Piperine, the active principle of black and long pepper, is now established as a bioavailability enhancer of various structurally and therapeutically diverse drugs and other phytochemicals(Reference Zutshi, Singh and Zutshi1, Reference Bano, Raina and Zutshi2). The potential of piperine to increase the bioavailability of drugs in humans is of great clinical significance. Studies have indicated that piperine is absorbed very quickly across the intestinal barrier. It exhibits passive diffusion with non-saturable absorption kinetics, short absorption clearance and a high permeability coefficient(Reference Khajuria, Zutshi and Bedi3). It is, therefore, reasonable to presume that since piperine is an apolar molecule, it may modulate membrane dynamics due to its easy partitioning in the hydrophobic core and so assist the easy permeation of solutes. The available research data point out that piperine increases the bioavailability of drugs either by promoting rapid absorption from the gastrointestinal tract, or by retarding the drug from being metabolised in the liver or by a combination of these two mechanisms.
The effect of piperine, the pungent principle of black pepper, on the absorptive function of the intestine has been studied in in vitro experiments which showed that piperine (25–100 μm) significantly stimulated γ-glutamyl transpeptidase activity, enhanced the uptake of radiolabelled amino acids and increased lipid peroxidation in freshly isolated epithelial cells of rat jejunum(Reference Johri, Thusu and Khajuria4). The kinetic behaviour of γ-glutamyl transpeptidase towards substrate and acceptor altered in the presence of piperine. The results suggested that piperine may interact with the lipid environment to produce effects leading to increased permeability of the intestinal cells. It is hypothesised that piperine's bioavailability-enhancing property may be attributed to increased absorption, which may be due to alterations in membrane lipid dynamics and changes in the conformation of enzymes in the intestine(Reference Khajuria, Thusu and Zutshi5). Results of membrane fluidity studies using an apolar fluorescent probe, pyrene (which measures the fluid properties of the hydrocarbon core), showed an increase in intestinal brush-border membrane (BBM) fluidity.
Piperine also stimulates leucine amino peptidase and glycyl-glycine dipeptidase activity, due to an alteration in enzyme kinetics(Reference Khajuria, Thusu and Zutshi5). This suggests that piperine could modulate membrane dynamics due to its apolar nature by interacting with surrounding lipids and hydrophobic portions in the protein vicinity, which may decrease the tendency of membrane lipids to act as steric constraints to enzyme proteins and thus modify enzyme conformation. Ultrastructural studies with piperine showed an increase in microvilli length with a prominent increase in free ribosomes and ribosomes on the endoplasmic reticulum in enterocytes, suggesting that synthesis or turnover of cytoskeletal components or membrane proteins may be involved in the observed effect. Thus, it is suggested that piperine may be inducing alterations in membrane dynamics and permeation characteristics, along with induction of the synthesis of proteins associated with cytoskeletal function, resulting in an increase in the small intestine absorptive surface, thus assisting efficient permeation through the epithelial barrier.
In the context of piperine, the pungent bioactive compound of black pepper, possibly promoting the absorption of other phytochemicals and drugs from the gastrointestinal tract by modulation of the ultrastructure, it is most relevant to examine if other pungent spices such as red pepper or its pungent principle (capsaicin) and ginger also exert similar influences on the ultrastructure and fluidity of the intestinal brush border. In the present investigation, an animal study examined the possible influence of the dietary intake of pungent spices (black pepper, red pepper and ginger) and pungent spice bioactive compounds (piperine and capsaicin) on: (i) membrane fluidity in intestinal BBM using a fluorescent probe, (ii) activity of intestinal enzymes whose activity is dependent on the interaction with the lipid microenvironment of the membrane, and (iii) ultrastructural alterations in the intestinal epithelium.
Experimental methods
Materials
Piperine and capsaicin, the active principles of black pepper and red pepper, were procured from Fluka Chemie AG (Buchs, Switzerland). Black pepper, red pepper and ginger were locally purchased and milled to fine powders. All other chemicals used were of analytical grade and were obtained from Sigma Chemical Co. (St Louis, MO, USA). The solvents were distilled before use. ADP, bovine serum album, 1,6-diphenyl-1,3,5-hexatriene (DPH), γ-glutamyl-p-nitroanilide, glycyl-glycine, ouabain, 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-ATP, β-glycerophospate, tripalmitin, Tris-HCl and thiobarbituric acid were purchased from Sigma Chemical Co. Triethanolamine, vitamin E acetate, vitamin A acetate, cholecalciferol and EDTA were obtained from Himedia Laboratories (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India). Maize starch, cane sugar powder and refined groundnut oil were purchased from a local market. Salt mixture (Bernhardt–Tommarelli modified) was purchased from SISCO Research Laboratories (Mumbai, India).
Animal treatment
Animal experiments were carried out taking appropriate measures to minimise pain or discomfort in accordance with standard guidelines for the care and use of animals for experimental purposes and with due approval from the Institutional Animal Ethics Committee. Male Wistar rats (ten rats per group) weighing 65–70 g and housed in individual stainless-steel cages were maintained on various experimental diets ad libitum for 8 weeks. The basal diet consisted of (%): casein, 21; cane sugar, 10; maize starch, 54; National Research Council (NRC) vitamin mixture, 1; Bernhart–Tommarelli modified NRC salt mixture, 4; refined groundnut oil, 10. The test spices or spice principles were incorporated into this basal diet, replacing an equivalent amount of maize starch to give the various experimental diets containing: black pepper (0·5 %); red pepper (3·0 %); ginger (0·05 %); piperine (0·02 %); or capsaicin (0·01 %).
At the end of the feeding trial, overnight fasted rats were killed under diethyl ether anaesthesia. Small intestines consisting of 15 cm long segments beyond 10 cm of the pyloric end were quickly excised and were flushed with cold physiological saline. These intestinal segments were everted over a glass rod and the mucosa was scraped off gently with the help of a glass slide. The mucosal scrapings were homogenised (10 %) in ice-cold 0·2 m-phosphate buffer (pH 7·4) using a Potter-Elvehjem homogeniser for 2 min and centrifuged for 10 min at 3000 g and the supernatant fractions were used for enzyme activity determinations(Reference Khajuria, Thusu and Zutshi5).
Enzyme activities in intestinal mucosa
Leucine amino peptidase activity in intestinal mucosa was assayed by measuring the amount of β-naphthylamine released using l-leucyl β-naphthylamine as substrate by following the rate of increase in absorbance at 340 nm according to Lee et al. (Reference Lee, Larve and Wilson6). The enzyme activity was expressed as μmol β-naphthylamine released/min per mg protein. Glycyl-glycine dipeptidase activity was determined by following the decrease in absorbance at 220 nm due to the hydrolysis of peptide bonds of glycyl-glycine according to Josefsson & Lindberg(Reference Josefsson and Lindberg7). Enzyme activity was expressed as μmol glycyl-glycine hydrolysed/min per mg protein. γ-Glutamyl transpeptidase (γ-GT) was assayed according to Indrani & Hill(Reference Indrani and Hill8) by using γ-glutamyl-p-nitroanilide as donor and glycylglycine as acceptor substrates and following the change in absorbance at 405 nm for 5 min. One unit of enzyme activity is the amount of enzyme that transforms 1 μmol substrate per min at 25°C and the activity was expressed as μmol substrate released/min per mg protein.
Na+,K+-ATPase activity was assayed according to Vajreshwari et al. (Reference Vajreshwari, Srinivasa Rao and Kaplay9). The released inorganic phosphate (Pi) by using ATP as substrate was estimated according to the method of Taussky & Shorr(Reference Taussky and Shorr10). The enzyme activities were expressed as μmol Pi formed/h per mg protein. Alkaline phosphatase activity was assayed according to Hubscher & West(Reference Hubscher and West11) by estimating the Pi liberated at pH 9·5 using β-glycerophosphate as substrate. The enzyme activities were expressed as μmol Pi formed/h per mg protein. Protein content was determined using the method of Lowry et al. (Reference Lowry, Rosebrough and Farr12).
Preparation of intestinal brush-border membrane
Intestinal BBM was isolated following the method of Kessler et al. (Reference Kessler, Acuto and Storelli13). Freshly excised intestines were flushed with cold physiological saline, everted, and the mucosa was scraped off gently with the help of a glass slide. Then 1 % mucosal homogenate in ice-cold 50 mm-mannitol plus 2 mm-Tris-HCl buffer (pH 7·1) was prepared, and filtered through 40 μm pore size nylon. To the filtrate, anhydrous CaCl2 was added with constant stirring on a magnetic stirrer to a final concentration of 10 mm. After 15 min, it was centrifuged at 2000 g, for 10 min at 4°C. The supernatant fraction was re-centrifuged at 27 000 g for 30 min. The pellet was suspended in 2 ml of 50 mm-mannitol in 10 mm-Tris buffer (pH 7·5) and re-centrifuged at 27 000 g for 30 min. The final pellet was re-suspended in 50 mm-buffer.
Membrane fluidity studies
Fluidity of the BBM was measured according to the method described by Livshin et al. (Reference Livshin, Mokady and Cogan14). A lipid-soluble fluoro probe (DPH) dissolved in tetrahydrofuran (final concentration of 1 μm) was mixed with BBM preparations (0·4 mg protein/ml) in stabilising buffer (50 mm-mannitol; 2 mm-Tris-HCl; pH 7·1) with vigorous agitation and incubated at 25°C for 30 min. Steady-state fluorescence polarisation was recorded in a Hitachi F-4000 spectrophotometer (Hitachi, Tokyo, Japan), operated in the ratio mode with 5 nm excitation and emission band pass with 355 nm excitation and 430 nm emission wavelengths. The polarisation of the fluorescence was expressed in terms of the fluorescence anisotropy ‘r’, and calculated according to:
where Ivv and IvH are the components of emitted light intensity, parallel and perpendicular respectively, with reference to the direction of polarisation of the excitation light, and G is the correction factor (G = Ivv/IvH) used to correct for unequal transmission in the optics. The anisotropy parameter, ((r0 /r) − 1)− 1, was calculated with the limiting anisotropy value of DPH of r0 = 0·362.
Membrane cholesterol and phospholipids
Total lipids were extracted from the intestinal brush border by the method of Folch et al. (Reference Folch, Lees and Sloane Stanley15). Cholesterol was estimated by the method of Searcy & Bergquist(Reference Searcy and Bergquist16). Phospholipids were quantified by the ferrous ammonium thiocyanate method using dipalmitoyl phosphatidylcholine as the standard(Reference Stewart17).
Scanning electron microscopic studies
From the freshly excised intestines, small pieces of jejunum (15 cm beyond 10 cm of the pyloric end) from control and spice-treated rats were processed for electron microscopy. Scanning electron microscopic studies of the intestinal brush border were done according to Daddow(Reference Daddow18). Intestinal sections were fixed by incubating in 1 % glutaraldehyde in 0·1 m-phosphate buffer (pH 7·4) for 3 h on ice with regular mixing and then thoroughly washed with water and dehydrated by stepwise washing with increasing concentrations of acetone (from 30 to 95 %). Dehydrated samples were gold coated by spattering and then examined under the scanning electron microscope (LEO 435VP; Leo Electron Microscopy Ltd, Cambridge, Cambs, UK) at different magnifications. The ultrastructure of the intestinal brush border was examined with particular reference to microvilli length and perimeter.
Light microscopic studies
From the freshly excised small intestine, small pieces of jejunum (spanning 15 cm beyond 10 cm of the pyloric end) were also processed for light microscopic studies by immediately fixing in 10 % formalin solution. These were processed further using alcohol, acetone and benzene. After paraffin infiltration, blocks were made. Sections of 5 μm thickness were cut and stained with haematoxylin and eosin. These were observed through a light microscope (Leitz Laborlux POL, Wetzlar, Germany). Intestinal microvilli height and perimeter were measured using an ocular micrometer (Erma, Tokyo, Japan).
Statistical analysis
Results are expressed as mean values with their standard errors and comparisons between groups were made by means of one-way ANOVA(Reference Dowdy and Weardew19). Comparisons among different groups were made by applying Dunnett's test. Differences were considered significant when P < 0·05.
Results
Effect of dietary spices on activities of enzymes associated with small-intestinal brush border
All the dietary spices, namely, black pepper, piperine, red pepper, capsaicin and ginger, stimulated the activities of glycyl-glycine dipeptidase, leucine aminopeptidase, γ-glutamyl transpeptidase and alkaline phophatase in the small-intestinal mucosa (Table 1). The activity of intestinal glycyl-glycine dipeptidase was significantly higher than that of the control group in all the test spice groups (32–62 % increase), with the highest increase found in black pepper and piperine groups. The activity of intestinal leucine amino peptidase was similarly increased by 30–64 % by all the tested dietary spices. Dietary red pepper produced the highest stimulation of this enzyme activity (64 %) followed by piperine (50 %). The activity of intestinal γ-glutamyl transpeptidase was also significantly higher than that of the control group in all the test spice groups (106–169 % increase). The increase in the enzyme activity was as high as 169 % as a result of piperine and ginger feeding. Dietary black pepper, piperine, red pepper, capsaicin and ginger effectively increased intestinal alkaline phosphatase (20–100 %), with piperine producing the maximum increase of 100 %.
Table 1 Influence of dietary spices on the activities of small-intestinal brush-border enzymes
(Mean values with their standard errors for eight animals per group)
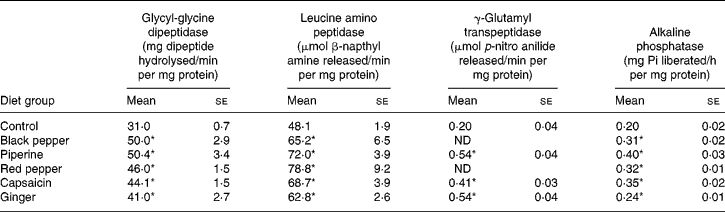
Pi, inorganic phosphate; ND, not determined.
* Mean value was significantly higher than that of the control group (P < 0·05).
Dietary black pepper, piperine, red pepper, capsaicin and ginger significantly stimulated the activities of intestinal ouabain-sensitive Na+,K+-ATPase (Table 2). The extent of stimulation in the activity of ouabain-sensitive Na+,K+-ATPase produced by dietary black pepper and red pepper was 111 and 95 %, respectively. Both piperine and capsaicin produced a similar increase of 52 % in the enzyme activity. Total Na+,K+-ATPase activity as well as the ouabain-insensitive fraction of Na+,K+-ATPase activity were lowered by all the test spices (Table 2). As a result of increasing the ouabain-insensitive portion of Na+,K+-ATPase, the percentage portion of ouabain-sensitive Na+,K+-ATPase in the total Na+,K+-ATPase was significantly higher in all the spice treatments.
Table 2 Influence of dietary spices on intestinal brush-border Na+,K+-ATPase (mg inorganic phosphate liberated/h per mg protein)
(Mean values with their standard errors for eight animals per group)
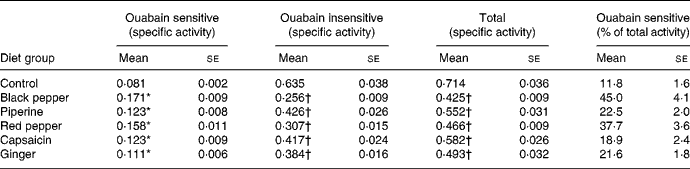
* Mean value was significantly higher than that of the control group (P < 0·05).
† Mean value was significantly lower than that of the control group (P < 0·05).
Effect of dietary spices on the fluidity of intestinal brush-border membrane
The effects of dietary spices on fluorescence anisotropy ‘r’ in microvillus membranes prepared from identical segments of the duodenum, jejunum and ileum of each group assessed with the lipid-soluble fluoroprobe DPH are illustrated in Table 3. Since there exists an inverse relationship between lipid fluidity and anisotropy parameter, lipid fluidity, as assessed by the fluorescence anisotropy of DPH, was significantly higher in spice-treated rats. Piperine produced 104 and 51 %, and capsaicin produced 72 and 108 % increases in fluidity in the ileum and jejunum, respectively, and ginger produced 49 and 25 % increases in fluidity in the ileum and jejunum, respectively. In the case of the duodenum, piperine and capsaicin produced decreases of 22 and 44 % in fluidity, while ginger had no effect on the same.
Table 3 Effect of dietary spices on intestinal brush-border membrane fluidity
(Mean values with their standard errors for eight animals per group)
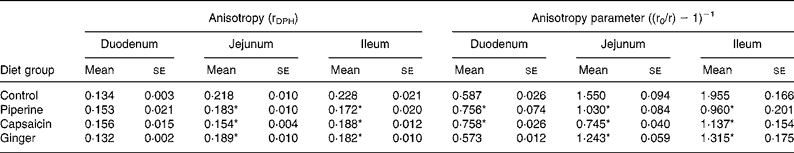
DPH, 1,6-diphenyl-1,3,5-hexatriene.
* Mean value was significantly different from that of the control group (P < 0·05).
Effect of dietary spices on intestinal membrane lipid composition
Intestinal membrane lipid composition was examined in order to determine the factors responsible for the foregoing differences in membrane fluidity. Cholesterol concentration was significantly lower in the villi of the jejunum portion in all the three spice-treated groups when compared with control animals (Table 4). Cholesterol concentration was also significantly lower in the villi of the ileum portion in capsaicin- and ginger-treated groups when compared with control animals. The cholesterol concentration of the duodenum portion of the intestine remained unaffected by the spice treatments. The phospholipid content of the villi of the duodenum and jejunum portions was significantly higher in capsaicin-fed animals when compared with control animals. Dietary piperine or ginger had no influence on the phospholipid content of the intestinal villi.
Table 4 Lipid composition of intestinal membrane in response to dietary spices
(Mean values with their standard errors for eight animals per group)

* Mean value was significantly different from that of the control group (P < 0·05).
A significant decrease in cholesterol in the test spice groups, both in the jejunum and ileum segments, with or without an increase in the phospholipid content, resulted in a relatively lower cholesterol:phospholipid ratio. The cholesterol:phospholipid ratio was significantly lower in the villi of the jejunum and ileum portions of all the three test spice groups as compared with the control group in the respective part of the small intestine (Table 4). The jejunum of the capsaicin-treated group showed a 30·2 % decrease in the cholesterol:phospholipid ratio, followed by piperine (19·3 %) and ginger (18·2 %). Similarly, the ileum of the capsaicin-treated group showed a 17·7 % decrease in the cholesterol:phospholipid ratio, followed by ginger (12·2 %) and piperine (9·8 %). The ratio of cholesterol:phospholipid remained unchanged in the duodenum as a result of the spice treatments. All these changes effected by dietary spices in the villi lipid profile are expected to increase the lipid fluidity of the membranes of the jejunum and ileum. These lipid compositional changes are concordant with the enhanced fluidity of the membranes (jejunum and ileum) as assessed by fluorescence anisotropy using the fluorescent probe DPH.
Effect of dietary spices on the ultrastructure of intestinal epithelium
Electron micrographs of control and spice-treated rat jejunum recorded at × 29 000 magnification displayed regular and well-formed microvilli (Fig. 1). All the three spice treatments generally induced an increase in microvilli length (Figs. 2 and 3). Among the test spices, capsaicin produced the maximum increase of 33 %, followed by piperine and ginger (10 %) (Fig. 3). A prominent increase in the perimeter of the villi was also observed. Among the test spices, capsaicin produced the maximum increase (29 %), followed by ginger (14 %) and piperine (12 %) (Fig. 3).

Fig. 1 Scanning electron microscopic photographs of intestinal cross sections showing enlarged microvilli in spice-treated animal groups ( × 150). (a) Control; (b) piperine; (c) capsaicin; (d) ginger.

Fig. 2 Light microscopic photographs of intestinal cross sections showing enlarged microvilli in spice-treated animal groups (haematoxylin–eosin × 100). (a) Control; (b) black pepper; (c) piperine; (d) red pepper; (e) capsaicin; (f) ginger.

Fig. 3 Effect of dietary spices on intestinal microvilli length and perimeter. (□), Control; (![]() ), piperine; (
), piperine; (![]() ), capsaicin; (■), ginger. Values are means of 100 microvilli per group, with standard errors represented by vertical bars. * Mean value was significantly higher than that of the control group (P < 0·05).
), capsaicin; (■), ginger. Values are means of 100 microvilli per group, with standard errors represented by vertical bars. * Mean value was significantly higher than that of the control group (P < 0·05).
Discussion
Compounds with high lipid solubility cause changes in membrane dynamics because of easy partitioning in a lipid matrix. The lipophilic compounds are capable of entering the hydrophobic region of both lipids and proteins in the membrane core(Reference Goldstein20). Permeability barriers in enterocytes are represented by different transport enzymes in the BBM(Reference Esposito and Csaky21) and any alteration in the membrane may affect its permeability characteristics and activity(Reference Le Grimellec, Friedlander and Yanddouziel22).
The BBM is the site of digestive and transport processes of the small intestine and there is considerable evidence that many of its protein-mediated functions are influenced by the composition and physical state of its lipids(Reference Schachter, Watts and De Point23). Glycyl-glycine dipeptidase and leucine amino peptidase are important hydrolysing enzymes of the intestinal BBM, located on the external surface of the BBM(Reference McDonald, Barrette, McDonald and Barrette24), whose activity is dependent on interaction with the lipid microenvironment of the membrane. They consist of a hydrophilic part (catalytic) protruding to the external surface and hydrophobic part embedded within the bilayer, important for maintaining optimal conformation of the enzyme(Reference Desnuelle25, Reference Ugolev, Mityushova and Egorova26). The present studies have shown that dietary spices caused enhancements in glycyl-glycine dipeptidase, leucine amino peptidase and γ-glutamyl transpeptidase activity in the jejunal mucosa. Increased activities of intestinal enzymes suggest that pungent spices could modulate membrane dynamics due to their apolar nature by interacting with surrounding lipids and hydrophobic portions in the protein vicinity, which may decrease the tendency of membrane lipids to act as steric constraints to enzyme proteins and thus modify enzyme conformation. This is also evident in the fluidity effect of tested spices or spice compounds on the membrane lipids. Thus, it is suggested that these dietary spices may be inducing alterations in membrane fluidity and passive permeability properties which results in the possible increased absorption of micronutrients from the small intestine.
Membrane fluidity studies using an apolar fluorescent probe, diphenyl hexatriene (which measures the fluid properties of the hydrocarbon core), showed an increase in intestinal BBM fluidity. The BBM is rich in many enzymes and transport proteins. Enzyme activity and certain transmembrane transport processes could be influenced by the physical state and lipid composition of the membranes. It is widely accepted that membrane functions are regulated by membrane fluidity(Reference Shinitzy and Shinitzky27–Reference Gupta and Waheed30). An increase in membrane disorder has been shown to contribute to the enhanced absorption of various drugs, for example, salicylates in epithelial and cancer cells(Reference Kaji, Horie and Hayashi31, Reference Pacilio, Florio and Pagnini32).
The effect of test spices on membrane fluidity may therefore be attributable to their easy partitioning in the membrane (due to the apolar nature of spice compounds) and the configuration that they assume with neighbouring molecules in the membrane, resulting in altered lipid dynamics. An inverse relationship exists between membrane lipid fluidity and the anisotropy parameter(Reference Shinitzky and Barenholz33). The fluorescence anisotropy of this probe in bilayer membranes is determined mainly by the maximal hindered anisotropy and provides an estimate of the static component of ‘fluidity’, i.e. of lipid order(Reference Van Blitterswijk, Van Hoeven and Van der Meer34). Because DPH partitions equally between gel and liquid crystal domains of membranes, its anisotropy gives a submicroscopical view of the overall membrane lipid order(Reference Van Blitterswijk, Van der Meer and Hilkmann35). This submicroscopic parameter is believed to be relevant for most physiological functions related to fluidity. Determinations of the fluorescence, which gives access to the rotational diffusion properties of the probe, have confirmed that DPH steady-state anisotropy measurements report essentially on the lipid order in membranes(Reference Sawyer, Aloia, Curtain and Gordon36).
Epithelial cells exhibit a marked morphological polarity and their vectorial transport function is achieved through a polarised distribution of transport systems between the apical and basolateral domains of the plasma membrane. Modifications of membrane fluidity are well recognised to affect the passive permeability properties of membranes(Reference Jain, Wagner, Jain and Wagner37, Reference Carruthers and Melchior38). The concept of control of membrane functions by lipid physical state supposes that fluidity (and/or composition) affects the activity of numerous proteins. This effect can result from a direct action on the conformation or on the conformational changes required by the function of a given protein or may involve the ease of proteins to associate or dissociate(Reference Shinitzy and Shinitzky27). It has been established that freedom of protein conformational changes can be regulated by lipid fluidity(Reference Wilson and Dahlquist39).
The term lipid fluidity may refer to the relative motional freedom of the lipid molecules in the membrane lipid bilayer(Reference Brasitus and Dudeja40), which include translational (or lateral) diffusion as well as rotational movement of the fatty acid tail of the phospholipids and sphingolipids. Lateral diffusion was measured by the excimer formation of the probe DPH that demonstrated an increased membrane fluidity in piperine-, capsaicin- and ginger-treated animals. This might have resulted due to the partial lipid removal and more motional freedom of the probe in the hydrocarbon phase.
A wide variety of substances such as glucose and amino acids are carried across the intestine through the ingested diet. Thus, any alteration in the biochemical or biophysical composition of the small intestine by dietary spices may affect its functional and dynamic aspect. In the present study, a significant increase in the intestinal brush-border enzymes (leucine aminopeptidase, glycyl-glycine dipeptidase, γ-glutamyl transpeptidase, ouabain-sensitive Na+,K+-ATPase and alkaline phosphatase) was noticed in the animals treated with all the tested pungent spices or their active principles. These increased activities of BBM enzymes can be due to a rise in the number of molecular enzyme proteins.
It has long been suggested that Na+,K+-ATPase activity in the BBM is dependent on the physical state of the membrane(Reference Grisham and Barnett41). The findings that purified Na+,K+-ATPase is associated with about 240 molecules of phospholipids including the sixty molecules constitutive of the lipid annulus, and that irreversible loss in activity occurred upon delipidation of the enzyme, strengthened the view of the lipid dependence of its activity(Reference Anner42). Decreasing the membrane fluidity of bovine kidney basolateral membranes by increasing their cholesterol content in vitro markedly inhibits Na+,K+-ATPase activity(Reference Tanaka and Teruya43). Lipid sensitivity of conformational changes associated with Na+,K+-ATPase activity was also confirmed by experiments under high hydrostatic pressure(Reference Fortes44). It has been reported that in intestinal basolateral membranes, methylation is associated with an increase in membrane fluidity and Na+,K+-ATPase activity(Reference Meddings, Desouza and Goel45). The present study has also evidenced increased activity of ouabain-sensitive ATPase along with increased fluidity of the BBM as a result of dietary spice treatment.
Cholesterol is a major constituent of the plasma membranes, and changes in the cholesterol content of biological membranes alter the fluidity of the lipid bilayer and concurrently influence a variety of plasma membrane functions including enzyme activities and ion transport processes(Reference Sawyer, Aloia, Curtain and Gordon36). The role of in vivo and in vitro alterations in cell membrane cholesterol content and/or fluidity in the modulation of Na+,K+-ATPase activity has been well studied(Reference Yeagle, Young and Rice46). The present data on the concurrent changes in the fluidity of the BBM, cholesterol:phospholipid ratio and the activity of ouabain-sensitive Na+,K+-ATPase in response to dietary treatments with piperine, capsaicin and ginger are in agreement with this earlier report.
Changes in microvillus membrane lipid fluidity estimated by fluorescence polarisation spectrometry directly paralleled the changes in the cholesterol:phospholipid ratio. In the case of the jejunum and ileum, membrane fluidity was increased and also the cholesterol:phospholipid ratio was considerably decreased in rats treated with test spices compared with the control group. Thus, the observed difference in BBM fluidity in the spice-fed animals may have resulted in part from differences in the cholesterol:phospholipid ratio.
Further, the tested dietary spices produced interesting changes in the ultrastructure of the intestinal epithelium. Scanning electronic microscopic examination of the intestinal villi in animals fed spices or spice principles revealed alterations in the ultrastructure, especially an increase in microvilli length and also perimeter. This would mean an increase in the absorptive surface area of the small intestine that may contribute to an increase in the absorption of micronutrients. Such an increased villi surface as a result of dietary spices suggests that synthesis or turnover of cytoskeletal components or membrane proteins may be involved in the observed effect. It has been suggested that the brush-border cytoskeleton is responsible for the shape, stability and motility of microvilli composed mainly of actin, myosin and filament-binding proteins(Reference Mooseker47). Very little is known about the mechanism that regulates microvilli length. Some researchers have reported that chemicals such as cycloheximide(Reference Le Count and Grey48) and colchicines(Reference Buschman49), as well as diet(Reference Williamson50), affect the topography of microvilli. It could be that synthesis and turnover of cytoskeletal components are involved in the increased microvilli length and perimeter by dietary spices.
The present animal study thus suggests that all the three pungent dietary spices (black pepper, red pepper and ginger) modulate BBM dynamics due to solubility characteristics of their bioactive compounds (piperine, capsaicin, and possibly 6-gingerol, respectively), by interacting or associating with lipids and hydrophobic portions in the protein vicinity. These effects may decrease the tendency of membrane lipids to act as steric constraints to enzyme proteins and modify enzyme conformation. These effects also induce an increase in microvilli length and perimeter, resulting in a beneficial increase in the absorptive surface of the small intestine, providing for an increased bioavailability of micronutrients. In conclusion, these dietary spices induce alteration in membrane dynamics (increased membrane fluidity) associated with increased microvilli length and perimeter, resulting in an increased absorptive surface of the small intestine. Such a modulation in the ultrastructure of the small intestine is likely to promote the permeation of nutrients through the epithelial barrier. The actual beneficial effect of these specific dietary spices in terms of increasing the absorption of micronutrients such as trace minerals and β-carotene needs to be evaluated in suitable animal models.
Acknowledgements
U. N. S. P. is thankful to the Indian Council of Medical Research, New Delhi for the award of a Senior Research Fellowship. The authors are thankful to Dr Sridevi A.Singh, scientist in this Institute, for help in the determination of anisotropy parameters.
U. N. S. P. was responsible for the bench work. K. S. supervised the whole animal experiment and analytical work. K. S. was also the project leader, who handled research planning, data interpretation and writing of the manuscript.
There are no conflicts of interest whatsoever among the authors.









