Alcohol abuse leads to several metabolic abnormalities inducing hepatic damage and varying degree of malnutrition. Ethanol manifests its toxic effect by increasing the oxidative stress in the liver, causing hepatic impairment(Reference Cederbaum1). It is also known to seriously affect the nutritional status by interfering with the digestion and absorption of nutrients(Reference Stickel, Hoehn and Schuppanet2). Physiological changes commonly occurring due to hepatic impairment are elevation in the plasma levels of hepatic enzymes, decrease in albumin synthesis, decrease in circulating branched-chain amino acids (BCAA) to aromatic amino acids (AAA) ratio, negative nitrogen balance, loss of lean tissue mass, malnutrition and increased rate of morbidity and mortality(Reference Plauth, Bernal and Dasarathy3). According to the WHO’s global status report on alcohol and health 2018, alcohol abuse causes 3 million death annually worldwide(4).
BCAA such as leucine, isoleucine and valine, have therapeutic value in liver disease conditions. The BCAA are primarily metabolised in the skeletal muscle instead of the liver, thereby providing rest to the compromised liver, improving liver function and quality of life(Reference Clemente5). Moreover, BCAA metabolism in the muscle produces glutamate, which in turn acts as a substrate for ammonia detoxification to glutamine(Reference Holeček6). Thus, BCAA are beneficial in the clinical condition of hepatic impairment as they can compensate ammonia detoxification for the impaired liver(Reference Kinny-Köster, Bartels and Becker7). Also, BCAA have shown to promote regeneration of hepatocytes and stimulate protein synthesis. They also prevent protein breakdown(Reference Andrea and Nair8). Due to these diverse physiological and metabolic roles of BCAA, they are recommended in liver diseases. The AAA such as phenylalanine, tyrosine and tryptophan are primarily metabolised in the liver, and in liver disease, they are not effectively metabolised and thus accumulate worsening the clinical outcome(Reference Shawcross and Jalan9).
Proteins with a higher Fischer ratio (ratio of BCAA to AAA) is recommended for patients with liver diseases(Reference Clemente5) for promoting protein synthesis and regeneration of hepatocytes. Production of high Fischer ratio peptide mixtures by enzymatic hydrolysis of maize gluten(Reference Ma, Lin and Sun10), sunflower protein(Reference Bautista, Corpas and Cremades11) and flaxseed protein(Reference Udenigwe and Aluko12) has been previously reported. The Fischer ratio of 4·7 has been recommended for patients with liver diseases(Reference Oomah13). A study on the infusion of modified amino acid mixture deficit in AAA and enriched with BCAA resulting in faster recovery of patients with chronic alcoholic cirrhosis has been previously reported(Reference Cerra, Cheung and Fischer14). The beneficial effect of the high Fischer ratio protein in advanced liver disease condition is studied; however, studies on the prophylactic effect of the same have not been reported.
Since alcoholism is habit-forming, total alcohol abstinence in alcohol-dependent patients cannot be achieved easily. Therefore, there is an increased need for hepatoprotective nutritional supplements to prevent hepatic damage, maintain health and nutritional status among alcoholics. The scarcity of such supplements paved the way for the current investigation. The objective of the investigation was to prepare and characterise the flaxseed protein hydrolysate with enhanced Fischer ratio and study its hepatoprotective effect in isolation and in combination with the antioxidant micronutrients (selenium and vitamin E) in the ethanol hepatotoxicity-induced rat model. The study also aims to explore the ameliorative effect of the flaxseed-based formulation containing enhanced Fischer ratio protein hydrolysate and antioxidant micronutrients against ethanol-induced hepatotoxicity.
Methods
Preparation of the flaxseed protein hydrolysate with enhanced Fischer ratio by dual enzymatic hydrolysis and charcoal treatment
Flaxseed protein hydrolysate having enhanced Fischer ratio was prepared by the method of Udenigew & Aluko(Reference Udenigwe and Aluko12), with slight modification in the enzyme to substrate ratio. Flaxseed protein suspended in water (5 % w/v) was first hydrolysed using thermolysin for 5 h followed by pronase for another 5 h under optimum temperature and pH conditions. The enzyme:substrate ratio used for the hydrolysis was 1:1000. The hydrolysed protein was freeze-dried. The flaxseed protein hydrolysate (FPH) (1 %, w/v) was suspended in water and mixed with activated charcoal (2·5 %, w/v) for 10 min to remove the cleaved AAA. The mixture was centrifuged at 7000 rpm for 30 min and the supernatant was filtered through Whatman No.42 filter paper to remove the traces of charcoal. Potato starch was added at the level of 10 % w/w of protein before lyophilisation to prevent water uptake by the hydrolysate after freeze-drying. The protein hydrolysate obtained was freeze-dried and termed as enhanced Fischer ratio FPH (EFR-FPH). The protein content of EFR-FPH estimated by Kjeldahl’s method(Reference Horwitz and Latimer15), was found to be 63·5 (sd 2·1) %.
Amino acid composition and mass spectrometric analysis of the enhanced Fischer ratio flaxseed protein hydrolysate
Amino acid analysis of the EFR-FPH was performed by acid hydrolysis under vacuum, followed by pre-column derivatization using phenylisothiocyanate. The phenyl thiocarbomyl amino acids were then analysed using a Waters Pico-Tag amino acid analysis system (Waters) as described by Bidlingmeyer et al. (Reference Bidlingermeyer, Cohen and Tarvin16). Tryptophan content of the protein was measured by the acid ninhydrin method(Reference Pinter-Szakacs and Molnar-Perl17).
For the reverse-phase LC-MS/MS analysis of EFR-FPH, 1 mg/ml sample was filtered through a 0·2 µm membrane, and an aliquot of 100 µl was dried under vacuum. The dried sample was resuspended in MS grade water (100 µl), and 20 µl of the sample was loaded on a C18 reverse-phase column (Zorbax eclipse plus; 50 mm × 2·1 mm; Agilent technologies) connected to an Ekspert ultra LC 100-XL system (Eksigent Technologies). The mobile phase comprised of water with 0·1 % formic acid (solvent A) and acetonitrile with 0·1 % formic acid (solvent B). A linear gradient of solvent B was used to elute the analytes as follows: 0–5 min, 5 %; 5–20 min, 5–95 %; 20–25 min, 95 %; 25·1–30 min, 5 %. The flow rate was maintained at 0·2 ml/min. The eluted analytes were subjected to Triple TOF 5600+ mass spectrometer (Sciex LP) in the positive mode. The method parameters followed in TOF-MS include ion spray voltage floating of 5500, temperature 400°C, collision energy of 10 eV, and declustering potential of 100. An MS2 (MS/MS) was obtained from the fragmentation of a precursor ion by information-dependent acquisition with rolling collision energy, the maximum number of candidate ions of 8 and >100 m/z having an intensity of >300 cps. The LC-MS/MS data were analysed using Peak View software (2.2. version, Sciex). The BCAA and AAA ions were extracted manually using extracted ion chromatogram option.
For matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis, the EFR-FPH was desalted using C18 ZipTip (Merck Millipore) as per the manufacturer’s protocol. The desalted sample was evaporated to dryness in a centrifuge tube. A saturated solution of matrix cyanocinnamic acid was prepared with 50 % acetonitrile/milli q water with 0·1 % trifluoroacetic acid. The dried sample in the centrifuge tube was resuspended in milli q water. An equal volume of sample and matrix (1 µL each) were mixed on a centrifuge tube cap and spotted on a MALDI plate and analysed using the UltrafleXtreme MALDI instrument (Bruker Daltonics). Reflection mode was used to acquire the spectra using Smart beam laser 2 kHz for ionization. Flex control (Bruker) software was used to obtain mass spectra. The mass spectra were analysed using Flex Analysis 3.1 software.
Preparation of the flaxseed-based hepatoprotective dietary supplement containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients
Flaxseed-based hepatoprotective dietary supplement containing EFR-FPH, selenium (Se) and vitamin E was formulated. The formulation was composed of roasted flaxseed (74 g), garlic (5·5 g), red chilies (5·5 g), jaggery (5·5 g), salt (3·3), oil (5·5 g), mustard seeds and curry leaves (0·6 g). The flaxseeds were roasted to 150°C and cooled, ground in a blender along with other ingredients and seasoned in oil with mustard and curry leaves. The EFR-FPH was added at three levels: 10, 15 and 20 % in the formulation. The nine-point hedonic test employing twenty-five semi-trained panel members was adopted to determine the optimum level of EFR-FPH substitution in the flaxseed-based formulation. The test was conducted in English language to determine the ‘degree of liking’ based on the overall acceptability of the formulation. The 10 % substitution of EFR-FPH in the formulation achieved the highest ‘degree of liking’ and therefore, this level was maintained for further studies. The formulation was fortified with Se and vitamin E to provide 40 μg and 50 mg of the nutrients, respectively, per 30 g product (to be consumed per day). The flaxseed-based formulation containing 10 % EFR-FPH, Se and vitamin E was evaluated for its sensory attributes by Quantitative Descriptive Analysis employing 10 trained panel members. Sensory evaluation of the product indicated good acceptability in terms of appearance, colour, texture, aroma and taste. The nutritional composition of the flaxseed-based hepatoprotective dietary supplement was analysed based on standard methods of the Association of Official Analytical Chemists(Reference Horwitz and Latimer15). The nutritional composition of the hepatoprotective supplement indicated the following values per 100 g product, protein, 23·97 (sd 0·89) g; carbohydrate, 24·58 (sd 0·69) g; fat, 33·7 (sd 0·23) g; fibre, 3·14 (sd 0·09) g and ash, 6·12 (sd 0·24) g.
Animal experimental design
The animal study was conducted after obtaining approval from the Institute Animal Ethical Committee of CSIR-CFTRI (IAEC No. FT/AHF/AI/77). Six-week-old healthy adult male Wistar rats weighing 140–160 g were procured from the institute’s animal house facility. The rats were housed in polycarbonate cages under appropriate temperature (25 ºC (sd 2)) and humidity (50–70 %). The 12 hours light/dark cycle was maintained. Thirty adult male Wistar rats were randomly grouped into five equal groups based on their body weight (BW) as described by Martin et al. (Reference Martin, Daly and Difonzo18) to study the effectiveness of the EFR-FPH alone and in combination with antioxidant micronutrients in countering the adverse effects of chronic ethanol administration. The details of the groups are as follows:
-
Group I: CON- control group not receiving ethanol
-
Group II: EtOH- ethanol-treated group
-
Group III: EtOH + EFR-FPH- ethanol-treated group receiving EFR-FPH
-
Group IV: EtOH + EFR-FPH + Se- ethanol-treated group receiving EFR-FPH and Se
-
Group V: EtOH + EFR-FPH + VITE- ethanol-treated group receiving EFR-FPH and vitamin E.
All the groups received the AIN-93M diet and had ad libitum access to feed and water. Ethanol (50 % v/v) at a dose level of 3·5 g/kg BW was orally administered by gavaging for 8 weeks. The treatment group III received EFR-FPH at a dose of 0·37 g/kg BW, group IV and group V received additionally Se (4·11 μg/kg BW), and vitamin E (5·13 mg/kg BW), respectively for 8 weeks along with ethanol (intervention). The dosage of EFR-FPH, Se and vitamin E was translated from human dose based on the formula recommended by FDA(Reference Reagan-Shaw, Nihal and Ahmad19). The dose of the EFR-FPH was fixed based on the protein content per serving of commercial nutritional supplements. The Se dose meets the Recommended Dietary Allowance (RDA) for this mineral. The vitamin E status of the alcoholics can be depleted due to prolonged exposure to ethanol-induced oxidative stress and thus a daily intake of 60–75 units of vitamin E is recommended(Reference Karimi and Karimi20) and studied in this investigation. The CON and EtOH groups received an equal amount of protein as in EFR-FPH in the form of casein. To ensure proper dosage, EFR-FPH, Se, and vitamin E were orally administered by gavaging after 6 h of ethanol administration.
The ameliorative effect of flaxseed based hepatoprotective dietary supplement containing EFR-FPH, Se and vitamin E against hepatotoxicity induced by chronic administration of ethanol in rats was also studied. Eighteen adult male Wistar rats were randomly grouped into three equal groups, based on the BW(Reference Martin, Daly and Difonzo18) and the details of the groups are, as follows:
-
Group 1: CON + PIBF- control group receiving AIN-93M diet supplemented with purified ingredient-based formulation (PIBF)
-
Group II: EtOH + PIBF- group receiving ethanol and AIN-93M diet supplemented with PIBF
-
Group III: EtOH + FBF- group receiving ethanol and AIN-93M diet supplemented with flaxseed-based formulation (FBF) containing EFR-FPH, Se and vitamin E
Purified ingredient-based formulation, providing macronutrients and calories equivalent to flaxseed based formulation, was formulated using maize starch, casein and groundnut oil. Ethanol was orally administered by gavaging for 8 weeks. The group received PIBF or FBF for 8 weeks, along with ethanol (intervention). The ingredients of FBF, such as the EFR-FPH, Se and vitamin E, were gavaged to ensure the proper dosage after 6 h of ethanol administration. The advocated quantity of the flaxseed based hepatoprotective dietary supplement was 30 g per d for a reference man. The dose of FBF, EFR-FPH, Se and vitamin E translated from human dose based on the formula recommended by FDA(Reference Reagan-Shaw, Nihal and Ahmad19) was 2·8 g, 0·31 g, 4·11 μg, 5·13 mg/kg BW, respectively.
The animals were acclimatised to oral gavaging of ethanol for a period of 10 d before the intervention. The confounding factors such as the order of the treatment and cage location were minimised as per the guidelines(Reference Percie du Sert, Hurst and Ahluwalia21). The study was blinded during intervention by coding the treatment and during measurement and analysis by coding the animals individually. There was no exclusion of animals during the analysis. The experimental procedures were as per the recommendations of the committee for the purpose of control and supervision of experiments on animals.
The feed intake was assessed every day. The animals were weighed at the beginning of the study and every 4 d throughout the investigation. At the end of the study, the animals were euthanised by carbon dioxide overdose after overnight fasting. A cardiac puncture was made to drain the blood into heparinised tubes. The liver was excised and weighed. A portion of the liver was homogenised in 50 mm phosphate buffer (pH 7·4) and centrifuged at 4°C, 15 000 g for 15 min to collect the supernatant for further analysis.
Assessment of biochemical markers in plasma and liver
The activities of hepatic enzymes such as aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) were quantified spectrophotometrically in the plasma using commercially available kits (Agappe Diagnostics Ltd). Based on the activities of these enzymes, their level in plasma was calculated and expressed as U/L. Total bilirubin in plasma was quantified based on the reaction between bilirubin and diazotised sulphanilic acid and was expressed as µmol/L. Plasma albumin was measured spectrophotometrically using a commercially available kit and expressed as g/dl (Agappe diagnostics, Ltd). Plasma AST, ALT, ALP, bilirubin and albumin were considered as the primary outcome measures to test the efficacy of the intervention.
Lipid peroxides in the plasma and liver homogenate were determined by quantifying the malondialdehyde (MDA) concentration as per the method described by Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi22). The catalase activity was measured based on the method described by Aebi(Reference Aebi23). The activity of glutathione reductase was evaluated by measuring the oxidation of NADPH(Reference Carlberg and Mannervik24). The glutathione peroxidase (GPx) enzyme activity was measured based on NADPH oxidation method of Flohe & Gunzler(Reference Flohé and Günzler25). The activity of superoxide dismutase (SOD) was measured according to the method described by Marklund & Marklund(Reference Marklund and Marklund26). The levels of MDA and activities of antioxidant enzymes were expressed per mg protein in the liver and per dl in plasma. The protein content of the liver homogenate was assessed based on the Lowry’s method(Reference Lowry, Rosebrough and Farr27).
Haematological parameters were analysed using an automated haematology analyser (Sysmex KX-21). Freshly drawn, unclotted blood was used to analyse various haematological parameters such as leucocytes, lymphocytes, erythrocytes,haemoglobin Hgb, haematocrit value (HCT), mean corpuscular volume, mean corpuscular Hgb, platelet, platelet distribution width (PDW), mean platelet volume (MPV), platelet large cell ratio (P-LCR) and plateletcrit (PCT).
Histopathological evaluation of the hepatic tissue
The liver samples were stored in 10 % formalin until the preparation of microscope slides of hepatic tissue. Graded ethanol was used to dehydrate the liver samples. The liver tissue of 5 µm thickness was sectioned and stained with haematoxylin and eosin to prepare the tissue slides. The hepatic tissue slides were observed under a light microscope (Olympus Optical Co. Ltd) to evaluate the morphological changes in the hepatic tissue.
Statistical analysis
The number of animals to be maintained in each group (sample size) was calculated by applying the following formula(Reference Charan and Kantharia28): sample size = 2 sd 2 (1·96 + 0·842)2/d 2, where sd (7·60) is the standard deviation and d (12·73) is the effect size (effect size = mean of control (101·4) – mean of treatment (28·20)/pooled sd (5·75) obtained from the previous study(Reference Ume Salma, Serva Peddha and Aswathanarayana Setty29). The mean of plasma AST values was used to determine the effect size. The desired power of the experiment was maintained at 80 % as recommended by Charan et al. (Reference Charan and Kantharia28). Based on the above-mentioned criteria, six rats were maintained in each group. Results of the parameters analysed are expressed as mean with a standard deviation of six rats. To analyse the differences between the groups, one-way ANOVA test (Tukey’s test with 95 % CI) was employed using Graph Pad software (Prism 5). The differences were considered statistically significant at a P-value of <0·05.
Results
Amino acid composition and mass spectrometric analysis of enhanced Fischer ratio flaxseed protein hydrolysate
The amino acid composition of EFR-FPH is given in Table 1. The EFR-FPH showed a significant increase in the total amount of BCAA by 23·6 %. There was a marked decrease in the total AAA by 72·5%, which was highly significant, compared with native flaxseed protein. The Fischer ratio of EFR-FPH was found to be 7·08. The HPLC chromatogram of the amino acid profile of EFR-FPH indicates a decrease in the concentration of phenylalanine and tyrosine, compared with native flaxseed protein. A decrease in the peak area of AAA in the HPLC chromatogram indicates their removal and subsequent increase in the Fischer ratio (Supplementary material).
Table 1. Amino acid composition and Fischer ratio of native flaxseed protein and enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH)
(Mean values and standard deviations)

AAA, aromatic amino acids; BCAA, branched-chain amino acids.
* Statistical difference highly significant compared with native protein (P < 0·001).
The EFR-FPH was analysed using reverse-phase LC coupled to a triple TOF mass spectrometer. The extracted ion chromatogram (Fig. 1) shows the relative abundance of free leucine/isoleuicne compared with phenylalanine and tyrosine. In the extracted ion chromatogram, the peaks for the masses 132·10 Da, 166·08 Da and 182·08 Da corresponded to leucine/isoleucine, phenylalanine and tyrosine, respectively. The MS/MS of m/z 132·10 revealed the presence of m/z 86·09 [(M + H) – HCOOH]+ and m/z 69·07 [(M + H) – HCOOH-NH3]+ ions confirming leucine and isoleucine, respectively (Fig. 2).

Fig. 1. Extracted ion chromatogram of LC–MS/MS analysis of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH). Peak 1 – leucine/isoleusine (mass divided by charge number (m/z) 132·10 (sd 0·0025) Da), peak 2 – phenylalanine (m/z 166·08 (sd 0·0025) Da), peak 3 – tyrosine (m/z 182·08 (sd 0·0025) Da).

Fig. 2. MS/MS of singly charged analyte at mass divided by charge number (m/z) 132·10 corresponding to leucine/isoleucine fragmentation. (a) Product ion at m/z 86·09 [M + H – HCOOH]+ corresponding to leucine fragmentation, (b) product ion at m/z 69·07 [M + H – HCOOH-NH3]+ corresponding to isoleucine fragmentation.
The LC-MS/MS analysis showed the presence of free amino acids. However, to determine the upper mass limit of the peptides, the EFR-FPH was subjected to MALDI-TOF analysis. The peptide was scanned for the mass above 600 Da. The peptide with the intensity of 2500 au (arbitrary unit) and above was considered. The MALDI-TOF analysis revealed the presence of peptide having the maximum molecular mass of 2338·27 Da (Fig. 3).

Fig. 3. MALDI-TOF spectrum of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH). MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight.
Effect of enhanced Fischer ratio flaxseed protein hydrolysate alone and in combination with antioxidant micronutrients on growth, plasma markers of hepatic damage and protein nutritional status of ethanol-treated rats
Chronic ethanol administration resulted in a significant decrease in feed intake of the rats of the EtOH group when compared with the control group animals (P < 0·05) (Table 2). There was no significant difference in the feed intake between EtOH and EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups. The rats of the EtOH group gained significantly lesser BW than the control group animals. There was a significant increase in the BW of the rats of EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups compared with the EtOH group. In comparison with the control group, the EtOH group showed significantly higher relative hepatic weight. The weight of the liver relative to the BW was significantly lower in EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups (P < 0·05).
Table 2. Effect of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH) alone and in combination with antioxidant micronutrients on feed intake, body weight gain and relative hepatic weight of ethanol-treated rats
(Mean values and standard deviation, n 6)

CON, control; EtOH, ethanol; Se, selenium; VITE, vitamin E.
a,b,c Mean values with different superscript letters are significantly different (P < 0·05).
The plasma markers of hepatic damage such as AST, ALT, ALP and bilirubin were significantly increased in the circulation of the rats of the EtOH group (P < 0·05) (Fig. 4). Treatment of rats with EFR-FPH significantly prevented the elevation of plasma levels of AST, ALT, ALP and bilirubin in the EtOH + EFR-FPH group by 35, 44, 44 and 66 %, respectively. An increase in the plasma levels of AST, ALT, ALP and bilirubin was significantly countered by 54, 50, 58 and 73 %, respectively, in the ethanol-treated rats receiving EFR-FPH and Se (EtOH + EFR-FPH + Se group). Treatment of rats with EFR-FPH and vitamin E also prevented the elevation of the plasma levels of AST, ALT, ALP and bilirubin by 51, 55, 59 and 73 %, respectively, which was statistically significant compared with EtOH group (P < 0·05). The rats receiving EFR-FPH, in combination with Se or vitamin E, showed statistically lower levels of plasma markers of hepatic damage than the rats receiving EFR-FPH alone. There was no statistical difference in the plasma hepatic damage markers between EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups.
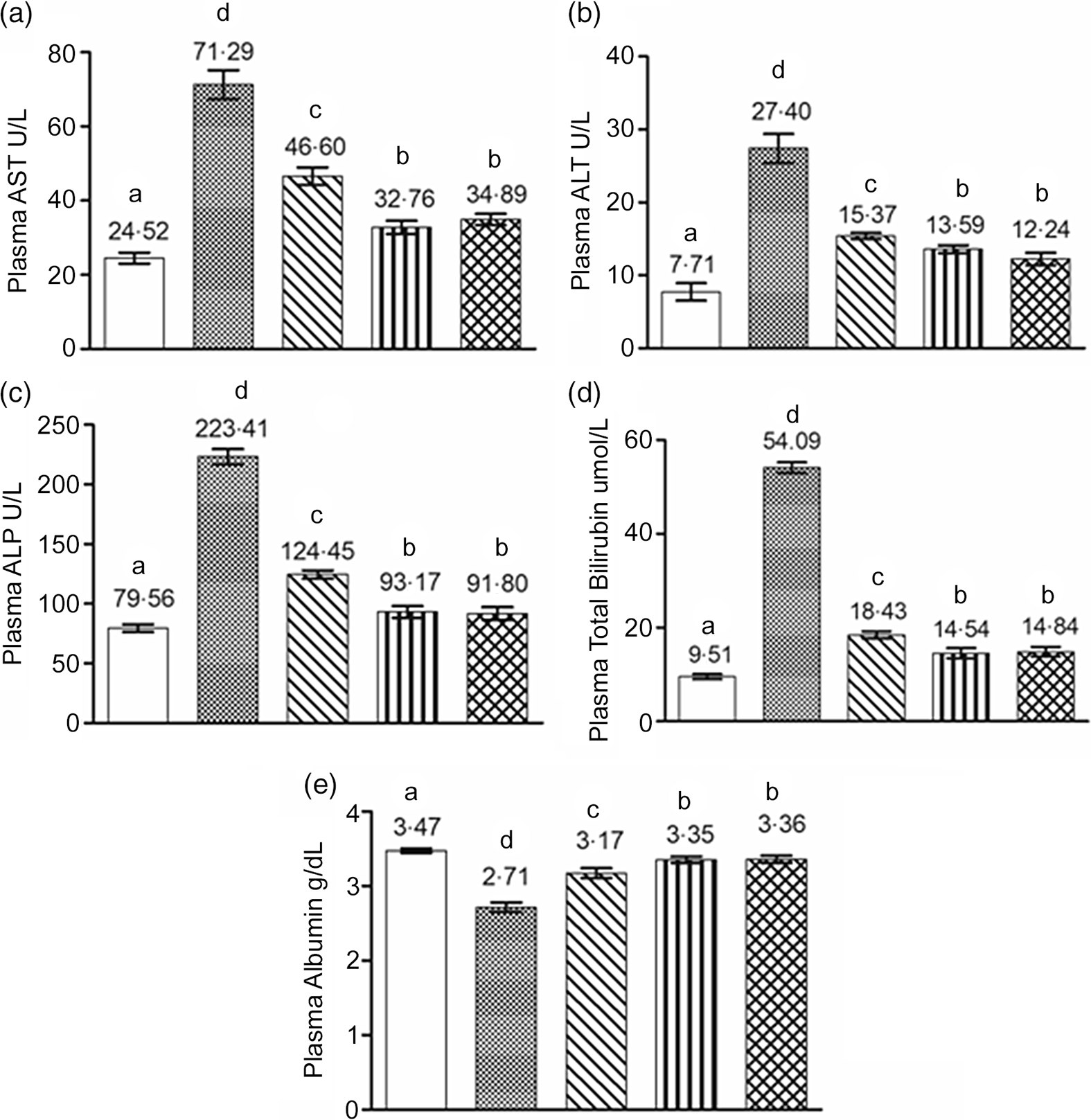
Fig. 4. Beneficial influence of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH) alone and in combination with antioxidant micronutrients on plasma markers of hepatic damage and protein nutritional status of ethanol-treated rats: (a) plasma AST, (b) plasma ALT, (c) plasma ALP, (d) plasma bilirubin and (e) plasma albumin. Values are means, with their standard deviation for six animals in each group. a,b,c,d Different alphabets indicate statistically significant difference (P < 0·05). I. CON; II. EtOH; III. EtOH + EFR-FPH; IV. EtOH + EFR-FPH + Se; V. EtOH + EFR-FPH + VITE. AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; CON, control; EtOH, ethanol; EFR-FPH, enhanced Fischer ratio flaxseed protein hydrolysate; Se, selenium; VITE, vitamin E.
The rats of the EtOH group showed significantly lower plasma albumin levels compared with the CON group. There was a significant increase in the plasma albumin levels of the rats of EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups in comparison with the EtOH group. The rats of EtOH + EFR-FPH + Se and ETOH + EFR-FPH + VITE showed significantly better protein nutritional status than the EtOH + EFR-FPH group. There was no significant difference in the plasma albumin levels between EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups.
Effect of flaxseed-based formulation on growth, plasma markers of hepatic damage and protein nutritional status of ethanol-treated rats
Feed intake and BW gain among the rats of the EtOH + PIBF group were significantly lower than the CON + PIBF group (P < 0·05) (Table 3). There was no significant difference in the feed intake between EtOH + PIBF and EtOH + FBF groups. The ethanol-treated rats receiving flaxseed-based formulation (EtOH + FBF) gained significantly higher BW compared with the ethanol-treated rats receiving purified ingredients based formulation (EtOH + PIBF). The rats of the EtOH + PIBF group showed a significant increase in relative hepatic weight compared with the CON + PIBF group. The rats of the EtOH + FBF group showed a significant restoration in relative hepatic weight.
Table 3. Effect of flaxseed-based formulation on feed intake, body weight gain and relative hepatic weight of ethanol-treated rats
(Mean values and standard deviation, n 6)

CON, control; EtOH, ethanol; FBF, flaxseed-based formulation containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients; PIBF, purified ingredient-based formulation.
a,b,c Mean values with different superscript letters are significantly different (P < 0.05).
A significant elevation in plasma total bilirubin and activities of plasma AST, ALT and ALP was observed in the rats of EtOH + PIBF (Fig. 5). The increase in the plasma levels of AST, ALT, ALP and bilirubin was significantly lowered by 47, 61, 55 and 78 %, respectively, in the rats of EtOH + FBF group receiving flaxseed-based formulation containing EFR-FPH and antioxidant micronutrients. Chronic exposure to ethanol affected protein nutritional status as indicated by a significant decrease in plasma albumin in the rats of the EtOH + PIBF group compared with the CON + PIBF group. The flaxseed-based formulation containing EFR-FPH and antioxidant micronutrients improved the protein nutritional status by significantly increasing the plasma albumin levels in the rats of the EtOH + FBF group.

Fig. 5. Beneficial influence of flaxseed-based formulation on plasma markers of hepatic damage and protein nutritional status of ethanol-treated rats: (a) plasma AST, (b) plasma ALT, (c) plasma ALP, (d) plasma bilirubin and (e) plasma albumin. Values are means, with their standard deviation for six animals in each group. a,b,c,d Different alphabets indicate statistically significant difference (P < 0·05). I. CON + PIBF; II. EtOH + PIBF; III. EtOH + FBF. AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; CON, control; PIBF, purified ingredient based formulation; EtOH, ethanol; FBF, flaxseed based formulation containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients.
Beneficial influence of enhanced Fischer ratio flaxseed protein hydrolysate alone and in combination with antioxidant micronutrients on lipid peroxidation and activities of antioxidant enzymes in the liver and plasma of ethanol-treated rats
Chronic administration of ethanol resulted in a significant increase in the lipid peroxidation as indicated by significantly higher levels of MDA in the plasma and liver homogenate of the EtOH group compared with the control group (P < 0·05) (Table 4). In comparison with the control group, the activities of antioxidant enzymes such as catalase, SOD, glutathione reductase and GPx were markedly lower in the plasma and liver homogenate of EtOH group (P < 0·05).
Table 4. Beneficial influence of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH) alone and in combination with antioxidant micronutrients on lipid peroxidation and activities of antioxidant enzymes in the liver and plasma of ethanol-treated rats (Mean values and standard deviation, n 6)

CAT, catalase; CON, control; EtOH, ethanol; GR, glutathione reductase; GPx, glutathione peroxidase; MDA, malondialdehyde; Se, selenium, SOD, superoxide dismutase; VITE, vitamin E.
a,b,c,d,e Mean values with different superscript letters are statistically different (P < 0.05).
Maximum protection against ethanol-induced lipid peroxidation was achieved by the group receiving EFR-FPH in combination with vitamin E (EtOH + EFR-FPH + VITE), followed by EtOH + EFR-FPH + Se and EtOH + EFR-FPH groups. In comparison with the EtOH group, there was a significant increase in the antioxidant enzyme activities in the plasma and liver homogenate of EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups. The activity of GPx enzyme in the plasma and liver homogenate was significantly higher in the group receiving EFR-FPH along with Se (EtOH + EFR-FPH + Se) compared with other ethanol-treated groups. The group receiving EFR-FPH along with vitamin E (EtOH + EFR-FPH + VITE) showed significantly higher activity of the enzyme SOD in the plasma and liver homogenate than other ethanol-treated groups. Treatment of rats with EFR-FPH in combination with Se or vitamin E was significantly more potent in mitigating ethanol-induced peroxidation and changes in the activities of antioxidant enzyme in the plasma and liver homogenate than the treatment with EFR-FPH alone.
Beneficial influence of flaxseed-based formulation on lipid peroxidation and activities of antioxidant enzymes in the liver and plasma of ethanol-treated rats
Chronic exposure to ethanol caused a significant increase in the levels of MDA in the plasma and liver homogenate of EtOH + PIBF group compared with CON + PIBF group (P < 0·05) (Table 5). A marked decrease in the activities of catalase, SOD, glutathione reductase and GPx were observed in the EtOH + PIBF group. In the plasma and liver homogenate of the group receiving flaxseed-based formulation containing EFR-FPH, Se and vitamin E, a significant increase in the activities of antioxidant enzymes was noted with a concomitant decrease in the levels of MDA in EtOH + FBF group.
Table 5. Beneficial influence of flaxseed-based formulation on lipid peroxidation and activities of antioxidant enzymes in the liver and plasma of ethanol-treated rats
(Mean values and standard deviation, n 6)

CAT, catalase; CON, control; EtOH, ethanol FBF, flaxseed-based formulation containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients; GPx, glutathione peroxidase; GR, glutathione reductase; MDA, malondialdehyde; PIBF, purified ingredient-based formulation; SOD, superoxide dismutase.
a,b,c Mean values with different superscript letters are statistically different (P < 0.05).
Effect of enhanced Fischer ratio flaxseed protein hydrolysate alone and in combination with antioxidant micronutrients on haematological parameters of ethanol-treated rats
In the EtOH group, there was a significant reduction in the number of leucocytes, lymphocytes, erythrocytes and Hg level of the blood. There was also a significant decrease in HCT value in the EtOH group compared with the control group (P < 0·05). A significant reduction in the platelet count and plateletcrit in the EtOH group was also observed. The EtOH group also showed various abnormalities in platelet indices, such as an increase in PDW, MPV, and P-LCR values (Table 6).
Table 6. Beneficial influence of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH) alone and in combination with antioxidant micronutrients on ethanol-induced haematological abnormalities (Mean values and standard deviation, n 6)

CON, control; EtOH, ethanol; HCT, haematocrit value; Hgb, haemoglobin; LYM, lymphocytes; MCH, mean corpuscular Hgb; MCV, mean corpuscular volume; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; P-LCR, platelet large cell ratio; PLT, platelet; Se, selenium; VITE, vitamin E.
a,b,c Mean values with different superscript letters are statistically different (P < 0.05).
Upon treatment with EFR-FPH alone or in combination with Se or vitamin E, there was a significant improvement in the blood parameters. There was a restoration of Hgb concentration in all the groups receiving EFR-FPH. An increase in the number of leucocytes, erythrocytes and lymphocytes in EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups was also observed. The group receiving Se along with EFR-FPH (EtOH + EFR-FPH + Se) showed maximum restoration of leucocytes and lymphocytes to normal values. A significant increase in the platelet count was also observed in EtOH + EFR-FPH, EtOH + EFR-FPH + Se and EtOH + EFR-FPH + VITE groups. Other platelet indices such as MPV, PDW, P-LCR and PCT also significantly improved upon treatment with EFR-FPH alone or in combination with Se or vitamin E.
Effect of flaxseed-based formulation on haematological parameters of ethanol-treated rats
A significant reduction in the leucocytes, lymphocytes, erythrocytes and Hgb was observed in the EtOH + PIBF group (Table 7), along with a significant decrease in the PCT value and platelet count. The irregularities in the platelet indices such as higher values of PDW, MPV and P-LCR were also observed in the EtOH + PIBF group. The diet supplemented with flaxseed-based formulation containing EFR-FPH, Se and vitamin E could significantly increase the production of leucocytes, lymphpcytes and erythrocytes along with improvement in the Hgb levels, which was observed among rats of EtOH + FBF groups. In the EtOH + FBF group, a significant increase in the platelet count was observed, and the platelet indices such as PDW, MPV and PCT were restored to normal values.
Table 7. Beneficial influence of flaxseed-based formulation on ethanol-induced haematological abnormalities
(Mean values and standard deviation, n 6)

CON, control; EtOH, ethanol; FBF, flaxseed-based formulation containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients; HCT, haematocrit value; Hgb, haemoglobin; LYM, lymphocytes; MCH, mean corpuscular Hgb; MCV, mean corpuscular volume; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; PIBF, purified ingredient-based formulation; P-LCR, platelet large cell ratio; PLT, platelet.
a,b,c Mean values with different superscript letters are statistically different (P < 0.05).
Effect of enhanced Fischer ratio flaxseed protein hydrolysate alone and in combination with antioxidant micronutrients on ethanol-induced histopathological alterations
Ethanol-induced alterations observed in the liver tissue are shown in Fig. 6. Chronic exposure to alcohol caused multifocal necrosis of the coagulative type in the centrilobular region of the liver tissue in the EtOH group. Infiltration of inflammatory cells was also observed in the periportal/peribiliary region. The photomicrographs of liver sections also revealed scar tissue indicating fibrosis in the liver tissue of the EtOH group. Steatosis, in the form of macrovesicular fatty infiltration, was also observed in the periportal and centrilobular regions of the liver. Other signs of damage observed in the liver tissue of EtOH group were dilation of the sinusoidal spaces and haemorrhage. The architecture of the liver tissue of rats receiving EFR-FPH alone or in combination with antioxidant micronutrients appeared normal with the normal portal triad.

Fig. 6. Photomicrographs of the liver tissue showing the ameliorative effect of enhanced Fischer ratio flaxseed protein hydrolysate (EFR-FPH) and antioxidant micronutrients against ethanol-induced histopathological alterations (scale bar = 100 µm): (a) normal hepatocytes of CON group, (b–g) EtOH group showing signs of damage such as (b) multifocal necrosis in the centrilobular region, (c) fibrosis in the periportal/peribiliary region, (d) periportal/peribiliary infiltration of inflammatory cells, (e) steatosis, (f) sinusoidal haemorrhage, (g) sinusoidal dilation, (h–j) normal portal triad and hepatocytes of the liver tissue of (h) EtOH + EFR-FPH, (i) EtOH + EFR-FPH + Se and (j) EtOH + EFR-FPH + VITE. CON, control; EtOH, ethanol; EFR-FPH, enhanced Fischer ratio flaxseed protein hydrolysate; Se, selenium; VITE, vitamin E.
Effect of flaxseed-based formulation on ethanol-induced histopathological alterations
Hepatic damage caused due to chronic exposure to alcohol is evident in the photomicrographs of the EtOH + PIBF group (Fig. 7). The signs of damage, such as necrosis and fibrosis, were observed in the liver tissue of the EtOH + PIBF group. Foci of necrosis with infiltration of inflammatory cells were noticed surrounding the peribiliary and perivesicular region. Severe fibrosis was also observed in the peribiliary and periportal area. Macrovesicular fatty degeneration was evident in the centrilobular region. There was severe haemorrhage noticed in the sinusoidal spaces in between the hepatocytes. These signs of damages were suppressed in the liver tissue of the EtOH + FBF group receiving flaxseed-based formulation containing EFR-FPH and antioxidant micronutrients.

Fig. 7. Photomicrographs of liver tissues showing ameliorative effect of flaxseed-based formulation against ethanol-induced histopathological alterations (scale bar = 100 µm): (a) normal portal vein and hepatocytes of CON + PIBF group, (b–e) EtOH + PIBF group showing histological alterations such as (b) foci of necrosis with infiltration of inflammatory cells surrounding the peribiliary and perivesicular region, (c) severe fibrosis in the peribiliary and periportal regions, (d) centrilobular region showing macrovesicular fatty degeneration, (e) severe sinusoidal haemorrhage, (f) EtOH + FBF showing normal portal triad and hepatocytes without any abnormalities. CON, control; PIBF, purified ingredient-based formulation; EtOH, ethanol; FBF, flaxseed-based formulation containing enhanced Fischer ratio flaxseed protein hydrolysate and antioxidant micronutrients.
Discussion
The protein hydrolysate from flaxseeds was prepared with enhanced Fischer ratio (EFR-FPH), and its efficacy in the amelioration of ethanol-induced hepatic damage was studied in a rat model. The protein hydrolysate had a Fischer ratio of 7·08, which was enhanced by 4·5 folds over the native flaxseed protein. The extracted ion chromatogram showed the presence of leucine/isoleucine, which has the same retention time and mass of m/z 132·10(Reference Zheng, Li and Chen30). The fragmentation pattern of the analyte at m/z 132·10 generated ions at m/z 86·09 and 69·07, which correspond to the presence of leucine and isoleucine, respectively. The fragmentation pattern observed in our study is in agreement with the findings of Zheng et al. (Reference Zheng, Li and Chen30). Thus, the LC-MS/MS analysis confirmed the presence of both leucine and isoleucine, which were present relatively higher than phenyalanine and tyrosine. The BCAA, such as leucine and isoleuicne, were abundantly present in the free form, whereas valine might be present as part of the peptides. The abundance of BCAA in comparison with AAA observed by the LC-MS/MS analysis is in corroboration with the amino acid composition analysis of EFR-FPH.
The peptides of higher mass occur as multiply charged species, and therefore analysis of larger peptides by LC-MS/MS becomes difficult. Thus, the MALDI-TOF technique, which gives the deconvoluted mass of the peptides, was adopted to determine the upper mass limit of the hydrolysate. The mass spectrometry analysis of EFR-FPH showed the molecular weight distribution ranging from free amino acids to peptides of mass 2338·27 Da. The peptides of EFR-FPH having low molecular weight can be efficiently transported through various peptide transporters across the enterocytes. Therefore, the low-molecular-weight peptides and free amino acid content of EFR-FPH made it easy to be digested and absorbed following oral intake and consequently it is suitable for the formulation of supplement for liver disease patients.
In the current study, the ethanol hepatotoxicity-induced rat model showed that the malnutrition and hepatic dysfunction remain at the forefront of the clinical implications of chronic alcohol abuse. Chronic exposure to ethanol caused a significant decrease in feed intake and BW gain. The possible reason for the ethanol-induced decrease in weight gain can be the induction of microsomal ethanol oxidising system, which breaks down alcohol without the production of chemical energy(Reference Lieber31). Moreover, ethanol alters the morphology and function of the mucosa of the gastrointestinal tract leading to impaired digestion and absorption of nutrients(Reference Stickel, Hoehn and Schuppanet2). Among the ethanol-treated rats receiving EFR-FPH, though there was a decrease in the feed intake, there was significantly more gain in mean BW than the EtOH group. Since ethanol has toxic effects on the epithelium, low-molecular-weight peptides and free amino acids of EFR-FPH can be efficiently absorbed and utilised to improve the nutritional status as observed in the groups receiving EFR-FPH(Reference Udenigwe and Aluko12). The diet supplemented with flaxseed-based formulation was also potent in increasing the BW and protein nutritional status. An increase in the hepatic weight relative to the BW was the classic feature of the ethanol-induced hepatotoxicity, which is associated with morphological changes such as hypertrophy of the liver occurring due to the chronic exposure to alcohol. The EFR-FPH alone and in combination with Se or vitamin E and the flaxseed-based formulation were potent in restoring relative hepatic weight by preventing ethanol-induced morphological changes.
The liver is the primary site of ethanol metabolism and, therefore, is highly susceptible to injury induced by chronic exposure to alcohol. In case of moderate intake, ethanol is metabolised by alcohol dehydrogenase system. Due to the chronic ingestion of ethanol, it is metabolised via cytochrome P450 of microsomal ethanol oxidising system. The induction of microsomal ethanol oxidising system generates reactive oxygen species(Reference Cederbaum1). The three mechanisms proposed to cause ethanol-induced hepatic damage are (1) acetaldehyde toxicity; (2) oxidative stress due to the accelerated production of reactive oxygen species and (3) activation of the immune response in the liver(Reference Han, Hashimoto and Fukushima32). Due to the described mechanisms, ethanol can perturb the structural integrity of hepatocytes causing cellular leakage of enzymes such as AST, ALP and ALT into the circulation. In the present study, we observed a significant increase in the activities of AST, ALT and ALP in the plasma of EtOH and EtOH + PIBF group rats compared with others, due to chronic exposure to alcohol in addition to hepatobilliary dysfunction.
The group receiving EFR-FPH showed significantly lower levels of plasma markers of hepatic damage than the EtOH group. The hepatoprotective effect of EFR-FPH can be attributed to its amino acid composition. In chronic alcoholic liver disease conditions, protein metabolism is severely affected as the liver is the primary site for amino acid metabolism, and there is also a rapid breakdown of muscle protein for gluconeogenesis, which generates ammonia(Reference Bemeur, Desjardins and Butterworth33). Ammonia is metabolised to urea in the liver. As the liver is under stress due to ethanol metabolism, the overproduction of ammonia exacerbates the hepatic impairment. The BCAA can be metabolised in the skeletal muscle, and therefore are preferred in liver disease conditions. Also, BCAA metabolism in the muscle produces glutamate, which then acts as a substrate for ammonia detoxification to glutamine(Reference Holeček6). Hence, BCAA have therapeutic value in liver disease conditions as they can compensate ammonia detoxification for the impaired liver(Reference Kinny-Köster, Bartels and Becker7) and providing sufficient rest to the compromised liver can improve its function and enhance recovery. The AAA are primarily metabolised in the liver, and they worsen the clinical outcome in liver disease conditions(Reference Shawcross and Jalan9) The EFR-FPH being high in BCAA and low in AAA has thus shown the potency of improving the liver function. Our investigation shows how FPH with the Fischer ratio of 7·08 is potent in improving liver function in chronic alcoholism in rats.
The hepatoprotective effect of the EFR-FPH was significantly enhanced when it was combined with Se, or vitamin E. Se is primarily required for the activity of GPx enzyme. The GPx eliminates free radicals such as hydrogen peroxides (H2O2) and lipid peroxides. Since ethanol-induced hepatic damage is exacerbated by oxidative stress, an increase in the activity of GPx can protect hepatocytes against free radicals. Our result is in agreement with Ozkol et al. (Reference Ozkol, Bulut and Balahoroglu34) and Adali et al. (Reference Adali, Eroglu and Makav35), who reported the efficacy of Se in reducing plasma markers of hepatic damage in ethanol-treated rats. Vitamin E, a lipophilic antioxidant also known as a chain-breaking antioxidant, prevents the progression of the free radical reaction. Vitamin E primarily protects the polyunsaturated fatty acid within the membrane by scavenging peroxyl radicals, thus offering protection to the hepatocytes against ethanol metabolism-induced oxidative stress. Our finding is in line with Lee et al. (Reference Lee, Young-Kim and Min36), who reported the role of vitamin E in lowering hepatic damage markers in the plasma of rats exposed to ethanol. The hepatoprotective effect of the flaxseed-based formulation can be attributed to the cumulative effect of the flaxseed, EFR-FPH and antioxidant micronutrients. The functional ingredients present in flaxseed, such as lignans, phenolics and n-3 fatty acid, make it a suitable ingredient for the formulation of the hepatoprotective supplement. The hepatoprotective effect of a flaxseed supplemented diet against ethanol-induced hepatotoxicity has been previously reported(Reference Ume Salma, Serva Peddha and Aswathanarayana Setty29).
The current study shows how prolonged exposure to ethanol accelerates the levels of MDA, indicating the impairment of antioxidant defense mechanisms. This is evident by a decrease in the activities of catalase, glutathione reductase, SOD, and GPx in the plasma and liver homogenate of ethanol-treated rats (EtOH and EtOH + PIBF). The reduction in the activity of antioxidant enzymes due to prolonged exposure to alcohol can be attributed to reactive oxygen species dependent inactivation of enzymes, depletion of co-substrates required for the enzyme activity, decrease in NADPH levels, accelerated generation of superoxide or increase in lipid peroxidation(Reference Abhilash, Harikrishnan and Indira37). Prathibha et al. (Reference Prathibha, Rejitha and Harikrishnan38) reported similar observation of decreased antioxidant enzyme activities in a chronic ethanol-induced hepatic fibrosis model.
The EFR-FPH was potent in preventing lipid peroxidation and enhanced the activity of antioxidant enzymes in the plasma and liver homogenate by effectively scavenging the free radicals generated in ethanol metabolism. Our findings suggest that protein hydrolysate having a higher Fischer ratio can exhibit antioxidant properties. Udenigwe et al. (Reference Udenigwe and Aluko12), showed how low-molecular-weight FPH having a high Fischer ratio strongly inhibited linoleic acid oxidation and scavenged superoxide anion and hydroxyl radical in an in vitro model. In our study, we confirmed the antioxidant activity of EFR-FPH using an in vivo ethanol-induced oxidative stress model of Wistar rats.
The EFR-FPH, combined with Se or vitamin E, offered better protection against lipid peroxidation and ethanol-induced changes in antioxidant enzyme activities. GPx activity was maximum in the group receiving EFR-FPH in combination with Se. Present in the active site of the enzyme, Se is essential for the activity of GPx. Prolonged exposure to ethanol can increase the requirement of Se to scavenge H2O2 and peroxides via GPx activity effectively. Se supplemented group also showed increased antioxidant enzyme activities due to the mitigation of free radical-dependent inactivation of antioxidant enzymes with a significant decrease in the levels of MDA in the plasma and liver. An increase in the activity of GPx upon Se supplementation in CCL4-induced hepatic damage in mice has been reported by Ding et al. (Reference Ding, Potter and Liu39).
The maximum protection against ethanol-induced lipid peroxidation was achieved by the group receiving EFR-FPH along with vitamin E. The lipophilic vitamin E functions as a potent antioxidant by transferring phenolic hydrogen to the peroxyl-free radical of the peroxidised polyunsaturated fatty acid of the membrane, minimising the lipid peroxidation. This is reflected in terms of lowered MDA levels in the plasma and liver of rats of vitamin E supplemented group. In response to a decrease in free radicals and lipid peroxidation, there was an enhancement in the antioxidant enzymes activities in the plasma and liver of the vitamin E supplemented group. Vitamin E supplemented group showed maximum SOD activity in the liver and plasma, which can be due to a decrease in the production of superoxide anions. As the hepatic damage progresses, the NADPH oxidase complexes are activated in the membrane of Kupffer cells, stellate cells and hepatocytes to produce superoxide anion(Reference Paik, Kim and Aoyama40). Vitamin E decreases the production of superoxide anion by interfering with the assembly of the NADPH oxidase complex in the membrane, which was demonstrated by Factor et al. (Reference Factor, Laskowska and Jensen41). Vitamin E induced increase in SOD activity in the alcohol model is previously reported by Kaur et al. (Reference Kaur, Shalini and Bansal42). The diet supplemented with flaxseed-based formulation also improved the antioxidant barrier, as evidenced by lower levels of MDA and an increase in antioxidant enzyme activities. The antioxidant potential of the flaxseed-based formulation is because of phenolics, lignan and secoisolariciresinol present in flaxseed in addition to the cumulative effect of EFR-FPH, Se and vitamin E present in the formulation against oxidative stress induced by ethanol.
Chronic exposure to ethanol adversely affected various parameters of blood directly due to its toxic effect on the bone marrow or indirectly by inducing nutritional deficiencies that impair the formation and function of blood cells(Reference Ballard43). Ethanol also caused a significant reduction in the platelet count resulting in a condition called thrombocytopenia, which can be attributed to ethanol-induced impairment of the liver, as the liver produces a protein called thrombopoietin required for the production of platelets(Reference Mitchell, Feldman and Diakow44). A decrease in the platelet count is evident from the sinusoidal haemorrhage seen in the liver tissue of the EtOH and EtOH + PIBF groups. Due to decreased production of platelets, the young platelets become bigger, causing an increase in MPV and P-LCR(Reference Budak, Polat and Huysal45). Ethanol also resulted in a condition called platelet anisocytosis, as indicated by a significantly higher PDW, which indicates a large variation in the size of platelets(Reference Budak, Polat and Huysal45).
A significant increase in the Hgb level was observed in the group receiving EFR-FPH. The EFR-FPH, due to its easy digestibility and absorption, causes improved protein nutritional status, which can, in turn, increase Hgb synthesis, as dietary protein is considered one of the essential regulators of Hgb synthesis(Reference Lewicki, Lesniak and Bertrandt46). With an improvement in the nutritional status, there was a concomitant increase in the number of leucocytes, erythrocytes and lymphocytes in all the groups receiving EFR-FPH. The group receiving EFR-FPH and Se showed maximum restoration in the number of leucocytes and lymphocytes. Yazdi et al. (Reference Yazdi, Masoudifar and Varastehmoradi47) showed how oral supplementation of Se increased lymphocytes count in BALB/c mice, implicating the beneficial influence of this mineral on the blood parameters, as observed in our current study. Treatment with EFR-FPH alone or in combination with antioxidant nutrients improved platelet production and other parameters of platelet due to the improvement in the liver function, which results in the hepatic production of thrombopoietin. Similar beneficial influence on the haemtaological parameters was seen due to the cumulative effect of EFR-FPH, Se and vitamin E in the group receiving flaxseed-based formulation.
Ethanol causes changes in the liver’s architecture primarily via triggering the production of inflammatory cytokines and activating the Kupffer cells(Reference Kawaratani, Tsujimoto and Douhara48). These mechanisms accelerate necrosis, apoptosis and formation of collagen, leading to fibrosis. The improper oxidation of fatty acid causes fat accumulation in the liver tissue due to chronic alcohol consumption, as noticed in the ETOH and EtOH + PIBF groups. In the groups receiving EFR-FPH alone or in combination with Se or vitamin E, ethanol-induced changes in the architecture of the liver were suppressed. BCAA have been reported to prevent Kupffer cell activation. Since the EFR-FPH has a substantial amount of BCAA, the histopathological alteration due to Kupffer cell activation might have been suppressed(Reference Kitagawa, Yokoyama and Kokuryo49). Moreover, treatment with EFR-FPH along with Se or vitamin E offers protection to mitochondria against oxidative stress as mitochondrial dysfunction due to prolonged oxidative stress interferes with the production of energy and lack of ATP results in cell death and necrosis. The prevention of mitochondrial dysfunction by the treatment of EFR-FPH, vitamin E and Se, in turn, resulted in oxidation of fat effectively, thereby countering the accumulation of fat in the liver tissue.
The diet supplemented with a flaxseed-based formulation containing EFR-FPH and antioxidant micronutrients could suppress ethanol-induced changes in the hepatic architecture. The flaxseed contains a substantial amount of n-3 fatty acid, which suppresses lipogenesis by reducing the sterol receptor element-binding protein-1c and upregulating fat oxidation, thus preventing fatty infiltration in the liver(Reference Davidson50). Other functional components of flaxseed-like phenolics, lignan and secoisolariciresinol prevent oxidative stress-induced lipid peroxidation of the cell membrane and thus prevent inflammatory responses and mitigate the progression to necrosis and fibrosis.
The present study highlights the consumption of EFR-FPH alone or with antioxidant micronutrients in a flaxseed-based formulation as a potential food-based approach in attenuating ethanol-induced hepatic damage. This could be the first report on the hepatoprotective effect of EFR-FPH in an in vivo model. The study’s important limitation is the traditional, two-dimensional visualisation and interpretation of the tissue slides instead of stereology, as the latter provides better insights into the architecture of the liver tissue. In this study, the hepatoprotective effect of EFR-FPH and the flaxseed-based dietary supplement are tested at a standard dose of ethanol consumption. The efficacy, however, needs to be confirmed with higher doses of ethanol.
Conclusion
The findings of this investigation suggest that flaxseed protein hydrolysate having a Fischer ratio of 7·08 is potent in improving the nutritional status, liver function and antioxidant defense mechanism in ethanol hepatoxicity-induced rats. The hepatoprotective effect can be significantly enhanced by combining protein hydrolysate treatment with Se, or vitamin E. This study also highlights the potential ameliorative ability of flaxseed-based hepatoprotective formulation containing enhanced Fischer ratio protein hydrolysate, Se and vitamin E against ethanol-induced hepatoxicity in rats. The beneficial application of EFR-FPH and flaxseed-based hepatoprotective formulation among chronic alcoholic human subjects, however, warrants clinical trials.
Acknowledgements
N.U.S. wishes to thank the Department of Science and Technology, Ministry of Science and Technology, Government of India, for awarding DST-INSPIRE fellowship. The authors thank the Central Instruments Facility and Services department of Cenral Food Technological Research Institute, Mysure, for mass spectrometry experiments. Authors are grateful to Mrs Sunitha Prakash, Proteomics Facility, Molecular Biophysics Unit, Indian Institute of Science, Bengaluru for MALDI-TOF experiment.
This investigation received no specific grant from any funding agency, commercial or not-for-profit sector.
N. U. S. is responsible for all the benchwork involved in this investigation, in addition to the data interpretation and writing of this manuscript. K. G is responsible for the methodology and data interpretation for the amino acid analysis. B. S. G. K contributed towards the methodology development and data curation related to mass spectrometric analysis. S. P. M designed the methodology for the animal studies. The study was conceptualised and supervised by A. J. L., who is also responsible for the methodology design and data interpretation.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452100115X