Cardiac hypertrophy is one of the major predictors of progressive heart disease and an independent risk factor for cardiac morbidity and mortality( Reference Drazner, Rame and Marino 1 ). It is the enlargement of the heart with an increase in the volume of cardiac cells, and prolonged hypertrophic status has been reported to be associated with the decompensation of heart function, the development of heart failure and sudden death in humans( Reference Liu, Cheng and Lee 2 ). One of the major signal transduction mechanisms leading to the development of cardiac hypertrophy is the overproduction of reactive oxygen species (ROS)( Reference Papparella, Ceolotto and Montemurro 3 ). An increase in ROS production both in vitro and in vivo is implicated in the development of cardiac hypertrophy and its pathophysiology( Reference Yao, Sun and Chang 4 ). Excessive ROS generation triggers cell dysfunction, lipid peroxidation and DNA mutagenesis, and can lead to irreversible cell damage or death( Reference Takimoto and Kass 5 ). Therapeutic intervention via suppression of oxidative stress and/or an increase in endogenous antioxidant enzymes may attenuate cardiac hypertrophy and associated complications. Recent studies have shown that treatment with antioxidants inhibits the hypertrophic response of cardiac myocytes( Reference Li, Wang and Huang 6 ).
Functional foods and nutraceuticals are becoming a part of everyday life and play an important role in maintaining human health. Plant foods can be considered as functional foods since they are all rich in phytochemicals or nutraceuticals, and they have been claimed to possess physiological benefits or provide protection against chronic diseases beyond their basic nutritional functions( Reference Alissa and Ferns 7 ). Epidemiological evidence suggests that diets rich in plant foods are associated with a lower incidence of CVD, diabetes, cancer and other degenerative diseases( Reference Ames, Shigenaga and Hagen 8 ). It has been proposed that a higher dietary intake rich in vegetables and fruits or antioxidant phytochemicals is associated with a lower risk of CVD and mortality( Reference Stampfer, Hu and Manson 9 ).
Boerhaavia diffusa L. from the family Nyctaginaceae is an important indigenous medicinal plant widely used in Ayurveda. It is commonly known as punarnava, has a long history of use by indigenous and tribal people( Reference Kaur and Goel 10 ) and is used against epilepsy in Nigerian folk medicine( Reference Srivastava, Saluja and Dwarakanath 11 , Reference Apu, Liza and Jamaluddin 12 ). B. diffusa has also been widely used for the treatment of dyspepsia, jaundice, enlargement of spleen, abdominal pain and fibrinolytic, and as an anti-stress agent( Reference Srivastava, Saluja and Dwarakanath 11 ). The plant has been reported to possess cardiotonic and antihypertensive potential( Reference Munasinghe, Seneviratne and Thabrew 13 , Reference Agrawal, Nandini and Sharma 14 ). Pharmacological studies have demonstrated that punarnava possesses antidiabetic( Reference Pari and Satheesh 15 ), immunomodulatory( Reference Manu and Kuttan 16 ), anticonvulsant, hepatoprotective, antibacterial, antiproliferative and anti-oestrogenic activities( Reference Kaur and Goel 10 , Reference Sreeja and Sreeja 17 ). The plant has been well demonstrated to have antimitotic activity in in vitro systems and inhibits the growth of several monocytic, lymphoblastoid, fibroblast and erythroleukaemic cell lines of mouse and human origin( Reference Sreeja and Sreeja 17 ). The plant possesses antioxidant potential, and experimental studies have demonstrated that B. diffusa could be effective in the prevention and treatment of diseases in which oxidants or free radicals are implicated( Reference Apu, Liza and Jamaluddin 12 , Reference Aviello, Canadanovic-Brunet and Milic 18 – Reference Pareta, Patra and Mazumder 21 ). B. diffusa is not only used as a medicinal plant but also used as a green leafy vegetable due to its nutraceutical properties in most of the Asian countries( Reference Apu, Liza and Jamaluddin 12 ).
The present study aimed to evaluate whether B. diffusa can ameliorate hypertrophy induced by angiotensin II in H9c2 cells, and its effects on oxidative stress and transcription factors such as NF-κβ and transforming growth factor β1 (TGF-β1).
Materials and methods
Preparation of Boerhaavia diffusa extract
B. diffusa was collected from the local areas of Thiruvananthapuram, India during the month of May, and identified and authenticated by Dr H. Biju, Taxonomist from the Jawaharlal Nehru Tropical Botanical Garden Research Institute, Palode, Thiruvananthapuram, Kerala. A voucher specimen was deposited in our herbarium for future reference (no. 01/05/2010 APNP/CSIR-NIIST). Extraction of the plant material was carried out according to the method described previously, with slight modifications( Reference Prathapan, Singh and Anusree 19 ). Briefly, the fresh whole plants were air-dried and extracted with ethanol at ambient temperature (27 ± 1°C) under stirring for 6 h, and the extraction process was repeated until the solvent became colourless. The supernatant was filtered through Whatman no. 1 filter paper and concentrated in vacuo under reduced pressure in a rotavapor (Heidolph) followed by lyophilisation. The lyophilised B. diffusa extract (BDE) was stored at 4°C until use. The yield of the extract was found to be 12·64 % (w/w). The same sample of the extract was used to conduct all the experiments.
The total phenolic content (TPC) of BDE was estimated using the Folin–Ciocalteu reagent( Reference Singleton and Rossi 22 ), and expressed as mg gallic acid equivalents/g extract. The total flavonoid content was determined using a colorimetric method( Reference Jia, Mengcheng and Wu 23 ), and expressed as mg catechin equivalents/g extract. The angiotensin-converting enzyme (ACE)-inhibitory potential of BDE was assayed as reported by Hernandez-Ledesma et al. ( Reference Hernandez-Ledesma, Martin-Alvarez and Pueyo 24 ). Briefly, different concentrations of BDE were added to 100 μl hippuryl-l-histidyl-l-leucine, prepared in 50 mm-HEPES buffer (pH 8·3) and then incubated for 5 min at 37°C. The reaction was initiated by adding 150 μl of 100 U/l ACE prepared in the same buffer and incubated for 1 h at 37°C. The enzyme reaction was stopped by the addition of 0·5 m-HCl and the released hippuric acid was extracted with ethyl acetate. The organic layer was taken and evaporated at 90°C for 15 min. The released hippuric acid was redissolved in distilled water and absorbance was measured at 228 nm using a UV–vis spectrophotometer (UV-2450PC; Shimadzu).
The inhibitory potential of xanthine oxidase (XO) was assayed spectrophotometrically according to the method of Owen & Johns( Reference Owen and Johns 25 ). The assay mixture consisted of 1 ml BDE at different concentrations, 2·9 ml of phosphate buffer (pH 7·5) and 0·1 ml of enzyme solution (0·01 units/ml in phosphate buffer, pH 7·5), which was prepared immediately before use. After pre-incubation at 25°C for 15 min, the reaction was initiated by the addition of 2 ml of substrate solution (150 mm-xanthine in the same buffer). The assay mixture was incubated at 25°C for 30 min. The reaction was then stopped by the addition of 1 ml of 0·5 m-HCl, and absorbance was measured at 290 nm using a UV–vis spectrophotometer (UV-2450PC; Shimadzu).
Cell culture
The H9c2 embryonic rat heart-derived cell line was obtained from the National Centre for Cell Science, Pune, India and cultured in Dulbecco's modified Eagle's medium (HiMedia) containing glucose (4·5 g/l), sodium bicarbonate (1·5 g/l) and sodium pyruvate (110 mg/l), supplemented with 10 % fetal bovine serum (Gibco) and penicillin (100 units/ml) and streptomycin (100 μg/ml) in a humidified incubator with 95 % air and 5 % CO2 at 37°C. The culture medium was changed every 2 d. After 4 d, cells were passaged and seeded at a density of 1·2 × 106 cells per 100 mm dish or 0·64 × 104 cells per 6·4 mm well of ninety-six-well plates. These cells were cultured for 3 d and then underwent treatments.
Cell treatment
H9c2 cells were treated with BDE for 6 h before angiotensin II treatment. Angiotensin II (100 nm; Sigma-Aldrich) was prepared in double-distilled water, diluted with culture media to induce hypertrophy and cultured for an additional 48 h( Reference Alissa and Ferns 7 ). The experimental group consisted of (1) control cells (2) BDE-alone-treated cells, (3) angiotensin II-alone-treated cells and (4) BDE+angiotensin II-treated cells.
Measurement of cell viability
To carry out this experiment, 1 × 104 cells plated in each well of twenty-four-well plates were placed in a 5 % CO2 incubator at 37°C and allowed to adhere to the substrate. Cells grown to 70–85 % confluence were exposed to various concentrations of BDE (1, 10, 25, 50, 75 and 100 μg/ml). BDE was dissolved in dimethyl sulphoxide and the final concentration of dimethyl sulphoxide used was less than 0·1 % (v/v) for each treatment. The same concentration of dimethyl sulphoxide was used in control cells as vehicle. The control and treated cells were incubated for 48 h. Cell viability was assayed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich) according to the standard protocol( Reference Wilson and Masters 26 ).
Lactate dehydrogenase release
Lactate dehydrogenase (LDH) activity of the cells from all experimental groups (treated with angiotensin II and different concentrations of BDE) was measured using an LDH assay kit (Cayman Chemical) following the manufacturer's instructions.
Measurement of cell size
Adherent cells were made to detach via trypsinisation and images of rounded cells were acquired using a Nikon TE2000-S microscope with an attached digital camera and a 40 × lens (Nikon). Measurements of cell diameter were made using Microsoft Office Document Imaging software (NIS Elements), and cell volume was calculated using the equation for the volume of a sphere (4/3πr 3). The diameter of individual cells was measured, and 100 cells per experimental group were measured randomly( Reference Merten, Jiang and Feng 27 ).
Measurement of protein content per cell
Cells in the dishes after the respective treatments were collected by trypsinisation with trypsin–EDTA (HiMedia) and washed twice in ice-cold PBS. Cells were then collected via centrifugation and lysed with cell lysis buffer (20 mm-Tris–HCl, 150 mm-NaCl, 1 mm-Na2EDTA, 1 mm-EGTA, 1 % Triton X-100, 2·5 mm-sodium pyrophosphate, 1 mm-β-glycerophosphate, 1 mm-Na3VO4, 1 μg leupeptin/ml and 1 mm-phenylmethylsulfonyl fluoride, pH 7·5) on ice for 30 min. The suspension was centrifuged at 14 000 g for 10 min at 4°C, and the supernatant was collected. Protein concentration in the total cell lysate was measured using the Bradford assay( Reference Bradford 28 ) with bovine serum albumin as the standard. Protein content per cell was determined by dividing the total amount of protein by the cell number( Reference Merten, Jiang and Feng 27 ).
Determination of atrial natriuretic peptide and B-type natriuretic peptide
Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) were measured using an ELISA kit (AssayPro).
Assay of endogenous antioxidant status and lipid peroxidation
Activities of various endogenous antioxidant enzymes were evaluated in the control and treated cells. Catalase activity was assayed by monitoring the disappearance of H2O2 at 240 nm, according to the method of Aebi( Reference Aebi 29 ). Superoxide dismutase activity was measured spectrophotometrically based on NADPH oxidation according to the method of Paoletti et al. ( Reference Paoletti, Aldinucci and Mocali 30 ). Glutathione peroxidase activity is based on the oxidation of reduced glutathione (GSH) by glutathione peroxidase coupled to the disappearance of NADPH by glutathione reductase( Reference Gunzler, Kramers and Flohe 31 ). The activity of glutathione reductase was measured by following the decrease in absorbance due to the oxidation of NADPH utilised in the reduction of oxidised glutathione according to the method of Goldberg & Spooner( Reference Goldberg, Spooner and Bergmeyer 32 ). GSH was quantified by using a fluorometric assay( Reference Hissin and Hilf 33 ). Protein carbonyls and glutathione S-transferase were measured using a kit (Cayman Chemical).
The thiobarbituric acid-reactive substance (TBARS) assay was used to determine lipid peroxidation and was performed with a modified method as described previously( Reference Choi, Chang and Cho 34 ) in order to reduce the limitations such as heat-induced lipid peroxidation, interference of Fe released during homogenisation and sucrose.
Detection of intracellular reactive oxygen species
Intracellular ROS levels were measured employing fluorescent 2′,7′-dichlorodihydrofluorescein diacetate as a probe( Reference Raghu and Cherian 35 ). Live cell bioimaging was done with a high-content bioimager (BD Pathway™ Bioimager System; BD Biosciences).
Expression of NF-κβ and transforming growth factor β1 using RT-PCR
Total RNA was isolated from H9c2 cells after the respective treatments using TRIzol reagent (Sigma-Aldrich) by the method described by Chomczynski & Sacchi( Reference Chomczynski and Sacchi 36 ).
The isolated RNA was used for RT-PCR to study the expression of NF-κβ and TGF-β1 at the mRNA level. Total RNA (1·5 μg) was used in the reversal transcription reaction with 0·5 μg oligo-dT16 (Fermentas), 10 mm of each of the four deoxynucleotide triphosphates, 25 mm-MgCl2, 10 U RNase inhibitor and 50 U RT (Fermentas) according to the manufacturer's instructions.
The primer sequences used were as follows: NF-κβ – forward 5′-CCTAGCTTTCTCTGAACTGCAAA-3′, reverse 5′-GGGTCAGAGGCCAATAGAGA-3′; TGF-β1 – forward 5′-GGC-CAG-ATC-CTG-TCC-AAA-CT-3′, reverse 5′-GCC-CTG-TAT-TCC-GTC-TCCTT-3′; glyceraldehyde 3-phosphate dehydrogenase – forward 5′-GCCAAAAGGGTCATCATCTCCGC-3′, reverse 5′-GGATGACCTGCCCACAGCCTTG-3′. The PCR mixture contains PCR buffer, 25 mm-MgCl2, 5 U DNA polymerase (Fermentas), 3 μl complementary DNA and 20 pmol of each primer for thirty-five cycles. The PCR products were electrophoresed in 1 % agarose gels containing 0·05 μg/ml of ethidium bromide. mRNA expression was quantified using a phosphoimager and accompanying Image Quant software (Bio Rad), and the relative expression was compared and normalised to the expression of glyceraldehyde 3-phosphate dehydrogenase in the same sample.
Statistical analysis
Results are expressed as means and standard deviations of the control and treated cells from three independent experiments in duplicate (n 6). Data were subjected to one-way ANOVA and the significance of differences between means was calculated by Duncan's multiple range test using SPSS for Windows, standard version 7.5.1 (SPSS, Inc.), and significance was accepted at P≤ 0·05.
Results
Cell viability
Cell viability analysis revealed that BDE did not possess any cytotoxicity in H9c2 cells (data not shown). Cells treated with angiotensin II alone and BDE with angiotensin II were also evaluated for their viability. Treatment with BDE protected the cells from angiotensin II-induced cell death in a dose-dependent manner, and BDE at a concentration of 75 μg/ml exhibited maximum activity (Fig. 1(a)). The activity of LDH increased significantly in the angiotensin II-treated hypertrophied cells while BDE+angiotensin II-treated cells showed reduced LDH activity, indicating the cytoprotective potential of BDE (Fig. 1(b)). There was no significant change in the activity of this enzyme in BDE-alone-treated cells when compared with the control cells. Since the BDE concentration of 75 μg/ml was found to be more protective, we used this dose for evaluating other parameters relevant to hypertrophy.
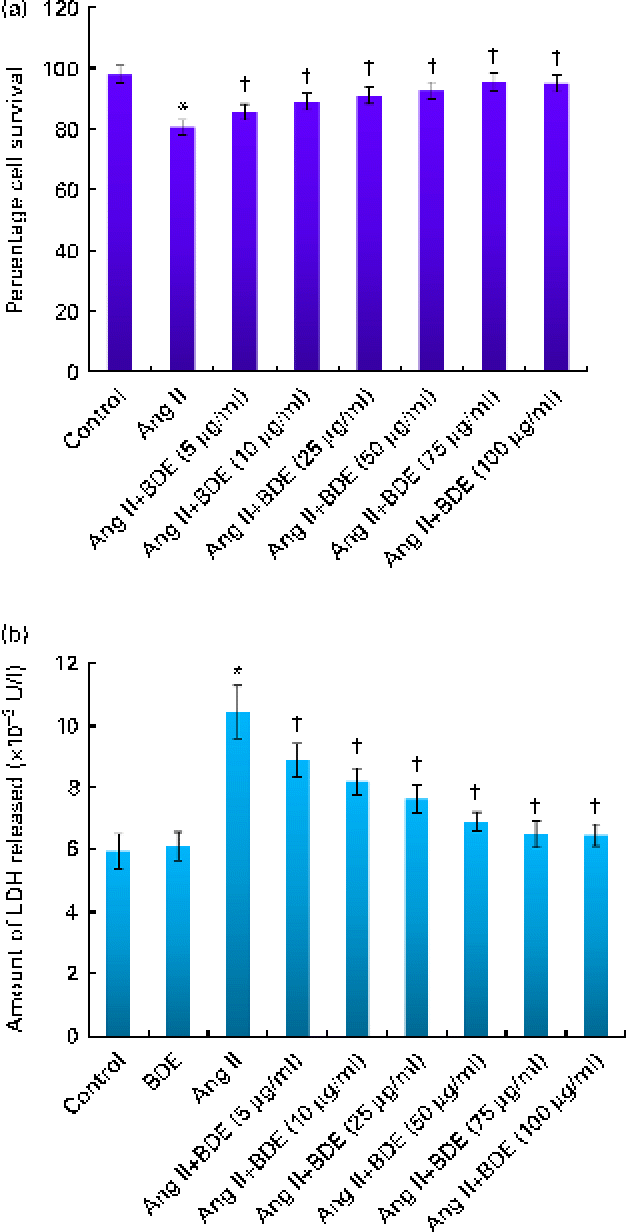
Fig. 1 (a) Viability of H9c2 cells treated with angiotensin II (Ang II) and different concentrations of Boerhaavia diffusa extract (BDE)+Ang II. (b) Activity of lactate dehydrogenase (LDH) in the control and treated cells. Values are means, with standard deviations represented by vertical bars (n 6). * Mean value was significantly different from the control cells (P≤ 0·05). † Mean values were significantly different from the Ang II-treated cells (P≤ 0·05). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Cell volume, protein content and the concentrations of atrial natriuretic peptide and B-type natriuretic peptide
Angiotensin II caused an increase in cell volume (69·26 (sd 1·21) %), protein content (48·48 (sd 1·64) %), ANP (81·90 (sd 1·22) %) and BNP (108·57 (sd 1·47) %) (Table 1). The BDE treatment significantly reduced cell volume, protein content and the concentrations of ANP and BNP (P≤ 0·05) in the angiotensin II-treated cells. This indicates the anti-hypertrophic potential of BDE.
Table 1 Change in cell volume, protein content, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) in the control and treated cells (Mean values and standard deviations, n 6)
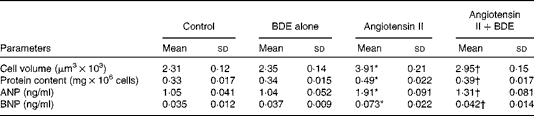
BDE, Boerhaavia diffusa extract.
* Mean values were significantly different from the control cells (P≤ 0·05).
† Mean values were significantly different from the angiotensin II-treated hypertrophied cells (P≤ 0·05).
Antioxidant status
Fig. 2(a) and (b) represents intracellular ROS production in the control and treated cells. The angiotensin II treatment caused increased ROS generation while the BDE treatment reduced the generation of ROS. Evaluation of endogenous antioxidant status during hypertrophy provides an indication of oxidative damage. For this purpose, we measured the concentration of TBARS and the activities of various antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase and glutathione S-transferase (Table 2). The concentration of TBARS was higher (92·71 (sd 1·21) %) in the hypertrophied cells when compared with the control cells, while the BDE+angiotensin II-treated cells showed a 39·86 (sd 2·1) % reduction in the concentration of TBARS when compared with the hypertrophied cells (P≤ 0·05). Depletion of GSH (38·22 (sd 1·92) %), a non-enzymatic antioxidant, was observed in the angiotensin II-treated cells, while the BDE pretreated cells significantly prevented the decrease in GSH. Activities of the antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase and glutathione S-transferase) were significantly reduced (42·65 (sd 1·33), 76 (sd 1·92), 46 (sd 1·51), 51 (sd .12) and 60 (sd 1·08) %, respectively) in the angiotensin II-treated cells when compared with the untreated control cells (P≤ 0·05), while the BDE treatment reversed these changes. This validates the antioxidant-mediated protection by BDE in the hypertrophied cells. Oxidative stress is also associated with protein oxidation and the concentration of protein carbonyls was also significantly higher (125·22 (sd 1·52) %) in the hypertrophied cells (Table 2). Here, the BDE treatment also significantly reduced the concentration of protein carbonyls when compared with the hypertrophied cells (P≤ 0·05).
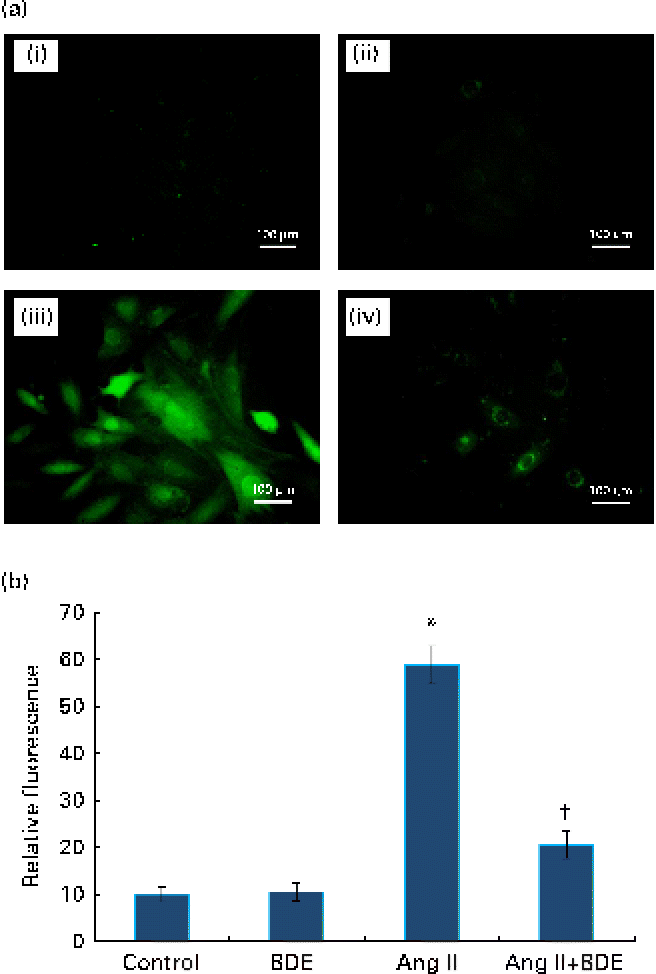
Fig. 2 (a) Effect of Boerhaavia diffusa extract (BDE) on intracellular reactive oxygen species (ROS) generation in the control and hypertrophied H9c2 cells. The representative photographs of ROS-induced fluorescence by the cells treated with angiotensin II (Ang II) and BDE. (i) Control cells, (ii) BDE (75 μg/ml)-alone-treated cells, (iii) Ang II (100 nm)-treated cells and (iv) Ang II+BDE-treated cells. (b) Amount of ROS release measured as fluorescence. Values are means, with standard deviations represented by vertical bars (n 6). * Mean value was significantly different from the control cells (P≤ 0·05). † Mean value was significantly different from the angiotensin II-treated cells (P≤ 0·05). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 2 Concentration of thiobarbituric acid-reactive substances (TBARS) and activities of antioxidant enzymes in the control and treated cells (Mean values and standard deviations, n 6)
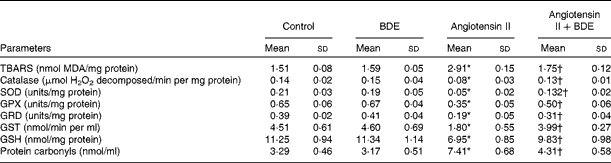
BDE, Boerhaavia diffusa extract; MDA, malondialdehyde; SOD, superoxide dismutase; GPX, glutathione peroxidase; GRD, glutathione reductase; GST, glutathione S-transferase; GSH, reduced glutathione.
* Mean values were significantly different from the control cells (P≤ 0·05).
† Mean values were significantly different from the angiotensin II-treated hypertrophied cells (P≤ 0·05).
Expression of NF-κβ and transforming growth factor β1
The mRNA expressions of NF-κβ and TGF-β1 were evaluated by RT-PCR (Figs. 3 and 4, respectively). The angiotensin II-treated cells showed a marked increase in NF-κβ and TGF-β1 expressions compared with the control cells (P≤ 0·05). The BDE treatment reduced the expression of the NF-κβ (Fig. 3(a) and (b)) and TGF-β1 (Fig. 4(a) and (b)) genes (P≤ 0·05). There was no change in their expression in the BDE-alone-treated cells.
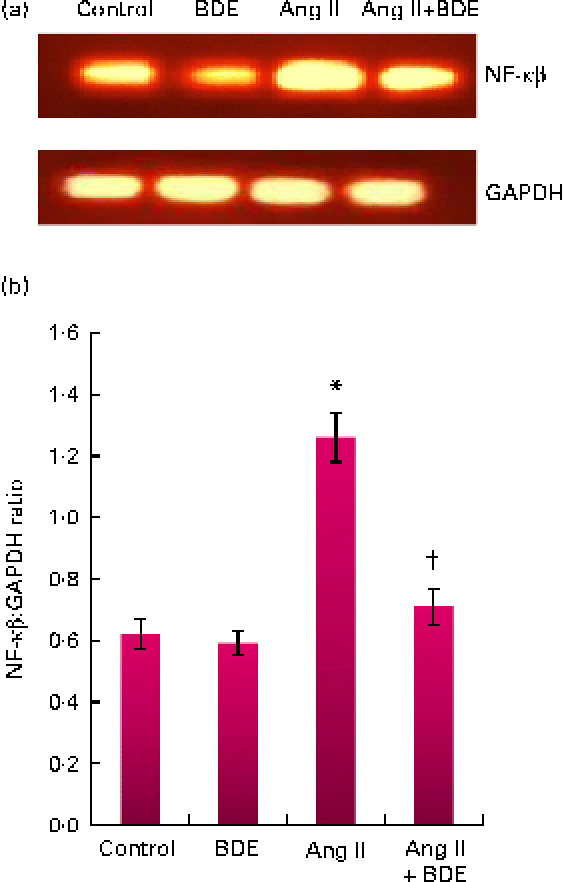
Fig. 3 (a) Expression of NF-κβ at the mRNA level using RT-PCR. (b) Graphical representation of the intensity of NF-κβ expression. Values are means, with standard deviations represented by vertical bars (n 6). * Mean value was significantly different from the control cells (Cont, P≤ 0·05). † Mean value was significantly different from the angiotensin II (Ang II)-treated cells (P≤ 0·05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; BDE, Boerhaavia diffusa extract. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
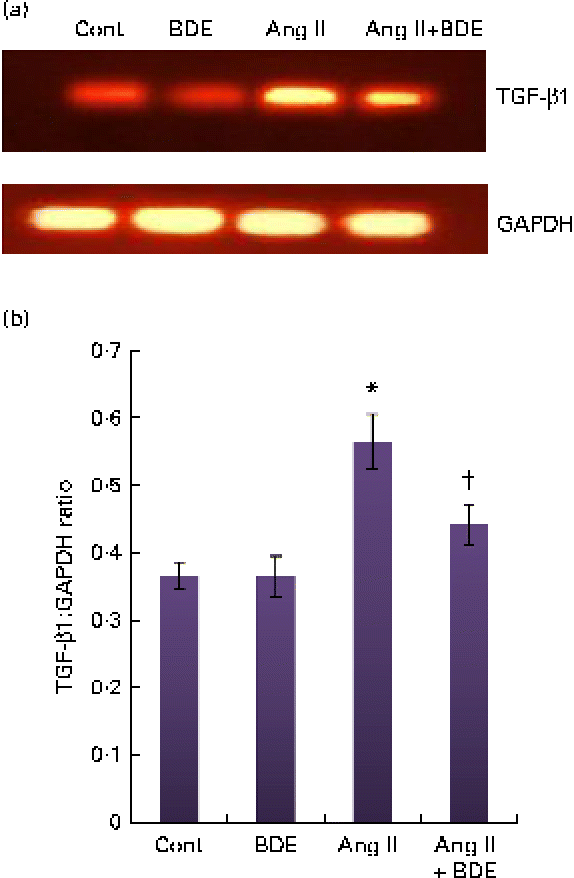
Fig. 4 (a) Expression of transforming growth factor β1 (TGF-β1) at the mRNA level using RT-PCR. (b) Graphical representation of the intensity of TGF-β1 expression. Values are means, with standard deviations represented by vertical bars (n 6). * Mean value was significantly different from the control cells (Cont, P≤ 0·05). † Mean value was significantly different from the angiotensin II (Ang II)-treated cells (P≤ 0·05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; BDE, Boerhaavia diffusa extract. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
The TPC and the total flavonoid content of BDE were estimated to be 125·02 (sd 1·56) mg gallic acid equivalents/g and 63·15 (sd 2·48) mg catechin equivalents/g extract, respectively. In addition, in vitro chemical assays revealed that BDE exhibited inhibitory potential against ACE and XO in a dose-dependent manner. The estimated 50 % effective concentration (EC50) value for BDE against ACE inhibition was 166·12 (sd 2·42) μg/ml and that of the standard compound captopril was 1·2 (sd 0·22) μg/ml. BDE showed XO inhibition with an estimated EC50 value of 60·05 (sd 1·54) μg/ml and that of the standard compound allopurinol was 6·11 (sd 1·27) μg/ml.
Discussion
The present study has shown the protective potential of B. diffusa against angiotensin II-induced cardiac hypertrophy in H9c2 cells. The H9c2 cell line was originally derived from the embryonic rat ventricular tissue( Reference Kimes and Brandt 37 ), which is important to study hypertrophy since cardiac hypertrophy resulting from hypertension mainly occurs in the ventricular muscle of the heart( Reference Watkins, Borthwick and Arthur 38 ). H9c2 cells show many similarities to primary cardiomyocytes, including membrane morphology, G-signalling protein expression and electrophysiological properties( Reference Hescheler, Meyer and Plant 39 , Reference Sipido and Marban 40 ). Importantly, they can display a wide range of hypertrophy-associated traits when stimulated with hypertrophic agents in vitro ( Reference Watkins, Borthwick and Arthur 38 ). Angiotensin II, the active octapeptide and circulatory hormone, is an important humoral factor responsible for cardiomyocyte hypertrophy( Reference Wollert and Drexler 41 ), and is emerging as an important molecule both in the development of cardiac hypertrophy and in the pathogenesis of progressive myocardial dysfunction leading to heart failure( Reference Unger 42 ). A reduction in cell size, protein content, LDH leakage and the down-regulation of ANP and BNP upon BDE treatment in the angiotensin II-exposed cells shows the beneficial effects of BDE against cardiac hypertrophy.
Direct evaluation of ROS yields a very good indication of oxidative damage to living cells( Reference Wang and Joseph 43 ). Elevated levels of ROS impair cardiomyocyte function by damaging ion channels as well as inhibiting contractility, and can disrupt the structural integrity of ion channels via membrane lipid peroxidation at the cellular level( Reference Hajjar and Leopold 44 ). Reduced generation of ROS upon BDE treatment shows the free-radical-scavenging potential of BDE. Since ROS act as a signalling molecule for the development of cardiac hypertrophy, agents that block the formation of ROS will have therapeutic importance against cardiac hypertrophy. Increased ROS generation is mainly due to the depletion of an endogenous antioxidant system( Reference Poljsak 45 ). This reduces the capacity of cells to scavenge various types of reactive radicals and cause oxidative stress in cells. Increased quantities of ROS initiate lipid peroxidation in cellular, mitochondrial and nuclear membranes along with protein oxidation( Reference Dinu, Bodea and Ceapa 46 ). The TBARS assay is the most commonly used method for measuring lipid peroxidation, but the method also suffers from some limitations( Reference Devasagayam, Boloor and Ramasarma 47 ). One of the limitations is interference from Fe released during homogenisation( Reference Devasagayam, Boloor and Ramasarma 47 , Reference Nelson, Bose and McCord 48 ) and another limitation is sporadic lipid peroxidation during heating( Reference Choi, Chang and Cho 34 ). In the in vitro experiments, sucrose can also interfere with the assay. In order to reduce the interference of Fe and heat-induced lipid peroxidation, the addition of an antioxidant such as butylated hydroxytoluene is advised( Reference Devasagayam, Boloor and Ramasarma 47 , Reference Nelson, Bose and McCord 48 ). To prevent sucrose interference, boiled samples can be extracted with butanol–pyridine solution( Reference Shlafer and Shepard 49 ). In the present study, in order to reduce the interference from heating and Fe-induced peroxidation, butylated hydroxytoluene was added to the reaction mixture and lipid peroxides were extracted with butanol. Protein carbonyls are the products of protein oxidation and are one of the most commonly used markers of protein oxidation( Reference Halliwell 50 ). An increased level of TBARS and protein carbonyls along with a reduced concentration of GSH in the angiotensin II-treated cells showed oxidative damage during hypertrophy. In addition, hypertrophied H9c2 cells showed reduced activities of antioxidant enzymes. Intracellular antioxidant enzymes act as a first line of defence against oxidative stress in the cell. Among the various antioxidant enzymes, superoxide dismutase catalyses the dismutation of the superoxide anion to H2O2 and molecular O2. H2O2 is decomposed to H2O by catalase and glutathione peroxidase. In the reaction catalysed by glutathione peroxidase, GSH is oxidised to oxidised glutathione, which can be subsequently reduced back to GSH by glutathione reductase( Reference Peng and Li 51 ). Decreased activities of GSH-dependent enzymes such as glutathione peroxidase and glutathione reductase in hypertrophied cells may be due to either free radical-dependent inactivation of enzyme or depletion of GSH. GSH is one of the major non-enzymatic antioxidants and a powerful nucleophile critical for cellular protective activities such as detoxification of ROS and control of the inflammatory cytokine cascade( Reference Abhilash, Harikrishnan and Indira 52 ). Depletion of GSH leads to the impairment of cellular defence against ROS and may lead to oxidative injury( Reference Kent, Harper and Bomser 53 ). GSH can also act as a cofactor for glutathione S-transferase. Glutathione S-transferase plays an important role in protecting cells against ROS-mediated injury through catalysing the decomposition of lipid peroxides produced during the ROS attack of cellular lipid molecules( Reference Peng and Li 51 ). The beneficial effects of BDE in reducing oxidative stress during hypertrophy are due to its antioxidant potential.
The results of the in vitro chemical assays revealed that BDE exhibits XO-and ACE-inhibitory potential. Generally, ROS are derived from the superoxide anion. One of the major enzymatic sources that generate the superoxide anion in the myocardium is XO, and previous studies have shown that the activity of XO was higher during cardiac dysfunction( Reference Hajjar and Leopold 44 ). The XO-inhibitory potential of BDE further confirms that the extract can scavenge ROS-producing superoxide anions and thereby protect the myocardium against oxidative stress-mediated hypertrophy. Likewise, the ACE-inhibitory potential of BDE also contributes to its protection against cardiac hypertrophy. It was established that ACE inhibition is associated with a significant improvement in cardiac hypertrophy secondary to hypertension and favourably affects coronary haemodynamics( Reference Ouyang, Takahashi and Komatsu 54 ).
In order to study the effects of angiotensin II-induced cardiac hypertrophy on transcription factors, mRNA expressions of NF-κβ and TGF-β1 were studied in H9c2 cells. NF-κβ is an inducible transcription factor that is activated by various inflammatory stimuli and growth factors, and is involved in inflammation, immune response and cell survival( Reference Ichikawa, Yagi and Tanaka 55 ). NF-κβ also influences the regulation of cell growth, and the activation of NF-κβ is closely linked with cardiac hypertrophy and required for the hypertrophic growth of cardiomyocytes both in vitro and in vivo ( Reference Purcell, Tang and Yu 56 , Reference Li, Ha and Gao 57 ). ROS can act as a strong stimulus for the activation of NF-κβ( Reference Baldwin 58 , Reference Jones, Brown and Wilhide 59 ) and treatment with antioxidants can abolish the angiotensin II-induced hypertrophic response of cardiomyocytes through inhibiting NF-κβ activation( Reference Li, Ha and Gao 57 ). A decrease in the mRNA expression of NF-κβ may be due to the decrease in the activation of NF-κβ. The decreased mRNA level of NF-κβ in BDE-treated cells indicates that BDE can down-regulate the expression of NF-κβ via reducing the generation of ROS. Reports have shown that ANP gene expression requires NF-κβ activation( Reference Li, Ha and Gao 57 ). We found in the present study that the concentration of ANP increased along with the activation of NF-κβ. TGF-β1 is another transcription factor involved in the regulation of development, differentiation, the maintenance and repair of various cells and tissues, and it has been shown to be expressed at high levels during the cardiac development and pathology of the heart( Reference Li, Li and Mickle 60 ). TGF-β1 is present in both cardiomyocytes and myocardial fibroblasts, and is an important mediator of the hypertrophic growth of the heart( Reference Schultz, Witt and Glascock 61 ). Also, in the present study, the mRNA expression of TGF-β1 was significantly increased in angiotensin II-treated cells, which is in agreement with previous reports( Reference Schultz, Witt and Glascock 61 ). Treatment with BDE reduced the expression of TGF-β1 mRNA levels, indicating that BDE can down-regulate the hypertrophic response induced by angiotensin II. Blocking the TGF-β1 pathway might be a pharmacological intervention in cardiac remodelling involving cardiac hypertrophy( Reference Yamazaki, Yamashita and Izumi 62 ).
From the overall results, it is clear that B. diffusa is a potent pharmacological agent that exerts significant protection against the angiotensin II-induced hypertrophic response in H9c2 cells by reducing oxidative stress and down-regulating transcription factors such as NF-κβ and TGF-β1. B. diffusa is a rich source of phenolic compounds that are potent natural antioxidants( Reference Prathapan, Singh and Anusree 19 , Reference Gulati, Harding and Palombo 20 ). In the present study, the TPC of B. diffusa was estimated to be 125·02 (sd 1·56) mg gallic acid equivalents/g extract, which is comparable with the study of Apu et al. ( Reference Apu, Liza and Jamaluddin 12 ) (TPC 163·14 (sd 1·95) mg/g extract). In contrast, others have reported less TPC values such as 24·28 (sd 3)( Reference Sreeja and Sreeja 17 ) and 5·54 (sd 0·24) mg/g extract( Reference Gulati, Harding and Palombo 20 ) and the reduced TPC content may be due to the differences in geographical distribution, the season of plant collection and the extraction of plant material. Polyphenols have recently attracted considerable attention for the prevention of oxidative stress( Reference Li, Wang and Huang 6 ), and epidemiological studies have shown that consumption of phenolics and flavonoids has modestly reduced the risks for CVD and acts as protective agents against CVD( Reference Stampfer, Hu and Manson 9 , Reference Prathapan, Singh and Anusree 19 , Reference Vita 63 ). Previous studies have shown that BDE contains a number of pharmacologically active compounds such as punarnavine, ursolic acid, punarnavoside, liriodendrin, eupalitin, rotenoids (boeravinones A, B, C, D, E, F and G), quercetin, kaempferol, etc.( Reference Agrawal, Nandini and Sharma 14 , Reference Ferreres, Sousa and Justin 64 , 65 ). Studies on B. diffusa have revealed that it can scavenge ROS and is effective in reducing diseases associated with oxidative stress( Reference Aviello, Canadanovic-Brunet and Milic 18 – Reference Gulati, Harding and Palombo 20 ). Among the various active compounds, quercetin is found to be effective in reducing left ventricular cardiac hypertrophy in a variety of experimental models( Reference Perez-Vizcaino and Duarte 66 ). Quercetin also exerts protection against endothelial dysfunction and exhibits antihypertensive effects( Reference Duarte, Jimenez and O'Valle 67 ). Ursolic acid and kaempferol are other active compounds from B. diffusa, which have also been reported to possess cardioprotective properties( Reference Calderon-Montano, Burgos-Moron and Perez-Guerrero 68 , Reference Senthil, Sridevi and Pugalendi 69 ). Liriodendrin isolated from B. diffusa exhibits Ca channel antagonistic properties in the heart cell, which is attributed to its cardioprotective and antihypertensive potential( Reference Lami, Kadota and Kikuchi 70 ). Boeravinone G, a rotenoid from B. diffusa, is a potent antioxidant and genoprotective agent( Reference Aviello, Canadanovic-Brunet and Milic 18 ). The observed beneficial effects of B. diffusa against hypertrophy may be due to the presence of these biologically active molecules.
In conclusion, the results obtained in the present study have shown that BDE protects H9c2 cardiac myocytes against angiotensin II-induced cardiac hypertrophy. The possible beneficial effect of BDE in reducing cardiac hypertrophy appears to be by the down-regulation of oxidative stress and transcription factors such as NF-κβ and TGF-β1. Since the plant is widely used as a vegetable, B. diffusa can be used as a nutraceutical/medicinal food for the prevention and management of cardiac hypertrophy and other associated disorders. However, further detailed studies are required to establish its clinical relevance/therapeutic potential.
Acknowledgements
A. P. thanks the Council of Scientific and Industrial Research for financial assistance in the form of Senior Research Fellowship. We are also thankful to the CSIR 12th 5-year plan project ‘THUNDER’ (BSC 0102) for partial financial assistance. We thank the Director, CSIR-NIIST and Head, Agroprocessing and Natural Products Division, CSIR-NIIST for providing necessary facilities. A. P. designed and conducted the experiments, analysed the results and prepared the manuscript. V. P. V. and P. A. A. also conducted the experiments and analysed the data. K. G. R. contributed to the conception and design of the study, the acquisition and interpretation of the data, performed the analysis and drafted the manuscript for important intellectual content. All the authors participated sufficiently in the work. The authors declare that there is no conflict of interest.








