Endometrial cancer (EC) is the sixth most common cancer worldwide and, after breast cancer, the second most common female cancer in developed countries. Its incidence has increased significantly over the last two decades(Reference Sheikh, Althouse and Freese1–Reference Lortet-Tieulent, Ferlay and Bray3). In 2020, EC caused 544 000 new cases and 260 000 deaths worldwide, with Northern America and Europe having the highest incidence and mortality rates(Reference Sung, Ferlay and Siegel2). It is currently estimated that the lifetime risk of EC in women is 3·1 %(Reference Crosbie, Kitson and McAlpine4). As the incidence of EC is increasing, it is critical to reduce the incidence of EC.
Ageing, obesity, type 2 diabetes mellitus (T2DM), insulin resistance and lifelong oestrogen exposure have now been established as EC risk factors(Reference Aune, Navarro Rosenblatt and Chan5–Reference Hu, Zhang and Ma7). According to a recent study, obesity-related factors have a strong correlation among the various risk factors for EC(Reference Raglan, Kalliala and Markozannes8), with obesity contributing to 34·0 % of the worldwide incidence of EC(Reference Arnold, Pandeya and Byrnes9). Excessive energy intake(Reference Swinburn, Sacks and Hall10) and reduced physical activity(Reference Ladabaum, Mannalithara and Myer11) are major contributors to obesity, while high sugar intake may cause excessive energy intake, which will lead to long-term weight gain(Reference Te Morenga, Mallard and Mann12) and an increased risk of T2DM(Reference Imamura, O’Connor and Ye13). Sugar intake increases the risk of cancer and can also be mediated by mechanisms such as inflammation(Reference Ma, Nan and Liang14) and increased insulin resistance(Reference Friberg, Wallin and Wolk15). Therefore, some scholars have speculated that the consumption of nutritional sweeteners is a potential cause of EC. The WHO recommends limiting sugar intake to less than 10 % of daily energy intake due to the detrimental health effects of excessive sugar consumption. Non-nutritional sweeteners, according to the College of Nutrition and Nutrition, can help limit energy intake as a tactic for weight and blood glucose control(Reference Fitch and Keim16), and we further speculated that it may reduce the incidence of EC(Reference Toews, Lohner and Küllenberg de Gaudry17). Additionally, non-nutritional sweeteners are compounds in their own right and their toxicity is of concern(Reference Qurrat ul and Khan18).
To sum up, this study investigated the relationship between sweetener exposure and the incidence of EC, both nutritional and non-nutritional, by collecting relevant studies.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines(Reference Moher, Shamseer and Clarke19) were followed in the planning, execution and reporting of this meta. All literatures up to December 2022 were searched in ‘PubMed’, ‘Web of Science’, ‘Scopus’ and ‘Ovid’ to identify relevant articles which reported the exposure of sweeteners and the risk of EC. The terms ‘sweetener’, ‘artificial sweetener’, ‘nutritional sweetener’, ‘non-nutritional sweetener’, ‘saccharin’, ‘sugar’ and ‘endometrial cancer’ were used as core words for retrieval. In order to avoid omitting any potentially relevant studies, references in the primary articles and related reviews were manually checked as well. This meta-analysis’s Prospero registration number was CRD42023400167.
Inclusion criteria and exclusion criteria
For inclusion, studies should satisfy the following criteria: (1) the design of case–control, prospective or retrospective cohort study was adopted; (2) participants were not having EC at the time of recruitment (cohort study) or who had no prior history of the disease (case–control); (3) the exposed group was exposed to any kind or dose of sweetener, while the non-exposed group was rarely exposed to sweetener (compared with other participants in the same study); and (4) the incidence of EC was taken as the outcome.
As per the exclusion criteria: (1) the full text cannot be obtained; (2) the research was not published in English; (3) research data cannot be extracted; and (4) if the cohort or participant is duplicated, the article should be included with the most recent information or the most thorough information.
Sweeteners are a type of substance that can add sweetness to food, including natural and added ingredients(Reference Johnson, Appel and Brands20). Based on their ability to generate heat and provide energy to the human body, sweeteners can be categorised as either nutritional or non-nutritional(21). Sugars and sugar alcohols, such as glucose, fructose, sucrose, xylitol and maltose which are the common sugars we come into contact with in life, are common nutritional sweeteners in food and beverages(Reference Fitch and Keim16). Non-nutritive sweeteners are almost energy-free but highly sweet and can be divided into two types: natural sources such as stevioside and ginsenoside, and synthetic sweeteners such as aspartame, sucralose, and saccharin(Reference Lohner, Toews and Meerpohl22). The non-nutritional sweeteners referred to in this article are mainly artificial sweeteners.
Quality assessment and data extraction
The Newcastle–Ottawa Quality Assessment Scale(Reference Stang23) was used to evaluate case–control and cohort studies. Selection, comparability, outcome or exposure were all part of the assessment. The article is of high quality when the score is greater than 6. If there is disagreement in evaluation, a third researcher will discuss and analyse it together.
Using a pre-made data extraction form, two authors separately extracted the characteristics of the articles. These significant data included author, year, country, exposure assessment, number of participants, type of sweeteners, adjust parameters, study designs, source of population, age at recruitment, the median age at the time of analysis, duration of experiment, median follow-up time, number of EC cases and sweetener dose measurement methods.
Statistical analysis
All statistical analyses of data were carried out by using Stata12.0 software. OR and 95 % CI were used to assess the relationship between exposure to sweeteners and the incidence of EC. The Chi-square test was used to identify any potential heterogeneity between studies, and I2 > 50 % was regarded to indicate a high level of heterogeneity(Reference Higgins, Thompson and Deeks24). It is important to take into account the complexity and diversity of exposure factors, such as different kinds of sweeteners, different methods of sweetener extraction, different exposure times and different follow-up times of each cohort. The included studies were chosen by the random effect model, while the proportion of each study was defined to increase the reliability of the results. Subgroup analysis was conducted concurrently to investigate the cause of heterogeneity. Begg’s test(Reference Peters, Sutton and Jones25) can be used to evaluate publication bias, and sensitivity analysis can be employed to evaluate the stability of studies. P < 0·05 was considered statistically significant.
Results
Literature search
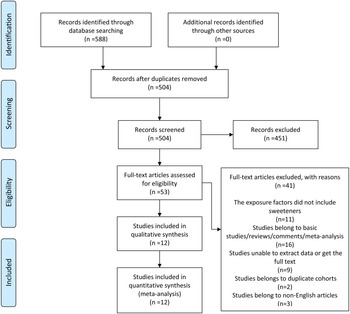
The research selection procedure in accordance with PRISMA guidelines is reported in Fig. 1. A total of 588 potential publications were identified from the electronic databases, such as PubMed, Web of Science, Scopus and Ovid. After removing duplicates, 504 articles were screened out, 451 of which were disqualified based on their titles and abstracts. Among the fifty-three eligible articles, forty-one were excluded due to the following reasons: eleven exposure factors did not include sweeteners, sixteen studies belonged to basic studies, reviews, comments, and meta-analyses, nine studies were unable to extract data or get the full text, two studies were duplicate cohorts and three studies belonged to non-English articles. Ultimately, twelve studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26–Reference Zhu, Li and Tang36) were included in the systematic reviews, including two case–control studies(Reference Bosetti, Gallus and Talamini28,Reference King, Chandran and Olson30) and ten cohort studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29,Reference Coleman, Kitahara and Murray31–Reference Zhu, Li and Tang36) , of which eleven mentioned nutritional sweeteners(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29–Reference Zhu, Li and Tang36) and four mentioned non-nutritional sweeteners(Reference Bosetti, Gallus and Talamini28,Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) .

Fig. 1. A schematic flow for the selection of articles included in this meta-analysis.
Study characteristics and quality evaluation
Of the twelve studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26–Reference Zhu, Li and Tang36) on the relationship between sweetener exposure and the incidence of EC, four studies were conducted in the USA(Reference Inoue-Choi, Robien and Mariani29–Reference Coleman, Kitahara and Murray31,Reference Zhu, Li and Tang36) , three in Canada(Reference Silvera, Rohan and Jain26,Reference Arthur, Kirsh and Mossavar-Rahmani34,Reference Willemsen, McNeil and Heer35) and one each in France(Reference Cust, Slimani and Kaaks27), Sweden(Reference Friberg, Wallin and Wolk15), Italy(Reference Bosetti, Gallus and Talamini28), Australia(Reference Hodge, Bassett and Milne32), and the UK(Reference Dunneram, Greenwood and Cade33). The experiments were conducted from 1982 to 2016, and the articles were published between 2005 and 2022. Most of the studies used FFQ to evaluate sweeteners. The cohort study enrolled 570 636 participants, of whom 3707 developed EC, with a total of 878 cases of EC and 2829 controls in the case–control study. The recruitment age of all studies was 20 years or older, with a median age at analysis ranging from 50·8 to 67·6 years and a median follow-up time ranging from 6·4 to 16·4 years. The types of sweeteners were roughly divided into total sugars, sugar-sweetened beverages, sugar-free beverages, artificially sweetened soft drinks, and so on. Among four studies on non-nutritional sweeteners(Reference Bosetti, Gallus and Talamini28,Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) , only one(Reference Bosetti, Gallus and Talamini28) was evaluated through food (excluding soft drinks), while the other three(Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) were evaluated through beverages. The main adjustment factors of the study included age, BMI, smoking, physical activity, energy intake, age at menarche, alcohol use, education and oral contraceptive use. Other characteristics are detailed in Table 1 and online Supplementary Table 1.
Table 1. Characteristics of included observational studies in the meta-analysis

No., number; HRT, hormone replacement therapy; OC, oral contraceptive; DHQ, diet history questionnaire; SEIFA, socio-economic indexes for areas; AHEI, alternate healthy eating index; PIR, poverty-to-income ratio.
Using the Newcastle–Ottawa Scale, the quality of each of the aforementioned case–control studies and cohort studies was evaluated. The end result complied with the quality standards of meta-analysis (online Supplementary Tables 2 and 3).
Total sweeteners and risk of endometrial cancer
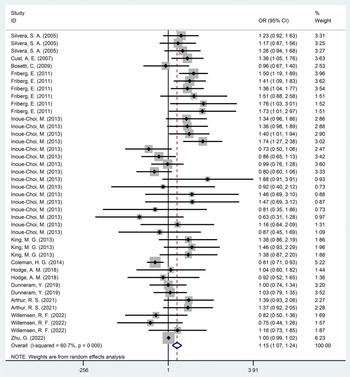
In this meta-analysis, compared with the non-exposed group, the incidence rate of EC was higher in the sweetener exposed group, with statistically significant results (OR = 1·15, 95 % CI 1·07, 1·24, P < 0·001) (Fig. 2). Excluding case–control studies(Reference Bosetti, Gallus and Talamini28,Reference King, Chandran and Olson30) , the statistical results of ten cohort studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29,Reference Coleman, Kitahara and Murray31–Reference Zhu, Li and Tang36) showed that the incidence rate of EC in exposed group was higher than in the non-exposed group, and the results were still statistically significant (OR = 1·14, 95 % CI 1·05, 1·24, P = 0·001) (online Supplementary Fig. 1). The heterogeneity in this study was high at 60·7 %.
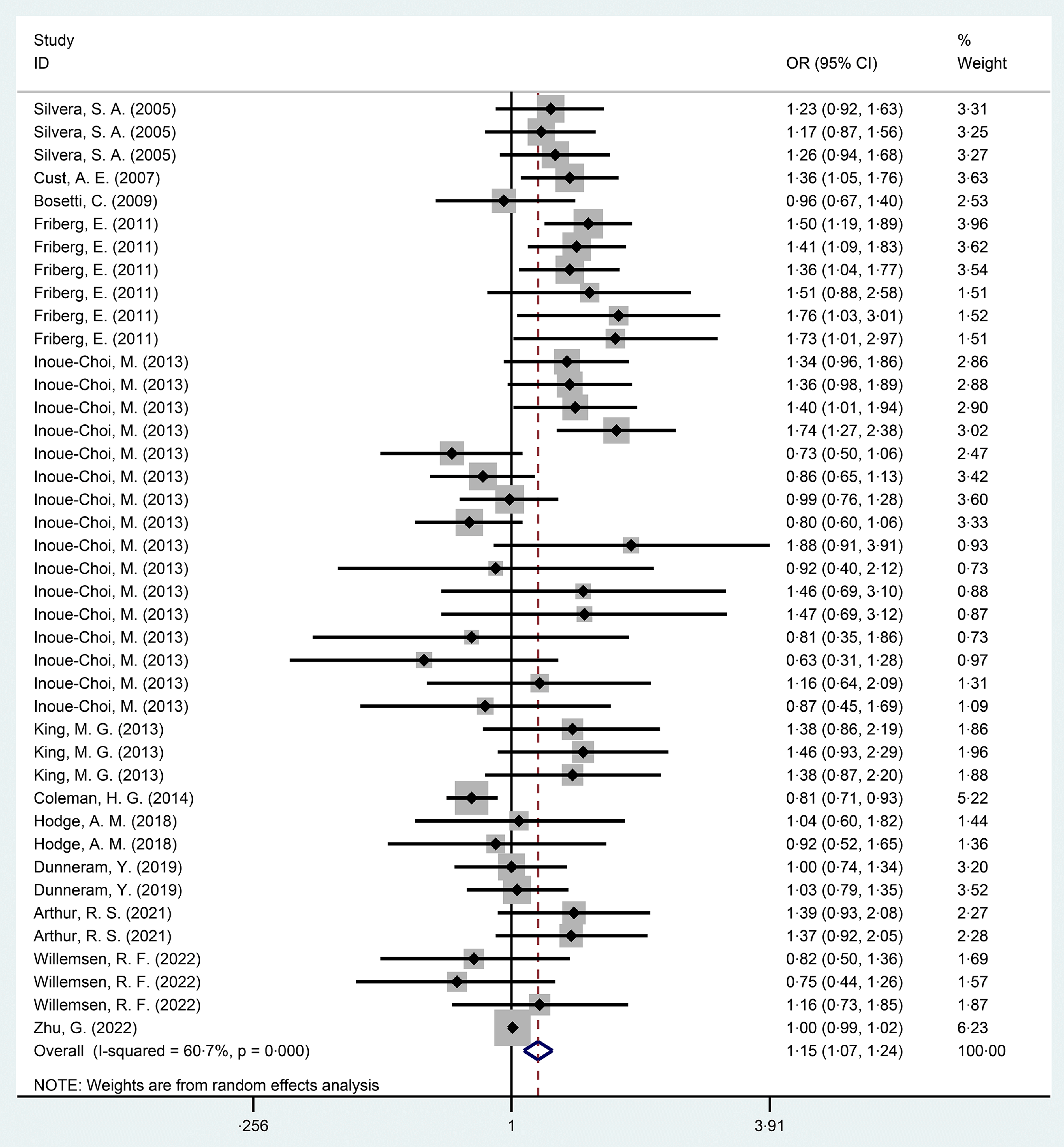
Fig. 2. Forest diagram of total sweeteners exposure and endometrial cancer incidence (P < 0·001).
Nutritional sweeteners
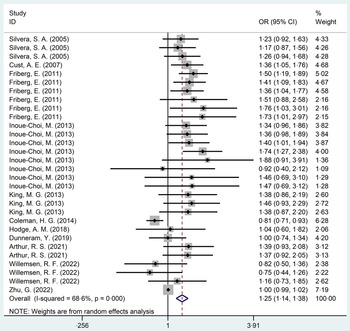
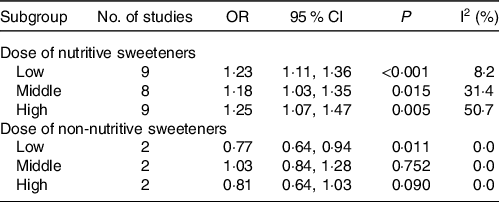
The relationship between nutritional sweeteners and EC was investigated in eleven studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29–Reference Zhu, Li and Tang36) , ten cohort studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29,Reference Coleman, Kitahara and Murray31–Reference Zhu, Li and Tang36) and one case–control study(Reference King, Chandran and Olson30), with a total of 571 458 participants. Statistical results showed that participants exposed to nutritional sweeteners had a higher incidence of EC than those not exposed to nutritional sweeteners (OR = 1·25, 95 % CI 1·14, 1·38, P < 0·001) (Fig. 3). Begg’s test showed no significant published bias (P = 0·08) (online Supplementary Fig. 2). After deleting each study individually, the statistical findings remained stable, according to the result of sensitivity analysis (online Supplementary Fig. 3). In addition, the relationship between the dose of nutritional sweetener exposure and the incidence of EC was further categorised into subgroups. The outcomes were as follows: compared with those who were not exposed to nutritional sweeteners, those who were exposed to low (OR = 1·23, P < 0·001), middle (OR = 1·18, P = 0·015) and high doses (OR = 1·25, P = 0·005) of nutritional sweeteners had higher incidence rate of EC, respectively (Table 2). After excluding case–control study(Reference King, Chandran and Olson30), ten cohort studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29,Reference Coleman, Kitahara and Murray31–Reference Zhu, Li and Tang36) showed that the incidence rate of EC in exposed group was higher than that in the non-exposed group (OR = 1·24, 95 % CI 1·13, 1·37, P < 0·001) (online Supplementary Fig. 4).

Fig. 3. Forest plot of nutritional sweeteners exposure and incidence of endometrial cancer (P < 0·001).
Table 2. Subgroup analysis of the association between sweeteners exposure and the incidence of endometrial cancer
No., number.
All references are rarely exposed to sweeteners.
Non-nutritive sweeteners
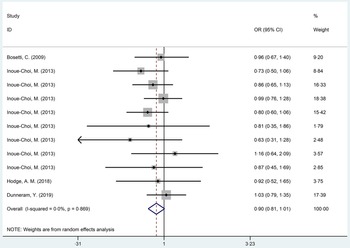
Three cohort studies(Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) and one case–control study(Reference Bosetti, Gallus and Talamini28) with a total population of 95 366 participants were conducted to explore the relationship between non-nutritional sweeteners and EC. According to statistical findings, there was no difference in the incidence of EC between population exposed to non-nutritional sweeteners and those not exposed to non-nutritional sweeteners (OR = 0·90, 95 % CI 0·81, 1·01, P = 0·067) (Fig. 4). Begg’s test showed no significant published bias (P = 0·276) (online Supplementary Fig. 5). In addition, sensitivity analysis showed that after removing each study one by one, the statistical results of the relationship between non-nutritional sweeteners and EC remained stable (online Supplementary Fig. 6). The further subgroup analysis of non-nutritional sweeteners was carried out according to the dosage. The results were as follows: compared with the population not exposed to non-nutritional sweeteners, population exposed to low-dose non-nutritional sweeteners had a lower EC incidence (OR = 0·77, 95 % CI 0·64, 0·94, P = 0·011), which only included two studies. Besides, there was no difference in the incidence of EC between the group exposed to middle (OR = 1·03, P = 0·752) and high doses (OR = 0·81, P = 0·090) of non-nutritional sweeteners and the unexposed group, respectively (Table 2). After excluding case–control study(Reference Bosetti, Gallus and Talamini28), three cohort studies(Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) showed that there was no significant difference in the incidence rate of EC between the exposed group and the non-exposed group (OR = 0·90, 95 % CI 0·80, 1·01, P = 0·064) (online Supplementary Fig. 7).

Fig. 4. Forest plot of non-nutritional sweeteners exposure and incidence of endometrial cancer (P = 0·067).
Discussion
A total of twelve studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26–Reference Zhu, Li and Tang36) (two case–control studies(Reference Bosetti, Gallus and Talamini28,Reference King, Chandran and Olson30) and ten cohort studies(Reference Friberg, Wallin and Wolk15,Reference Silvera, Rohan and Jain26,Reference Cust, Slimani and Kaaks27,Reference Inoue-Choi, Robien and Mariani29,Reference Coleman, Kitahara and Murray31–Reference Zhu, Li and Tang36) ), including 572 820 participants, were included in this study to evaluate the relationship between sweeteners and EC. According to the statistical results, the consumption of sweeteners was positively correlated with the risk of EC. After subgroup analysis of sweeteners, we discovered that nutritional sweeteners increase the incidence of EC, whereas non-nutritional sweeteners may do not.
Nutritional sweeteners can burden heat and consist mainly of sucrose, glucose and maltose(Reference Fitch and Keim16,21) . Due to their high concentration of readily absorbed carbohydrates, nutritional sweeteners can raise the risk of diabetes(Reference Yoshida and Simoes37). Numerous studies showed that T2DM was related to the occurrence of EC(Reference Friberg, Orsini and Mantzoros38–Reference Saed, Varse and Baradaran42). Compared with women without T2DM, women with T2DM have a 62 % increased risk of EC(Reference Saed, Varse and Baradaran42). The metabolic disorders caused by T2DM, such as dyslipidemia and hyperinsulinemia, were thought to be responsible for EC(Reference García-Jiménez, García-Martínez and Chocarro-Calvo43,Reference Samuel, Varghese and Varghese44) . Hyperlipidemia can lead to increased levels of cholesterol and non-esterified fatty acid levels, which activate cancer signal pathways, membrane synthesis and ATP(Reference Samuel, Varghese and Varghese44), further increasing the incidence of EC.
By obesity, nutritional sweeteners can also raise the risk of EC(Reference Augustin, Franceschi and Jenkins45). Due to their high added sugar content, low satiety and potential for insufficient compensation for total energy, nutritional sweeteners may cause excessive energy intake, resulting in weight gain(Reference Malik, Popkin and Bray46,Reference DiMeglio and Mattes47) . Some studies showed that obesity increased the risk of EC by 2·6 times(Reference Shaw, Farris and McNeil48). Inflammation, insulin resistance, hyperinsulinemia, elevated steroid hormone bioavailability and oxidative stress response are some of the mechanisms by which obesity is associated with cancer(Reference Roberts, Dive and Renehan49). Specifically, insulin acts on endometrial tissue through mitosis and anti-apoptotic growth factors, promoting cell proliferation and tumour growth(Reference Cust, Slimani and Kaaks27,Reference Kaaks, Lukanova and Kurzer50,Reference Calle and Thun51) . Insulin can also promote tumorigenesis directly through insulin receptors in the endometrium(Reference Kaaks, Lukanova and Kurzer50). Hyperinsulinemia will promote the occurrence of ovarian hyperandrogenism, which in turn leads to the release and aromatisation of androgens from adipose tissue, resulting in an increase of oestrogen(Reference Kaaks, Lukanova and Kurzer50). Research showed that the risk of EC is mainly related to hormone levels. When oestrogen is not inhibited by progesterone, it will increase the stimulation of endometrial epithelium and further increase the probability of EC(Reference Key and Pike52). Obesity also leads to the decrease of hepatic sex hormone-binding globulin, which in turn increases the diffusion of bioavailable oestrogen to the endometrium(Reference Calle and Kaaks53).
The mechanism of obesity-induced EC differs in women with different menstrual statuses. The fundamental reason for this is that oestrogen is not inhibited by progesterone in premenopausal women. It might be because adipose tissue becomes the primary component of oestrogen production in postmenopausal women(Reference Yue, Yager and Wang54). Obesity caused by high glucose also means having more adipose tissue, and this additional glandular transformation of adipose tissue increases the production of oestrogen(Reference Baglietto, English and Hopper55,Reference Kaye, Folsom and Soler56) . It increases the level of oestrogen in the blood, thereby inhibiting cell apoptosis, boosting endometrial cell proliferation, encouraging angiogenesis and increasing the risk of EC(Reference Amant, Moerman and Neven57). In a word, our study shows that nutritional sweeteners will increase the risk of EC.
However, a recent Mendelian randomisation study on the relationship between dietary factors and EC yielded inconsistent results. Mendelian randomisation studies showed that sugar can reduce the risk of EC(Reference Wang, Glubb and O’Mara58). This may be due to the following reasons: first, the study only included European participants. Second, it did not take into account macronutrients according to type, making it impossible to assess whether there are differences in causal effects between certain subtypes and the overall nutrients. Additionally, it was accomplished by using a relative macronutrient intake that may be within the dose range for safety. Finally, Mendelian randomisation was not consistent with our study methodology, and the final conclusion needs further evaluation.
Non-nutritional sweeteners, which primarily consist of stevia glycosides, sucralose, aspartame, etc., have nearly no energy content but are sweeter(21,Reference Lohner, Toews and Meerpohl22) . Compared with nutritional sweeteners, the use of non-nutritional sweeteners may reduce the incidence of T2DM and obesity(Reference Walbolt and Koh59). In order to control body weight and blood glucose levels, obese patients frequently use non-nutritional sweeteners to reduce energy content and carbohydrate intake(Reference Shwide-Slavin, Swift and Ross60). Research showed that, compared with sugary drinks, sugar-free soda water was less related to diabetes(Reference Imamura, O’Connor and Ye13). According to Higgins’ findings, sucralose, a non-nutritional sweetener, could cause weight loss and a decrease in energy intake in the non-nutritional sweetener group, while sucrose or saccharin caused weight gain in the nutritional sweetener group(Reference Higgins and Mattes61).
However, according to the latest use of non-sugar sweeteners: WHO guideline, issued by WHO. This report suggests higher incidence of obesity, higher BMI, higher risk of T2DM, based on prospective observational studies(62). Because non-nutritional sweeteners are synthetic, there are always safety concerns. Some by-products of non-nutritional sweeteners, such as formaldehyde, a metabolite of aspartame, are certain carcinogens. At present, its reasonable biological mechanism has been determined through experimental research. To be detailed, formaldehyde can cause DNA damage, chromosome aberration and mitotic error(Reference Rycerz and Jaworska-Adamu63,Reference Yılmaz and Uçar64) . Some researchers have also speculated that non-nutritional sweeteners may interact with both identified and undiscovered taste receptors. These receptors have an affinity for non-nutritional sweeteners found in the intestine and are related to the ability to glucose absorption and homoeostasis(Reference Pepino and Bourne65), indicating that they are somewhat comparable to sugar sweeteners. Alternatively, non-nutritional sweeteners can also directly affect the intestinal epithelium to modify intestinal epithelial functions, such as the production of adhesins and intestinal barrier function(Reference Van den Abbeele, Van de Wiele and Verstraete66–Reference Shil, Olusanya and Ghufoor69), which normally control the formation and metabolism of the gut microbiota(Reference Santos, Caria and Gotardo68,Reference Tailford, Crost and Kavanaugh70) . Overall, non-nutritional sweeteners can have an impact on the human body through gut microbiota, including glucose intolerance and metabolic changes. Furthermore, a meta-analysis including nine cohort studies found that there was no relationship between non-nutritional sweeteners intake and body weigh(Reference Miller and Perez71). Simultaneously, some scholars have conducted relevant studies on the dose of non-nutritional sweeteners. The results showed that compared with the consumption of higher doses of non-nutritional sweeteners, lower doses of non-nutritional sweeteners consumption can reduce weight gain(Reference Toews, Lohner and Küllenberg de Gaudry17). The specific mechanism is not clear. According to our study, non-nutritional sweeteners are not associated with EC, but the intake of low doses (< 0·4 servings/week or 1–3 times/month) of non-nutritional sweeteners may help reduce the incidence of EC.
Advantages, limitations and prospects of current experiments
As far as we know, this is the first systematic review and meta-analysis to investigate the relationship between sweeteners and EC, which distinguishes between nutritional sweeteners and non-nutritional sweeteners. Due to the heterogeneity among the included studies, such as different types of sweeteners, different follow-up times of each cohort and so forth, a random effect model was adopted to improve the reliability of the results. However, there are certain limitations on this study as well. It is important to acknowledge that there was significant heterogeneity, which may result from variations in methods, populations, sweetener kinds and their intake dose. There is no way to do future research due to the small number of articles included in this study, particularly those pertaining to non-nutritional sweeteners. Despite the fact that we have divided sweetener doses into groups, it is difficult to clearly distinguish between low, middle and high doses due to the various dose evaluation methods in each study. Among the four studies(Reference Bosetti, Gallus and Talamini28,Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) including non-nutritional sweeteners, only three(Reference Inoue-Choi, Robien and Mariani29,Reference Hodge, Bassett and Milne32,Reference Dunneram, Greenwood and Cade33) considered the intake of non-nutritional sweeteners in beverages, resulting in incomplete data. Due to the inconsistent adjustment of confounding factors in each study, for example, only four studies(Reference Friberg, Wallin and Wolk15,Reference Bosetti, Gallus and Talamini28,Reference Inoue-Choi, Robien and Mariani29,Reference Dunneram, Greenwood and Cade33) excluded or adjusted for coffee as a confounding factor. As coffee has been proven to be associated with a decrease in EC(Reference Bandera, Williams-King and Sima72), it may also have a certain impact. Most of the included studies used the FFQ, which is a self-reported questionnaire, making it likely to have memory bias in data collection. Most of the systematic reviews were conducted in North America, while the rest were conducted in Northern Europe and Australia. Therefore, the results of this study may not be suitable for direct extrapolation to other countries.
Due to the limitations of this study, especially the non-nutritional sweeteners, more and larger-scale studies are needed for further discussion. In addition, our research shows that the incidence of EC is different between nutritional sweeteners and non-nutritional sweeteners. Consequently, it is advised to distinguish between nutritional and non-nutritional sweeteners by considering the danger of sweeteners and cancer risk in the future.
Conclusion
This study reported that consumption of sweeteners as well as nutritional sweeteners may increase the risk of EC, whereas the exposure to non-nutritional sweeteners is not associated with the incidence of EC. Besides, it is worth noting that consuming low doses of non-nutritional sweeteners may lessen the incidence of EC.
Acknowledgements
None.
The author(s) reported there is no funding associated with the work featured in article.
All authors had read and approved the manuscript. Huiping Li and Yeyuan Zhang contributed to writing the manuscript; Yujing He contributed to perform procedures and data analysis; Jianing Huang and Jie Yao contributed to writing the manuscript; Xieyan Zhuang contributed to drafting conception and design.
No potential conflict of interest was reported by authors.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523001484