Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterised by persistent deficits in social communication and interaction, as well as restricted, repetitive patterns of behaviours, interests or activities.1 People with ASD are frequently diagnosed with co-occurring physical and mental health conditions including intellectual disability, seizure disorders, depression, anxiety, psychosis, obsessive–compulsive disorder and attention-deficit hyperactivity disorder.Reference Croen, Zerbo, Qian, Massolo, Rich and Sidney2–Reference Nah, Brewer, Young and Flower4 Rates of psychotropic prescribing are also high in this group; surveys of medication use in the USA and Canada found that up to 64% of children and adults with ASD were prescribed at least one psychotropic medicationReference Aman, Lam and Van Bourgondien5–Reference Coury, Anagnostou, Manning-Courtney, Reynolds, Cole and McCoy8 and that 12–29% were prescribed two or more medications simultaneously.Reference Martin, Scahill, Klin and Volkmar6, Reference Coury, Anagnostou, Manning-Courtney, Reynolds, Cole and McCoy8 There is a growing body of evidence to suggest that psychotropic medications are prescribed to people with ASD and/or intellectual disability for the purposes of managing behaviour.Reference Glover, Williams, Branforc, Avery, Chauhan and Hoghton9–Reference Tsakanikos, Costello, Holt, Sturmey and Bouras11 This is despite limited evidence of the effectiveness of psychopharmacological strategies for this purposeReference Taylor12, Reference Siegel and Beaulieu13 and current clinical recommendations that first-line psychological interventions be used to manage core symptoms of ASD.14
Although a number of research studies have examined psychotropic prescribing to people with ASD, most have focused on children and adolescents,Reference Esbensen, Greenberg, Seltzer and Aman7, Reference Coury, Anagnostou, Manning-Courtney, Reynolds, Cole and McCoy8, Reference Birch, Foley, Pollack, Britt, Lennox and Trollor15 or were conducted with international cohorts (for example in the UK or USA) where prescribing practices may differ as a result of geographical or cultural factors, or differences in clinical practice.Reference Aman, Lam and Van Bourgondien5–Reference Coury, Anagnostou, Manning-Courtney, Reynolds, Cole and McCoy8, Reference Hsia, Wong, Murphy, Simonoff, Buitelaar and Wong16 There is currently a paucity of data relating to psychotropic prescribing for adults with ASD from other countries, including Australia. Yet, such data is required to highlight areas where improvements in prescribing practices are required. Here we use baseline data from the Cooperative Research Centre for Living with Autism's (Autism CRC) Australian Longitudinal Study of Adults with Autism (ALSAA) to investigate the neuropsychiatric profile and patterns of psychotropic prescribing in a cohort of adults with ASD in Australia. The aims of the current report are to: (a) describe the baseline neuropsychiatric profile of a subsample of the Autism CRC's ALSAA cohort with a formal diagnosis ASD (i.e. the diagnosis was reported to have been made by an appropriate health professional) and compare this with non-autistic controls; (b) determine whether ASD status is a predictor of psychotropic prescribing when accounting for increased rates of neuropsychiatric disorders; (c) examine patterns of prescribing in respondents with ASD (i.e. the proportion of participants taking a particular psychotropic medication with a corresponding indication reported in their medical history).
Method
The Autism CRC's ALSAA is a nationwide questionnaire-based longitudinal study of adults with ASD aged 25 years or older that uses an inclusive research approach. Those invited to participate are adults with ASD, informants/carers of a person with ASD and non-autistic controls. Participants (adults with ASD, informants/carers of adults with ASD and non-autistic controls) are recruited through advertisement and contact with autism-, disability-, education- or employment-related organisations, and through social media. Exclusion criteria for all participants include being aged under 25 years or not being fluent in English. For informants/carers it is also a requirement that the carer is aged over 18 years. Baseline questionnaires were completed online or in hard-copy format from 2015 to 2017. Ethics approval for the study was granted by the UNSW Sydney Human Research Ethics Committee (project number HC15001). Informed consent was obtained from all participants prior to completing the survey. This manuscript reports on a subsample of the baseline ALSAA cohort who reported that they had received a formal diagnosis of ASD from an appropriate health professional.
Detailed information including demographic characteristics and medical history was obtained from self- and informant-report questionnaires. Demographic items included age, gender, marital status and socioeconomic index for areas (SEIFA) score (calculated as a binary variable for the top and bottom 50% areas of residence). Autism symptoms were measured using the Autism Quotient (AQ), specifically the AQ-ShortReference Hoekstra, Vinkhuyzen, Wheelwright, Bartels, Boomsma and Baron-Cohen17 or a 28-item parent/carer adaptionReference Baron-Cohen, Hoekstra, Knickmeyer and Wheelwright18 for participants not self-reporting.
Current and lifetime history of neurological and psychiatric disorders, a comprehensive medical history and current medications were obtained by self- or informant-report. We considered psychotropic medications to be those listed within the following categories of the Anatomical Therapeutic Chemical Classification system: N03 (antiepileptics); N04 (anti-Parkinson medications); NO5 (psycholeptics – antipsychotics, anxiolytics, hypnotics/sedatives); N06 (psychoanaleptics – antidepressants, psychostimulants). We also considered clonidine and propranolol as potential psychotropic agents given that these medications may be prescribed to manage behavioural features including irritability and aggression. Because of the small number of respondents currently taking anti-Parkinson medications, this class was collapsed into the ‘other’ category.
Statistical analyses
Sample characteristics for the ASD and non-autistic control groups were summarised using descriptive statistics. Characteristics of the ASD group were compared with controls using independent t-tests (continuous variables) or non-parametric equivalents where data were not normally distributed, and χ2 analyses (categorical variables). Logistic regression was used to determine whether ASD was associated with increased odds of having ‘any psychiatric diagnosis’, adjusting for age, intellectual disability status, marital status, gender and SEIFA score. Two further logistic regression models were built to determine whether ASD status was a predictor of psychiatric medication use, controlling for age, intellectual disability status, marital status, gender, SEIFA score and the presence of any psychiatric or neurological disorder. For these models, the outcomes were defined as: (a) currently taking at least one psychotropic medication; (b) currently taking two or more psychotropic medications.
Prescribing patterns were determined by noting the presence or absence of indications approved by the Therapeutic Goods Administration (TGA) in respondents' medical histories. This was followed by individual file review by a neuropsychiatrist (J.N.T.) to determine whether a clinical indication for prescription was present for a participant even if this was outside the scope of TGA guidelines (for example use of quetiapine for management of anxiety, or clonidine for management of hyperactivity). Prescribing patterns were then summarised using descriptive statistics. Hypnotics were not included in the prescribing patterns analysis as insomnia was not specifically assessed in the Autism CRC's ALSAA baseline survey.
Results
Sample characteristics
The baseline ALSAA subsample included in this report comprised a total of 303 respondents aged 25–80 years. This included 188 respondents with ASD (aged 25–80 years; n = 29 by informant-report), and 115 non-autistic controls (aged 25–79 years). Sample characteristics for these groups are presented in Table 1. As expected, total AQ-Short scores were significantly higher in the ASD group than controls (Mann–Whitney U-test 3923.5, P < 0.001). The proportion of women in the ASD group was smaller than in the control group (χ2 = 21.427, P < 0.001). Note that this reflects an over-representation of females in the control group rather than an under-representation of women in the ASD group; the proportion of women in the ASD group was in fact higher than that which would be expected based on the estimated male-to-female ratio of 3:1. Fewer participants in the ASD group were married or in a defacto relationship (χ2 = 44.601, P < 0.001). Intellectual disability (χ2 = 14.766, P < 0.001) and epilepsy (χ2 = 4.361, P = 0.028) were more common in the ASD group than in the control group. There were no significant differences between groups for age, SEIFA score, or the rate of Parkinson's disease or related disorders.
Table 1 Participant characteristics
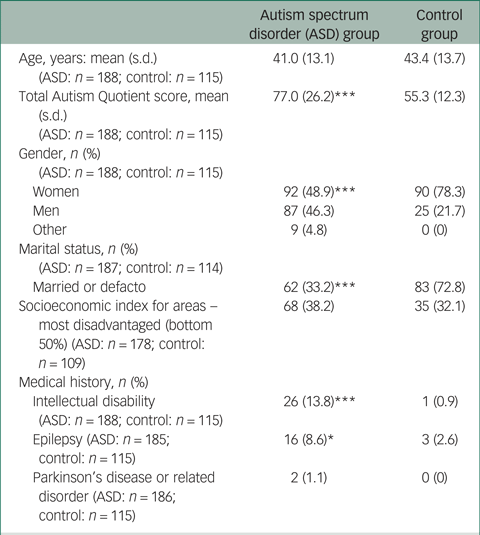
* P < 0.05; ***P < 0.001.
Mental health profile (lifetime history or current psychiatric diagnoses)
Individuals with ASD were significantly more likely to have received a psychiatric diagnosis during their lifetime than controls (adjusted odds ratio (OR) = 10.08, 95% CI 4.62–22.00). Overall 86.7% of respondents with ASD had a current or lifetime history of any psychiatric disorder compared with 48.7% of controls. The proportions of individuals within each group with specific psychiatric diagnoses are provided in Table 2.
Table 2 Psychiatric diagnoses (lifetime history or current)
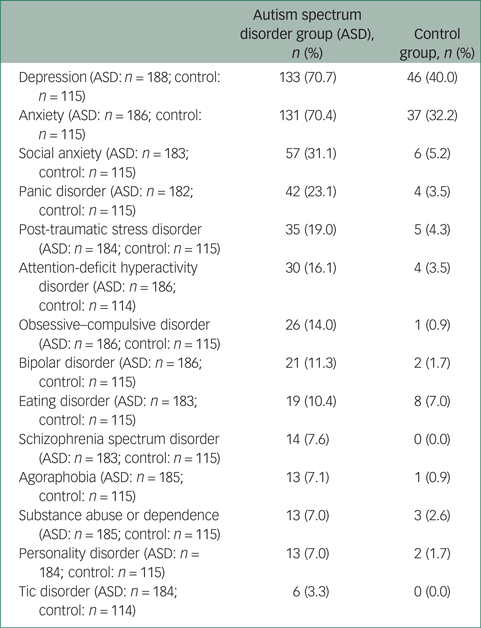
Psychotropic medication use
The number of current psychotropic medications prescribed ranged from 0 to 5 in the ASD group and from 0 to 3 in the control group. The proportions of participants within the ASD and control groups currently taking psychotropic medication within each drug class are shown in Table 3.
Table 3 Current psychotropic medications
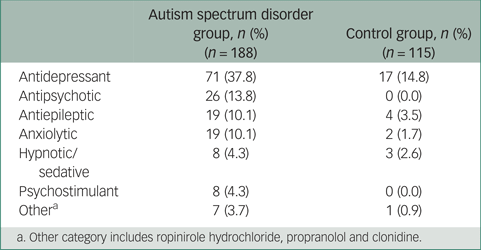
a. Other category includes ropinirole hydrochloride, propranolol and clonidine.
Participants with ASD were more likely to be currently taking at least one psychotropic medication compared with controls (adjusted OR = 4.54, 95% CI 2.26–9.10), even when accounting for diagnoses of any psychiatric or neurological disorder. Similarly, a diagnosis of ASD was associated with significantly greater odds of being prescribed two or more psychotropic medication when controlling for other confounding factors (adjusted OR = 3.13, 95% CI 1.09–9.01). Overall 54.8% of respondents with ASD and 18.3% of controls were taking at least one psychotropic medication. In total, 22% of respondents with ASD were taking two or more psychotropic medications compared with 4.3% of controls.
Prescribing patterns
As a result of the small number of psychotropic medications prescribed from each drug class in the control group, prescribing patterns are presented for the ASD group only. A total of 174 psychotropic medications were prescribed to the ASD group. Of the 103 individuals with ASD currently taking at least one psychotropic medication, 59.2% (n = 61) were taking one, 24.3% (n = 25) were taking two, 8.7% (n = 9) were taking three, 4.9% (n = 5) were taking four and 2.9% (n = 3) were taking five psychotropic medications simultaneously. Rates of co-prescription with other medication classes were highest for stimulants (87.5%) and antipsychotics (76.9%), both of which were most frequently co-prescribed with antidepressants. Rates of co-prescription for other medication classes were as follows: antiepileptics (68.4%); anxiolytics (63.2%); hypnotic/sedatives (62.5%); antidepressants (42.3%); other (71.4%). The overlap between medication classes prescribed to individuals with ASD is depicted in supplementary Fig. 1 available at https://doi.org/10.1192/bjo.2018.64.
Indications approved by the TGA were reported in the medical histories of respondents for 78.7% of medications prescribed; additional clinical indications were noted for 6.9%. Of the psychotropic medications prescribed to adults with ASD, 14.4% had no corresponding indication. There were no approved indications reported in participants' medical histories for 25% of antiepileptics and antipsychotics prescribed to adults with ASD. The proportions of medications with TGA approved or clinical indications by drug class are presented in Fig. 1.

Fig. 1 Summary of indication status by class of psychotropic medications prescribed to individuals with autism spectrum disorder.
Discussion
Main findings
This paper describes the neuropsychiatric profile and rates of psychotropic medication use in a community-based sample of adults with ASD and non-autistic controls. Consistent with previous research,Reference Croen, Zerbo, Qian, Massolo, Rich and Sidney2, Reference Nah, Brewer, Young and Flower4, Reference Aman, Lam and Van Bourgondien5, Reference Esbensen, Greenberg, Seltzer and Aman7 adults with ASD were more likely to have been diagnosed with a psychiatric disorder during their lifetime and were more likely to be currently taking psychotropic medication than controls. Of note, ASD was associated with increased odds of psychotropic medications even when controlling for the presence of any neurological or psychiatric disorder. This indicates that higher rates of psychotropic medications are not adequately explained by the presence of psychiatric disorder in this group. Although the majority (≥95%) of antidepressants, anxiolytics and psychostimulants taken by adults with ASD were linked to corresponding TGA approved or clinical indications in respondents' medical histories, there were no reported indications for a quarter of antiepileptics and antipsychotics prescribed. This is important given the potential side-effects of these medications and impact on cardiometabolic health and highlights important considerations for practitioners treating individuals with ASD.
Comparison with findings from previous studies
In line with previous researchReference Croen, Zerbo, Qian, Massolo, Rich and Sidney2 individuals with ASD were significantly more likely than controls to have been diagnosed with at least one psychiatric disorder during their lifetime, and were also more likely to be currently taking at least one psychotropic medication. Importantly, high rates of psychiatric disorder and psychotropic medication use in the ASD group are not attributable to co-occurring intellectual disability, as intellectual disability status was included as a covariate in all analyses. Because of the small number of cases within each category it was not possible to compare rates of specific diagnoses or drug classes while controlling for relevant covariates (for example marital status, intellectual disability status), but the descriptive findings are consistent with previous findings of high rates of depressive and anxiety disorders in individuals with ASD,Reference Nylander, Axmon, Björne, Ahlström and Gillberg3, Reference Nah, Brewer, Young and Flower4, Reference Joshi, Wozniak, Petty, Martelon, Fried and Bolfek19 as well as high prescription rates of antipsychotics and antidepressants.Reference Nylander, Axmon, Björne, Ahlström and Gillberg3, Reference Aman, Lam and Van Bourgondien5–Reference Esbensen, Greenberg, Seltzer and Aman7, Reference Hsia, Wong, Murphy, Simonoff, Buitelaar and Wong16
Possible factors contributing to our findings
Notably, the increased odds of currently taking at least one psychotropic mediation were not entirely explained by higher rates of neurological or psychiatric disorder among those with ASD. Even when controlling for the presence of any neurological or psychiatric disorder, individuals with ASD were still more than four times as likely to be currently taking at least one psychotropic medication compared with non-autistic controls. There are a number of possible factors that may have contributed to this finding, including issues considered to be barriers to rational prescribing. Barriers to rational prescribing described by primary care providers (referring to the general population) include: difficulties with accurately diagnosing a psychiatric disorder; poor communication; a lack of time during consultations; difficulty accessing appropriate non-pharmacological therapies; a lack of knowledge or awareness of appropriate first-line therapies and inadequate education about potential non-pharmacological therapies.Reference Hedenrud, Svensson and Wallerstedt20
Features associated with ASD (i.e. complex and atypical mental health presentations, particularly among those with intellectual disability; communication difficulties; high rates of physical comorbidities) likely compound these issues further, highlighting the importance of accessible ASD-specific clinician education and training in assessment and management of mental health disorders. Priorities for training should include considerations for assessment and management, communication strategies, valuing neurodiversity, identification of physical and mental health comorbidities, working within a multidisciplinary framework, assessment of challenging behaviours (including discerning the underlying causes), appropriate adaptation of non-pharmacological therapies where necessary and recommendations for responsible prescribing.Reference Foley and Trollor21
Our investigation into the prescribing patterns among those with ASD revealed that most (≥95%) antidepressants, anxiolytics and psychostimulants were linked to corresponding TGA approved or clinical indications in respondents' medical histories. However, there were no reported indications for a quarter of antiepileptics and antipsychotics prescribed. Propranolol, a beta-blocker approved for the management of a number of conditions including angina, hypertension, migraine and essential tremor, was the only medication in the ‘other’ category for which there was no reported indication in participants' medical histories. In these cases, it is likely that antipsychotics (such as risperidone), antiepileptics and propranolol were being used for management of behavioural features, including self-injurious behaviours, irritability and aggression.
Our findings align with investigations into psychotropic prescribing in the UK indicating high rates of psychotropic prescribing to people with intellectual disability and/or ASD in the absence of a diagnosed mental disorder,Reference Glover, Williams, Branforc, Avery, Chauhan and Hoghton9 and highlight important considerations for clinicians when prescribing psychotropic medication to manage behaviour. These considerations form the foundation of the ‘Stopping Overmedication of People with a Learning Disability, Autism or Both’ (STOMP) campaign initiated by NHS England, which aims to reduce inappropriate prescribing in people with intellectual disability and ASD in general practice and hospitals.Reference Sheehan, Strydom, Morant, Pappa and Hassiotis22 Although no such campaign has been implemented in Australia, clinical recommendations described by STOMP and other resources provide a framework for safe and appropriate prescribing to people with ASD.
Recommendations regarding responsible prescribing in this group
First, clinicians should undertake a comprehensive physical and psychiatric assessment of people with ASD presenting with mental health or behavioural concerns, with support from an informant or carer where appropriate. Second, clinicians should be familiar with current clinical guidelines for best treatment practices, which recommend that first-line psychological interventions be used to manage core symptoms of ASD.14 Although pharmacotherapy may increase potential for adults with ASD to engage with and benefit from psychological therapy in some cases,Reference Volkmar, Siegel, Woodbury-Smith, King, McCracken and State23 the potential benefit for the person with ASD should be considered against possible side-effects. Third, when prescribing psychotropic medication, clinicians should proactively monitor risk for adverse outcomes and work in collaboration with specialists, the person with ASD and their support network, to ensure that regimens are appropriate and individualised. Finally, clinicians should undertake regular medication reviews, and consider reducing or stopping psychotropic medications where clinically appropriate (for further recommendations, see Foley &TrollorReference Foley and Trollor21, Trollor et al Reference Trollor, Salomon, Curtis, Watkins, Rosenbaum and Samaras24 and van Dooren et al Reference van Dooren, Nicollet and Lennox25).
Strengths and limitations
Strengths of the current study include the reasonable sample size, the community-based recruitment method and the comparison of neuropsychiatric diagnoses and psychotropic medications with an appropriate non-autistic control group. However, there are some limitations to consider. First, although we controlled for the impact of intellectual disability status in logistic regression analyses, the small number of people with intellectual disability precluded an analysis of the impact of intellectual disability on prescribing patterns. This is important given the challenges associated with assessing and diagnosing psychiatric disorders in people with more severe intellectual disability, and evidence that psychotropic medication use increases with level of disability.Reference Aman, Lam and Van Bourgondien5
Second, self-report measures of physical and mental health problems are vulnerable to recall bias, and/or intentional non-disclosure of specific diagnoses or medications. Third, prescribing practices tend to rely on a number of factors that were not captured by this report. These factors include the professionals involved, individual patient characteristics and the setting in which medications are prescribed (for example primary versus secondary or tertiary care). Future research using medical records data (for example the Medicare Benefits Schedule and Pharmaceutical Benefits Scheme) in conjunction with self-report scales would help to address issues related to recall bias and non-disclosure. Further, the inclusion of items to collect information about other factors relevant to prescribing practices (such as the role and specialty of the prescribing practitioner and a history of other pharmacological or non-pharmacological interventions trialled to address the presenting issue) would provide a more comprehensive snapshot of prescribing practices in this area.
Implications
In conclusion, the findings of this study suggest substantial prescribing for the management of behaviour in adults with ASD despite limited evidence to support this practice. This is important given the potential side-effects of psychotropic medications including cardiometabolic risks, which may compound the known higher rates of cardiovascular disease in adults with ASD.Reference Cashin, Buckley and Trollor26 It is imperative that primary care providers and specialists are familiar with current clinical guidelines and receive adequate ASD-specific education and training to support responsible prescribing for this group.
Funding
Financial support from the Cooperative Research Centre for Living with Autism (Autism CRC), established and supported under the Australian Government's Cooperative Research Centres Program.
Acknowledgements
The authors would like to thank members of the Autism CRC ALSAA Research Advisory Network: Matthew Bennett, Bob Boyce, Jen Harland, Julianne Higgins, Michael Knight, Joanne Mahony, Andrea Michael, Gabriel Nakhel, Cheryl Strangio and Chris Tanner for their review of findings and interpretations of this work.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2018.64.







eLetters
No eLetters have been published for this article.