further adjusted for race/ethnicity (white, black or other), education (less than high school, high school graduate including some college, college graduate or graduate school), income ( < $20 000, $20 000–34 999, $35 000–49 999 or ≥ $50 000), married (yes or no), current smoking (yes or no), BMI (linear term), total exercise (metabolic equivalents h/week, linear term), physical function score (linear term), systolic blood pressure (linear term), diastolic blood pressure (linear term), history of high cholesterol, high blood pressure, diabetes, myocardial infarction, coronary revascularisation and atrial fibrillation, use of off-study post-menopausal hormone therapy, use of diuretics, β-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and WHI study arm. Model 3 additionally adjusted for alcohol intake (none, 0–10 g/d or >10 g/d) and quartiles of Na, Ca (Mg and K models), Mg (Ca and K models), K (Ca and Mg models), protein, saturated fat, monounsaturated fat, polyunsaturated fat and fibre. Because intake of Ca, Mg and K may reduce blood pressure, adjusting for factors related to blood pressure may be an over-adjustment. We therefore constructed a final model adjusted for all of the factors in model 3, except for systolic and diastolic blood pressure, history of high blood pressure and use of diuretics, β-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Collinearity was examined using the variance inflation factor. None of the variance inflation factors exceeded 4·1, below the threshold of 10, which has been proposed as an indicator of important collinearity(22). Tests for linear trend were performed by including the median of each quartile as a continuous variable in the models. Potentially non-linear associations were explored using penalised splines(Reference Thurston, Eisen and Schwartz23).
In sensitivity analyses, we excluded participants with self-reported HF at study entry because pre-existing HF may alter diet (n 303), participants with a history of cancer at baseline or during follow-up because of the cardiotoxic effects of some chemotherapeutic agents (n 654) and participants in the DM or Ca plus vitamin D trials because these participants may have altered their intake in ways not fully captured by the FFQ (n 1215). Finally, we excluded participants who did not have a physician diagnosis of HF at the index hospitalisation, because of the potential for misclassification of disease (n 251). We stratified results by whether the participants had a history of IHD prior to the index HF hospitalisation (history of myocardial infarction or revascularisation) and tested whether the associations between the micronutrients and mortality varied by history of IHD by including the product of an indicator variable for IHD and the median values of the micronutrient quartiles.
We tested the proportional hazards assumption by including the product of micronutrients and the natural logarithm of time as a term in the models; there was no evidence of violation of the proportional hazards assumption. Statistical analyses were conducted using SAS version 9.2 and R version 2.12.1. Two-sided P values < 0·05 were considered statistically significant.
Results
Over a median of 4·6 years of follow-up (interquartile range 1·5–6·9 years) after HF hospitalisation, 1433 women died (42·9 %). Characteristics of the study population are described in Table 1. The women who died were older, had lower physical activity, were more likely to use diuretics, and were more likely to have diabetes, hypertension, myocardial infarction, coronary revascularisation and atrial fibrillation. Dietary Ca intake was moderately correlated with dietary Mg (r 0·48) and K (r 0·54). Dietary intake of Mg and K were highly correlated (r 0·84). Among the 929 women with diet assessment before and after HF hospitalisation, correlations between pre- and post-HF hospitalisation nutrient intakes were moderate (r 0·49 for Ca, 0·56 for Mg and 0·54 for K).
Table 1 Characteristics of 3340 Women's Health Initiative (WHI) participants with heart failure hospitalisation by mortality (Mean values and standard deviations or percentages)

MET, metabolic equivalents; ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
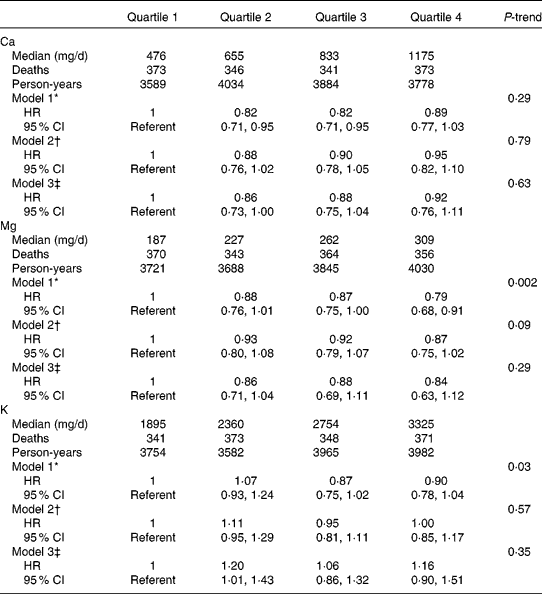
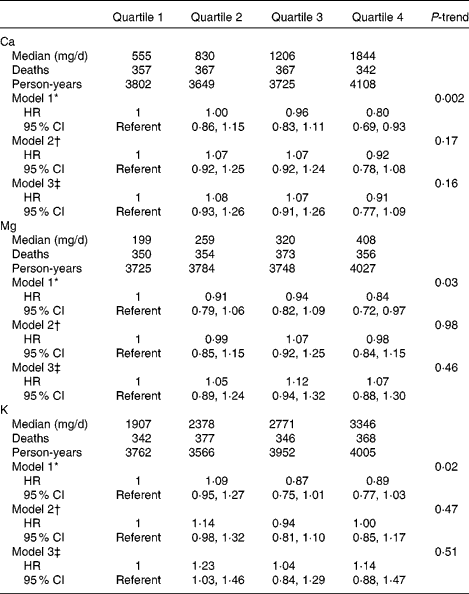
In age- and energy-adjusted models, dietary intake of Mg and K were inversely associated with mortality (Table 2). However, after further multivariable adjustment, there was no significant association between any of the micronutrients and mortality. When dietary Ca, Mg and K were considered as continuous variables using penalised splines, there was no significant linear or non-linear association. Total intake (from foods and supplements) of Ca, Mg and K was associated with mortality in age- and energy-adjusted models, but the associations were not evident after multivariable adjustment (Table 3). The findings were the same when we did not adjust for blood pressure-related variables. Results after excluding participants with baseline self-reported HF, participants with a history of cancer, participants in the DM or Ca plus vitamin D trials or participants who did not have a physician diagnosis of HF at the index hospitalisation were not materially different. No significant associations of Ca, Mg or K with mortality were observed among the women with a history of IHD (n 860) or those without a history of IHD (n 2480), and tests for interaction between the nutrients and history of IHD were not statistically significant.
Table 2 Calcium, magnesium and potassium from foods and survival among women with heart failure (Hazard ratios (HR) and 95 % confidence intervals)

* Model 1 adjusted for age at heart failure hospitalisation (linear) and total energy intake (linear).
† Model 2 adjusted for variables in model 1 and race/ethnicity (white, black or other), education (less than high school, high school graduate/some college, college graduate or graduate school), income ( < $20 000, $20 000–34 999, $35 000–49 999 or ≥ $50 000), married (yes or no), current smoking (yes or no), BMI (linear term), total exercise (metabolic equivalents h/week, linear term), physical function score (linear term), systolic blood pressure (linear term), diastolic blood pressure (linear term), history of high cholesterol, high blood pressure, diabetes, myocardial infarction, coronary revascularisation and atrial fibrillation, use of off-study post-menopausal hormone therapy, use of diuretics, β-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and Women's Health Initiative study arm.
‡ Model 3 adjusted for variables in model 2 and alcohol intake (none, 0–10 g/d or >10 g/d), and quartiles of Na, Ca (Mg and K models), Mg (Ca and K models), K (Ca and Mg models), protein, saturated fat, monounsaturated fat, polyunsaturated fat and fibre.
Table 3 Calcium, magnesium and potassium from foods and supplements and survival among women with heart failure (Hazard ratios (HR) and 95 % confidence intervals)

* Model 1 adjusted for age at heart failure hospitalisation (linear) and total energy intake (linear).
† Model 2 adjusted for variables in model 1 and race/ethnicity (white, black or other), education (less than high school, high school graduate/some college, college graduate or graduate school), income ( < $20 000, $20 000–34 999, $35 000–49 999 or ≥ $50 000), married (yes or no), current smoking (yes or no), BMI (linear term), total exercise (metabolic equivalents h/week, linear term), physical function score (linear term), systolic blood pressure (linear term), diastolic blood pressure (linear term), history of high cholesterol, high blood pressure, diabetes, myocardial infarction, coronary revascularisation and atrial fibrillation, use of off-study post-menopausal hormone therapy, use of diuretics, β-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and Women's Health Initiative study arm.
‡ Model 3 adjusted for variables in model 2 and alcohol intake (none, 0–10 g/d or >10 g/d), and quartiles of Na, Ca (Mg and K models), Mg (Ca and K models), K (Ca and Mg models), protein, saturated fat, monounsaturated fat, polyunsaturated fat and fibre.
Discussion
We did not find significant associations between dietary or total intake of Ca, Mg and K and mortality in WHI participants with HF. We had hypothesised that these nutrients would be associated with mortality based on previous research on HF and other CVD. For example, supplementation with Ca, Mg and K may reduce blood pressure, a risk factor for HF(11–14). Extremely low Ca levels can cause HF in rare instances(Reference Mikhail, El-Bialy and Grosser24). However, Ca supplements have been associated with increased risk of myocardial infarction and stroke in some studies(Reference Bolland, Grey and Avenell16). High dietary intake of Mg has been inversely associated with the development of hypertension and type 2 diabetes(Reference Song, Manson and Buring25, Reference Song, Sesso and Manson26), risk factors for HF and with markers of inflammation and endothelial function(Reference Chacko, Song and Nathan9, Reference Song, Li and van Dam10). Patients with HF are more likely to have low serum Mg than other older individuals, and low serum Mg has been associated with all-cause mortality in HF patients(Reference Arinzon, Peisakh and Schrire27, Reference Adamopoulos, Pitt and Sui28). Higher K excretion, which in part reflects greater intake, is associated with a lower rate of CVD(Reference Cook, Obarzanek and Cutler15). Low serum K has been associated with mortality in the context of HF(Reference Ahmed, Zannad and Love29, Reference Bowling, Pitt and Ahmed30). In addition, diet patterns and foods with high Ca, Mg and K content have been associated with incident HF(Reference Levitan, Wolk and Mittleman31–Reference Pfister, Sharp and Luben34).
There are a number of potential explanations for the lack of associations observed in the present study. The present hypothesis that intake of Ca, Mg and K are associated with all-cause mortality in women with HF relied on the assumption that availability of these nutrients in the body is related to dietary consumption. HF patients are often aggressively treated with multiple medications, which could confound or mask any influence of diet. Diuretics, which are a mainstay of HF treatment, can directly make an impact on circulating levels of K and Mg through urinary excretion(Reference Hunt, Abraham and Chin35). We did not have data to examine how dietary intake influences serum levels of Ca, Mg and K in the present population. Prior to HF hospitalisation, 42 % of the population were treated with diuretics. The use of diuretics could weaken the relationship between intake of the micronutrients and the effective exposure to them. In fact, the women who died following hospitalisation for HF were more likely to have hypertension and to use diuretics. We adjusted for diuretic use and other medication use prior to hospitalisation, but we were not able to capture medication use following hospitalisation. Additionally, kidney dysfunction is very common in HF and may influence the retention and availability of Ca, Mg and K. We did not have measures of kidney function in the present population.
The present dietary assessment was not ideal. FFQ are known to have substantial errors, which reduces the power to detect modest effects of diet. In addition, we used dietary assessment prior to HF hospitalisation rather than dietary assessment at the time of HF hospitalisation, and the correlations between pre- and post-HF hospitalisation intake of nutrients were moderate. This could lead to misclassification of diet during the relevant time period, biasing results towards no association if the misclassification is not related to mortality. However, in the present study, the errors in exposure assessment may be related to mortality, and the resulting bias would depend on the relationship between the error and underlying likelihood of death. We were not able to rule out unmeasured or residual confounding, for example, by severity of HF or kidney function, which were not assessed. We did not have information on left ventricular systolic function or adjudicated HF aetiology; the relationship between diet and mortality in patients with HF could plausibly differ by these characteristics. We did examine the relationship between the micronutrients and mortality in women with and without evidence of ischaemic disease prior to HF hospitalisation, and we did not find associations of Ca, Mg or K in women with or without history of IHD. Finally, dietary intake of these nutrients, rather than serum levels, simply may not affect mortality in HF patients. In addition to the limitations of the present study, there were important strengths. These included the racially and ethnically diverse population, the extensive information collected on the WHI participants, the large number of HF hospitalisations among these participants and the relatively long follow-up for post-HF hospitalisation mortality.
In summary, we did not find significant associations between intake of Ca, Mg or K and post-HF hospitalisation mortality in WHI participants. These results do not challenge the present dietary recommendation for HF patients. Further research is needed to conclusively determine whether nutrient intake can reduce mortality in HF patients.
Acknowledgements
All authors were involved in the design of the study. J. M. S., L. G. S., L. W. M., J. D. C. and C. E. L. collected the data. E. B. L. performed the statistical analysis and wrote the paper. All authors revised the paper and are responsible for the final manuscript. The authors declare no conflict of interest. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32 and 44221. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford and Nancy Geller. Clinical Coordinating Centre: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix and Charles Kooperberg.
Investigators and Academic Centres: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.