The trace element magnesium (Mg2+) has an essential role in hundreds of enzymatic reactions.Reference De Baaij, Hoenderop and Bindels1, Reference McLean2 The Mg2+ in our bodies is derived from food such as cereals, nuts and (green) vegetables.Reference Bouzari, Holstege and Barrett3–Reference Volpe5 Insufficient intake of Mg2+ can cause hypomagnesaemia (i.e. an Mg2+ level of <0.7 mmol/L).Reference Talapatra, Ray and Sen4, Reference Ford and Mokdad6–Reference Al-Ghamdi, Cameron and Sutton9 Hypomagnesaemia can also develop owing to the use of diuretics, defects in absorption or diarrhoea.Reference De Baaij, Hoenderop and Bindels1, Reference Volpe5 About 2–15% of the general population has hypomagnesaemia. In some populations this percentage is even higher, e.g. it is 14–48% in patients with type 2 diabetes.Reference Pham, Pham, Pham, Miller and Pham10 Mg2+ deficiency may pose a risk to metabolic and cardiovascular health.Reference Sarrafzadegan, Khosravi-Boroujeni, Lotfizadeh, Pourmogaddas and Salehi-Abargouei11, Reference Yamori, Sagara, Mizushima, Liu, Ikeda and Nara12
Mg2+ and mental health
For over 50 years, the idea has existed that Mg2+ deficiency may also pose a risk to mental health,Reference Boyle, Lawton and Dye13 in particular with respect to (pathological) low mood.Reference Nielsen14–Reference Murck16 One hypothesis, which attempts to explain this association, is that Mg2+ deficiency affects brain chemistry, membrane fluidity and inflammation,Reference De Baaij, Hoenderop and Bindels1, Reference Murck17, Reference Romani18 all of which are associated with psychiatric illnessesReference Serefko, Szopa, Wlaź, Nowak, Radziwoń-Zaleska and Skalski19 and the response to antidepressants.Reference Murck17 Furthermore, Mg2+ may protect neurons against cell death owing to its regulating effects on calcium dynamics.Reference De Baaij, Hoenderop and Bindels1 Mg2+ is also involved in the glutamatergic system, regulating learning, memory, neuroplasticity and perhaps antidepressant activity.Reference Marsden20
Animal studies
Some preclinical experiments have shown that Mg2+ deficiency is related to the functioning of limbic brain areas and to behaviour in rodents that some conceptualise as ‘depression-like’.Reference Murck16, Reference Winther, Jørgensen, Elfving, Nielsen, Kihl and Lund21 The administration of Mg2+ supplements,Reference Poleszak, Szewczyk, Kędzierska, Wlaź, Pilc and Nowak22 magnesium sulphateReference Fromm, Heath, Vink and Nimmo23 and magnesium chlorideReference Cardoso, Lobato, Binfaré, Ferreira, Rosa and Santos24 has been shown to alter this behaviour. However, owing to a lack of validity of the behavioural read-outs, the translational value of such experiments is questionable.Reference Molendijk and de Kloet25, Reference De Kloet and Molendijk26
Human studies
There is a considerable amount of human data on the topic. Some studies evaluated whether the prevalence (cross-sectional) or the incidence (longitudinal) of depression differs as a function of dietary Mg2 intake.Reference Jacka, Overland, Stewart, Tell, Bjelland and Mykletun27, Reference Yary, Lehto, Tolmunen, Tuomainen, Kauhanen and Voutilainen28 Others have investigated Mg2+ in bodily fluids as a function of mood disorder status.Reference Frazer, Ramsey, Swann, Bowden, Brunswick and Garver29, Reference Styczeń, Siwek, Sowa-Kućma, Dudek, Reczyński and Szewczyk30 Some experiments have also investigated whether Mg2+ supplementation can serve as an antidepressant.Reference Bhudia, Cosgrove, Naugle, Rajeswaran, Lam and Walton31, Reference Rajizadeh, Mozaffari-Khosravi, Yassini-Ardakani and Dehghani32
Conflicting findings
However, the findings from these studies appear to be inconclusive,Reference Derom, Sayón-Orea, Martínez-Ortega and Martínez-González33 and the two meta-analyses on the topic to date do not provide a high level of evidence either. Cheungpasitporn and colleaguesReference Cheungpasitporn, Thongprayoon, Mao, Srivali, Ungprasert and Varothai34 pooled data from three studies on blood Mg2+ levels with two studies on dietary Mg2+ intake and concluded from this heterogeneous pool of data that hypomagnesaemia is related to depression (odds ratio (OR) = 1.34). Li and colleaguesReference Li, Lv, Wang and Zhang35 pooled nine cross-sectional and two prospective studies on dietary Mg2+ intake and found a relative risk of 0.81 for depressive symptoms in people who adhered to a diet high in Mg2+. However, they did not differentiate between cross-sectional and longitudinal designs, leaving it open to interpretation whether dietary Mg2+ intake is a risk factor for depressive symptoms versus a concomitant phenomenon or a consequence of it.
The conflicting findings in this field may be attributable to moderators, such as the way in which dietary information is acquired or the blood component in which Mg2+ is measured (e.g. measurement methods and absolute values of Mg2+ are different for plasma and serum,Reference Saris, Mervaala, Karppanen, Khawaja and Lewenstam36 which may present an additional source of between-study heterogeneity in outcome). They may also stem from the differing methodological characteristics of individual studies (e.g. sample size, participant characteristics, medication effects) or from general issues such as publication bias.
The current study
One way to provide a more definitive answer to the question of whether Mg2+ and mood disorders are related, as well as explaining the potential causes of heterogeneity in the findings, is to carry out a systematic review with meta- and moderator analyses covering the broad literature on this topic. We set out to present such analyses on the following associations: (a) mood disorder prevalence or incidence by dietary Mg2+ intake, (b) Mg2+ levels in bodily fluids by mood disorder status and severity, and (c) the effects of Mg2+ supplements on mood.
Method
This project was reported following the guidelines of PRISMAReference Moher, Liberati, Tetzlaff and Altman37 and MOOSE.Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie38 PRISMA and MOOSE checklists can be found in Appendices 1 and 2, respectively. The review protocol is presented in appendix 3.
Search strategy
We searched PubMed, Web of Science, and Embase (from their commencement to 22 December 2017) for eligible papers using the following terms: (Magnesium OR Mg*) AND (depression OR depress* OR affect* OR mood OR mania OR bipolar). The reference lists of identified articles were scrutinised, as were the references that were made to the two seminal papers on the topicReference Nielsen14, Reference Malleson, Frizel and Marks15 (to which, at the date of our latest search, 65 and 5 references were made respectively).
Study selection
We included human studies that reported original findings on the following associations: (a) prevalence and/or incidence of depression as a function of dietary Mg2+ intake, (b) Mg2+ levels in bodily fluids/blood components as a function of mood disorder status and/or severity, and (c) changes in mood disorder status as a function of Mg2+ supplementation. Studies had to be published in peer-reviewed journals (including advance online publication) and written in English, French, German, Spanish or Dutch in order to be included.
In case of overlap among study samples, we excluded the study that reported on the fewest participants.
Data extraction
From each eligible article, we extracted data on a range of demographic, clinical and methodological variables, as well as raw numbers or effect-size estimates (with corresponding 95% confidence intervals) on the associations of interest. Data extraction is specified in Supplementary Table S1, available at https://doi.org/10.1192/bjo.2018.22. Authors of articles in which data necessary to our investigations were missing were contacted by e-mail to request these data.
Assessment of the eligibility of each publication and data extraction were performed independently by two of the authors. Cases of disagreement were resolved by discussion and consensus.
Quality assessment
The methodological quality of cross-sectional and case–control studies was assessed using the Newcastle–Ottawa scale,Reference Wells, Shea and O'Connel39 and that of prospective studies was assessed using the method proposed by Lievense et al. Reference Lievense, Bierma-Zeinstra, Verhagen, van Baar, Verhaar and Koes40 The methodological quality of treatment trials was assessed using the method of evaluation of (randomised) trials provided by the US Department of Health and Human Services.41
Data analyses
Analyses were performed in STATA version 13.Reference StataCorp42 Associations were tested for statistical significance at a two-tailed confidence interval of 95%. Summary tables on characteristics of eligible papers were created.
Random-effects meta-analyses were used in all cases to pool the data. In case of binary outcomes (e.g. incidence of depression), we calculated the OR as an effect-size estimate. When continuous data served as the outcome and group membership as the predictor (e.g. Mg2+ concentrations in patients and healthy control participants), we calculated Hedge's g as the measure of effect. Associations between continuous variables (e.g. Mg2+ concentration and depression severity) were quantified using Pearson's r.
Heterogeneity in outcome was quantified using the I 2 measure and its statistical significance was assessed using the χ 2 statistic.Reference Sterne, Bradburn and Egger43 In cases of heterogeneity, moderator analyses were performed. Predictors of heterogeneity were, where applicable: the medium in which Mg2+ was determined, type of diagnosis, male/female ratio and mean age of the sample, type of medication, duration of follow-up, and the estimated methodological quality of the study. The sensitivity of our results was further tested by excluding each single study at a time.
Publication bias was assessed by means of visual inspection of funnel plots and Egger's test.Reference Sterne, Bradburn and Egger43 When evident, trim-and-fill procedures were applied to estimate pooled effect sizes while taking bias into account.Reference Duval and Tweedie44
Results
We identified 4110 articles after duplicates were removed. Of these, 4053 articles were excluded, leaving 58 that reported on at least one of the associations of interest. The study selection process, from initial search to final selection, is presented in Figure 1. Table 1 and Supplementary Table 10 list the articles that were included in our meta-analysesReference Nielsen14, Reference Malleson, Frizel and Marks15, Reference Jacka, Overland, Stewart, Tell, Bjelland and Mykletun27–Reference Rajizadeh, Mozaffari-Khosravi, Yassini-Ardakani and Dehghani32, Reference Bjørum45–Reference Tarleton, Littenberg, MacLean, Kennedy and Daley94 and provide information on their characteristics.
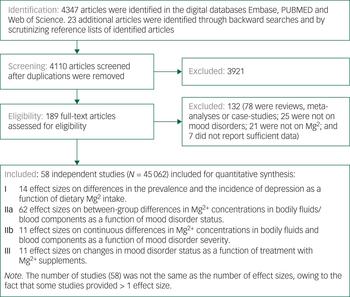
Fig. 1 Flowchart on identification, screening and inclusion of eligible articles.
Table 1 Characteristics of the included studies. Studies are presented by year of publication and in alphabetical order
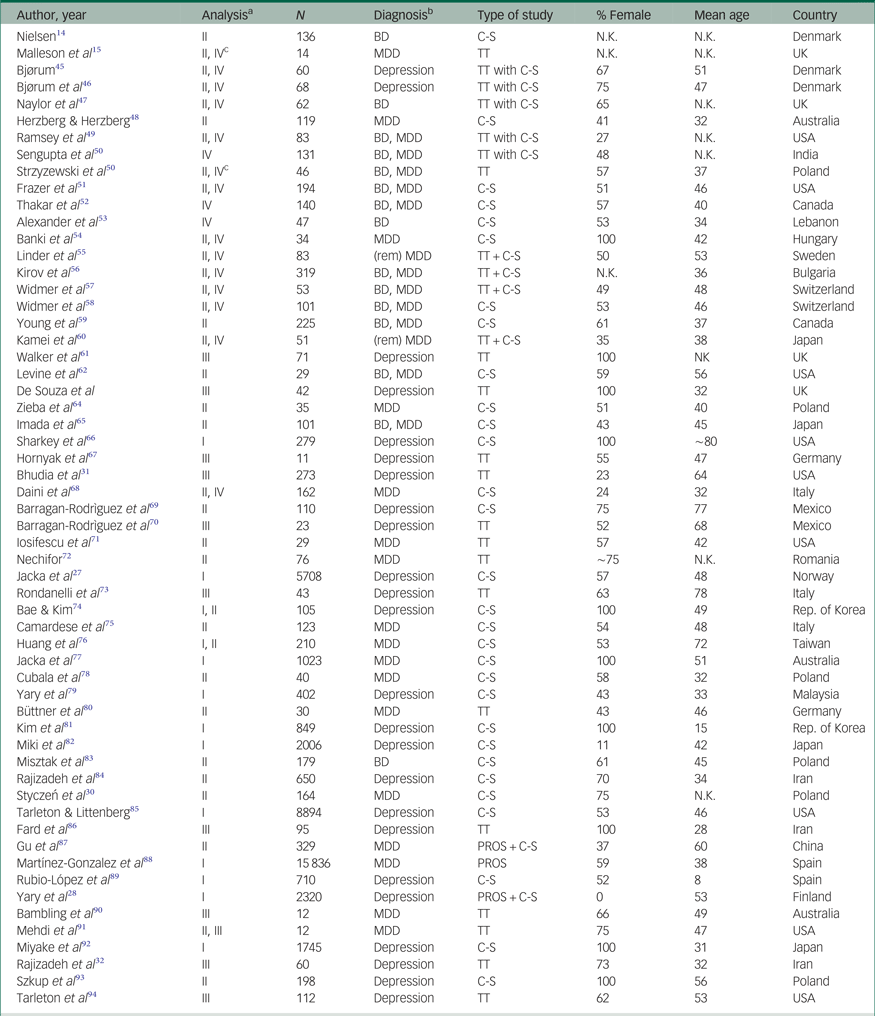
ADs, antidepressants; BD, bipolar disorder; C-S, cross-sectional; MDD, major depressive disorder; PROS, prospective; REM, remitted; TT, treatment trial.
a. This column indicates in which meta-analysis the study in the corresponding row was included:
I Dietary Mg2+ in relation to mood disorder prevalence and incidence; II Mg2+ in bodily fluids of patients and healthy control subjects or Mg2+ in relation to symptom severity; III Mg2+ supplements as an antidepressant; IV additional analyses ([1] differences in Mg2+ levels in bodily fluids between patients with mood v. other psychiatric disorders, [2] pre-post treatment (with antidepressants and/or mood stabilisers) differences in Mg2+ levels in bodily fluids, and [3] Mg2+ ATPase in erythrocytes or platelets; see Results section).
b. We distinguish depression from MDD here. Depression refers to self-reported symptoms, MDD to the diagnosed syndrome.
c. This study reported on changes in Mg2+ levels over the course of treatment in a single patient sample only.
Methodological quality of the included studies
In the online Supplementary Tables 2–9, we provide details on the quality assessment tools that we used. The assessment of study quality showed a high degree of agreement (~83% agreement; see the online supplement for more information) among two independent assessors (D.P. and M.M.). Item and total quality scores per eligible study are provided in Supplementary Tables 2–9. Methodological quality was not used as a criterion for inclusion or exclusion.
The overall methodological quality of the included studies was modest. In general, most studies applied valid statistical techniques, although statistical power was seldom reported. Methodological quality also was hampered by a lack of data on the representativeness of the sample, and drop-out and response rates. Most studies adjusted for confounding, ranging from almost absent adjustment to - in our view - thorough adjustment. Finally, for the treatment studies, no paper reported on the adequacy of randomisation and allocation concealment.
Dietary Mg2+ and the prevalence or incidence of unipolar depression/depressive symptoms
Adherence to a diet high in Mg2+ was associated with a lower prevalence of depression in cross-sectional studies (OR (highest versus lowest category) = 0.66, 95% CI = 0.51–0.81; P < 0.01, k = 12, n = 21 927), but not in longitudinal cohorts that assessed the incidence of new-onset depression (OR = 0.71, 95% CI = 0.40–1.02; P = 0.10, k = 2, n = 18 156).
Between-study heterogeneity in outcome was present in the cross-sectional studies assessing the association between dietary Mg2+ intake and depression prevalence, as was as evidence of publication bias (Figure 2A). Sample size was the only variable (Table 2) that was associated with between-study heterogeneity; smaller samples on average yielded stronger associations between dietary Mg2+ and mood disorder prevalence. The strength of this association, in terms of Spearman's rho (ρ), was 0.61. Correction for the presence of publication bias led to an attenuated, yet statistically significant, effect size estimate (OR = 0.84, 95% CI = 0.70–0.98).
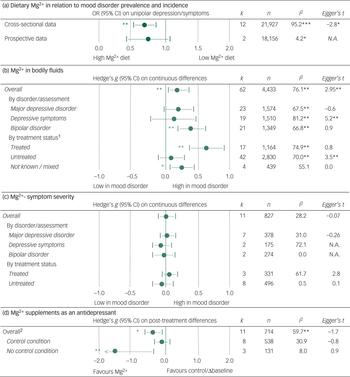
Fig. 2 Results of the meta-analyses, heterogeneity, and publication bias assessment. A: dietary Mg2+ intake was associated with prevalence of depression but not with incidence of depression. B: patients with mood disorders on average had higher levels of Mg2+, and this effect was driven by treatment status. C: Non-significant associations between the amount of Mg2+ in bodily fluids and mood disorder severity. E: Change in mood disorder symptoms over the course of treatment with Mg2+ supplements. 1: The effect-size estimate for differences in Mg2+ between patients with a mood disorder and healthy control subjects was significantly different for treated v. non-treated patients. 2: The effect-size estimate for changes in mood disorder symptoms was statistically significantly different at P < 0.01 when comparing studies that applied a (placebo) control v. those studies that compared pre- v. post-treatment scores.
Table 2 Meta-regression coefficients and standard error on the relation between study characteristics and effect-size estimates, separately for the different indicators that are in use to operationalise the hypothesis of Mg2+ involvement in mood disorders
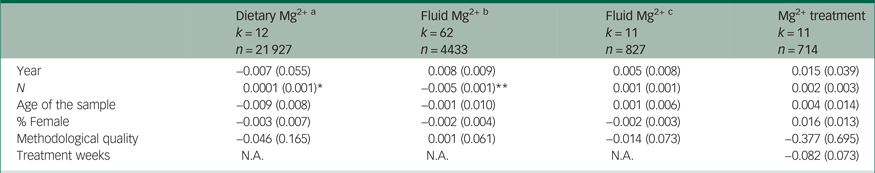
N.A., not applicable.
In order to aid with interpretation, we include a synopsis. Sample size was positively associated with the effect-size estimates in dietary studies; this indicates that smaller samples on average yielded stronger associations between dietary Mg2+ and depression prevalence (the strength of this association in terms of Spearman's rho (ρ) was 0.61). Sample size was negatively associated with the effect-size estimates in studies investigating differences in Mg2+ in bodily fluids between patients and healthy control subjects. This means that smaller samples on average yielded larger differences (the strength of this association was ρ = −0.42).
a. Results are presented for cross-sectional data only. There were only two prospective studies available and hence separate meta-regression analyses were not possible. Results from the analyses were no different when the prospective studies were pooled with the cross-sectional.
b. Mean differences in bodily fluid Mg2+ levels between patients with a mood disorder and healthy control subjects.
c. Continuous differences in bodily fluid Mg2+ levels as a function of mood disorder symptom severity.
* P < 0.05; **P < 0.01.
Between-study heterogeneity and publication bias could not be assessed in the analysis of depression incidence owing to the small number of studies.
There were no studies which reported on the effects of dietary Mg2+ on symptoms of bipolar disorder.
Mg2+ levels in bodily fluids as a function of mood disorder status
Sixty-two effect-size estimates were found for Mg2+ levels in bodily fluids by mood disorder status. Pooling these data showed higher Mg2+ levels in patients with a mood disorder, relative to healthy controls (g = 0.19, 95% CI = 0.05–0.36; P < 0.001, k = 62, n = 4433).
There was between-study heterogeneity (Figure 2B). A large part of this was due to treatment status, as Mg2+ levels in bodily fluids were particularly high in patients who were treated with antidepressants and/or mood stabilisers (P < 0.01 for the difference between treated and untreated samples). In fact, Mg2+ levels of untreated patients were no different from those of controls. Diagnostic status was also associated with heterogeneity, as the differences between patients and controls were larger for samples composed of bipolar depressed patients (Figure 2B) relative to patients with depressive symptoms/major depression. No evident heterogeneity resulted from the medium in which Mg2+ levels were determined (e.g. plasma versus serum).
A significant association between sample size and effect-size estimate was observed, indicating that smaller samples on average yielded larger differences in Mg2+ concentrations between patients and controls (ρ = −0.42; Table 2). Egger's t-tests and funnel plots suggested the presence of publication bias. Correcting for this led to non-significant between-group differences overall.
Mg2+ levels and symptom severity
Pooling 11 effect-size estimates that reported on continuous associations between Mg2+ levels and scores on mood disorder severity scales showed no evident association between these variables. In some instances, heterogeneity in outcomes was observed. However, this remained unexplained in subgroup and sensitivity analyses (Figure 2C).
Changes in mood disorder status following treatment with Mg2+ supplements
Eleven studies showed that Mg2+ supplementation was associated with a decline in symptoms (g = −0.44, 95% CI = −0.68 to −0.20; P < 0.01, k = 11, n = 714). This effect was restricted to uncontrolled studies (g = −1.62, 95% CI = −2.81 to −0.40) and was not observed in placebo-controlled studies (g = −0.22, 95% CI = −0.48–0.17; Figure 2D). The difference between effect-size estimates for controlled versus uncontrolled studies was significant. The remaining heterogeneity could not be explained by the specified moderators or publication bias (Figure 2D; Table 2).
Dosage of Mg2+ supplementation (range 225–4000 mg) and number of weeks of treatment (range 1–12) were unrelated to outcome.
Additional analyses
Three meta-analyses were performed which were not a priori defined but driven by the data that we encountered.
The first analysis explored between-group differences in Mg2+ levels in bodily fluids between patients with mood disorders versus other psychiatric disorders. Pooling 11 associations (n = 508) showed little evidence for the existence of such an association (g = −0.07, 95% CI = −0.47–0.33; P = 0.47).
The second analysis quantified pre–post treatment (with antidepressants and/or mood stabilisers) changes in Mg2+ levels in bodily fluids. A total of 17 effect-size estimates on this association (n = 223) showed no evidence for the existence of such changes (g = −0.09, 95% CI = −0.27–0.10; P = 0.36).
Finally, we pooled 13 effect-size estimates from three studies (n = 545) on between-group differences in Mg2+-ATPase (the enzyme that mediates the transport of Mg2+ across the cell membrane).Reference De Baaij, Hoenderop and Bindels1, Reference Niggli, Adunyah, Penniston and Carafoli95 We found higher Mg2+-ATPase activity in patients with depression relative to controls (g = 0.69, 95% CI = 0.42–0.93; P < 0.001).
Discussion
We quantitatively pooled the available human data on the involvement of Mg2+ in the pathophysiology of mood disorders. A summary and discussion of our results is presented below, arranged by the type of association investigated.
Dietary Mg2+ and the prevalence and incidence of mood disorders
We found that adherence to a diet high in Mg2+ was negatively associated with prevalence of depression in cross-sectional studies. Note that all studies investigated associations with major depression or depressive symptoms, but not bipolar disorder. This suggests that dietary Mg2+ intake may play a part in the pathology of depression. However, the cross-sectional design of these studies precludes any causal association or conclusions being made regarding the direction of the effect.
Furthermore, the sources of heterogeneity that we observed weaken the rationale for this association. Considerable between-study heterogeneity in outcome was observed, and sample size was the only variable which moderated this heterogeneity; studies that included fewer subjects tended to report a stronger association between dietary Mg2+ and prevalence of depression. We found evidence of publication bias when we used formal tests to assess this bias, which is in keeping with this small-study effect.Reference Nüesch, Trelle, Reichenbach, Rutjes, Tschannen and Altman96
The belief in an association between dietary Mg2+ intake and depression may be further weakened by the lack of a significant association between dietary Mg2+ intake and the incidence of depression in longitudinal studies (epidemiological cohorts). However, the number of longitudinal studies was limited, and not only was the point estimate for the effect from these studies rather similar to the pooled estimate for cross-sectional studies (ORs of 0.71 and 0.66, respectively), but their confidence intervals were also widely overlapping. This, together with the observation of between-study heterogeneity, leaves it open to debate on whether the effect is sufficiently strong as to be clinically relevant.
A lack of statistical evidence for the existence of an association in longitudinal studies could suggest reverse causation, i.e. in the depressed state, the likelihood of adhering to a diet low in Mg2+ may be increased. This is in line with evidence which demonstrates that mood disorders set the stage for a low-quality diet, which by extension is low in Mg2+.Reference Volpe5, Reference Darmon and Drewnowski97, Reference Stunkard, Faith and Allison98 Additionally, the evidence indicating that the quality of the diet may cause – de novo – depression is suggestive, but limited and not fully consistent.Reference Molendijk, Molero, Sánchez-Pedreño, van der Does and Martínez-González99 On the other hand, the results from two recent randomised trials Reference Jacka, O'Neil, Opie, Itsiopoulos, Cotton and Mohebbi100, Reference Parletta, Zarnowiecki, Cho, Wilson, Bogomolova and Villani101 suggest that dietary advice may alleviate depressive symptoms in patients who already are depressed, although it may be questioned whether this effect is solely due to a change of diet or to other factors such as selective expectancies.Reference Molendijk, Fried and van der Does102
Mg2+ levels in bodily fluids as a function of mood disorder status
Against expectations, we found higher Mg2+ levels in bodily fluids in patients with a mood disorder relative to healthy control subjects. This effect was moderated by treatment status; Mg2+ levels were high in patients treated with antidepressants and/or mood stabilisers and were not so in untreated patients. Perhaps this observation reflects the hypothesis that an increase in Mg2+ may underlie the clinical efficacy of (fast-acting) antidepressants.Reference Murck17 However, alternative explanations may account for this finding. Dehydration for instance is one; antidepressants and mood stabilisers decrease renal water reabsorption,Reference Vestergaard, Schou and Thomsen103 which can lead to dehydration, a common side-effect of antidepressants.Reference Kalisch Ellett, Pratt, Le Blanc, Westaway and Roughead104 This may result in artificially high concentrations of trace elements. Other potential confounding factors are presented below.
Notwithstanding the lack of a clear and single explanation for the higher levels of Mg2+ in treated patients, the similar Mg2+ levels in untreated patients and healthy control subjects suggest little involvement of (peripheral) Mg2+ in the pathophysiology of mood disorders.
Changes in mood following treatment with Mg2+ supplements
In line with expectations, we found that treatment with Mg2+ supplements was associated with a decline in depressive symptoms. This effect was moderated by study type. The supposed therapeutic efficacy of Mg2+ supplements on mood was only observed in uncontrolled studies; in controlled studies, they did not have a superior effect compared with placebo. Therefore, the effect of Mg2+ supplements on mood may merely represent a placebo effect. This finding does not corroborate the hypothesis that Mg2+ affects the pathophysiology of mood disorders.Reference Murck17, Reference Serefko, Szopa, Wlaź, Nowak, Radziwoń-Zaleska and Skalski19
Additional analyses
We performed three additional meta-analyses that were driven by the data that we encountered. The first of these showed no group differences in Mg2+ levels in bodily fluids in patients with mood disorders versus patients with other psychiatric disorders. The second provided no evidence for differences in Mg2+ levels pre- and post-treatment with an antidepressant and/or mood stabiliser. Finally, Mg2+–ATPase, the enzyme that mediates the transport of Mg2+ across the cell membrane,Reference De Baaij, Hoenderop and Bindels1, Reference Tarleton, Littenberg, MacLean, Kennedy and Daley94 showed higher activity in patients relative to healthy controls. The effect size of this association was large, but it was derived from only three studies.
We will not discuss these findings further given the limited number of studies and their exploratory nature.
Comparison with previous meta-analyses
Our findings stand out from two previous meta-analyses in that our analysis included a more comprehensive collection of articles, which were pooled by type of association.
Cheungpasitporn et al Reference Cheungpasitporn, Thongprayoon, Mao, Srivali, Ungprasert and Varothai34 pooled data from three studies on blood Mg2+ levels and two studies on dietary Mg2+ intake and concluded that hypomagnesaemia was related to depression. Our results are not in line with their conclusion. This discrepancy may be due to the heterogeneous nature of the studies pooled by Cheungpasitporn et al. Reference Cheungpasitporn, Thongprayoon, Mao, Srivali, Ungprasert and Varothai34 Furthermore, we do not speak in terms of hypomagnesemia, because the data do not allow that. As mentioned previously, hypomagnesemia refers to <0.7 mmol Mg2+l/L blood,Reference Al-Ghamdi, Cameron and Sutton9 and the included studies on Mg2+ in blood do not report on this; they report on continuous values instead. Additionally, information on hypomagnesemia cannot be estimated from diet. Hence, Cheungpasitporn et al Reference Cheungpasitporn, Thongprayoon, Mao, Srivali, Ungprasert and Varothai34 probably refer to low levels of Mg2+ when using the term hypomagnesemia.
Our findings from cross-sectional dietary data are similar to those reported by Li et al. Reference Li, Lv, Wang and Zhang35 What we add is the crucial separation between cross-sectional and prospective data. As we have shown, results from these two types of data are clearly distinct, with evidence for an association between dietary Mg2+ and depression in cross-sectional but not prospective studies.
Limitations
Our results should be interpreted in light of the following limitations, many of which relate to measurement error and confounding. In the case of confounding, it is likely that in our meta-analyses we overestimated the strength of associations. By contrast, with regards to measurement error, it is more likely that the effect-size estimates we reported on the associations of interest are an underestimation of the true effect. In extreme cases, measurement error may even have led to a lack of construct validity and an inability to assess certain associations.
Most studies that we reviewed were observational in nature, except for some treatment studies; therefore, our results may have been affected by residual confounding. For example, Mg2+ is derived from diet,Reference Bouzari, Holstege and Barrett3, Reference Talapatra, Ray and Sen4 and diet is influenced by income-related disparitiesReference Darmon and Drewnowski97, Reference Darmon and Drewnowski105 and many other such variables. Each of these variables may have effects on the outcome that are difficult to distinguish from the effects of Mg2+ intake. Another limitation related to the dietary data was that only one single assessment of dietary practices was applied in each of the included studies. One single assessment may not be enough to capture dietary habits and the dietary changes that may have occurred. Finally, the investigators of the included studies calculated the Mg2+ in nutrients in order to reach an overall Mg2+ estimate and in doing so ignored a relevant source of dietary Mg2+; tap and bottled water.Reference Azoulay, Garzon and Eisenberg106
The Mg2+ measurements in bodily fluids, as they were performed in the included studies, were also limited. First, they were all taken in peripheral tissues, while the pathophysiology of the mood disorders is believed to reside in the brain. Although positive correlations have been reported between central and peripheral Mg2+ parameters, there clearly is not a one-to-one relationship between them.Reference Hallak, Berman, Irtenkauf, Evans and Cotton107, Reference Morris108 Furthermore, the included studies extracted isolated Mg2+ parameters (e.g. Mg2+ levels from blood serum). This is a limitation because Mg2+ levels and receptor systems interact and as such probably define biological outcome; single measurements may simply not be rigorous or elaborate enough, and as such the findings in this field of study may lack construct validity.
A general limitation is that the mood disorders are highly heterogeneous, whereas in the included studies they were not conceptualised as such. Perhaps, subtypes of mood disorders exist in which Mg2+ plays an important part, and this is overlooked when broad disorders are included and presented as if they were the same outcome variable. Finally, the populations under study were largely Caucasian, sample sizes were generally quite small and follow-up periods were relatively short.
Future work
Future studies could assess multiple dietary and Mg2+ parameters at multiple time points and define their potential interacting effect on mood disorder incidence, course and subtype while accounting for time-related changes in other variables such as body mass index. Such an investigation would aid construct validity by reducing the potential influence of measurement error. Moreover, the study of Mg2+ and the mood disorders could use a certain amount of control, for instance in the form of randomly assigned long-term dietary interventions. This may reduce the potential influence of residual confounding on outcome. Ideally, such studies would be based on validated animal models and specific knowledge of the potential underlying mechanisms.
Conclusion
The question of interest here was whether Mg2+ is involved in the pathophysiology of the mood disorders. This association seems plausible, yet the results of our analyses by and large do not provide compelling evidence for the involvement of Mg2+ in mood disorders. Although this conclusion is based on the largest and most comprehensive body of human data to date, there are methodological and practical limitations that may have hindered valid assessment of the associations of interest. Future studies should aim to reduce confounding and measurement error in order to increase knowledge on the potential role of Mg2+ in the pathophysiology of the mood disorders.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2018.22.
Acknowledgements
We thank the authors who, upon request, provided us with data.
Appendices
Appendix 1 PRISMA checklist
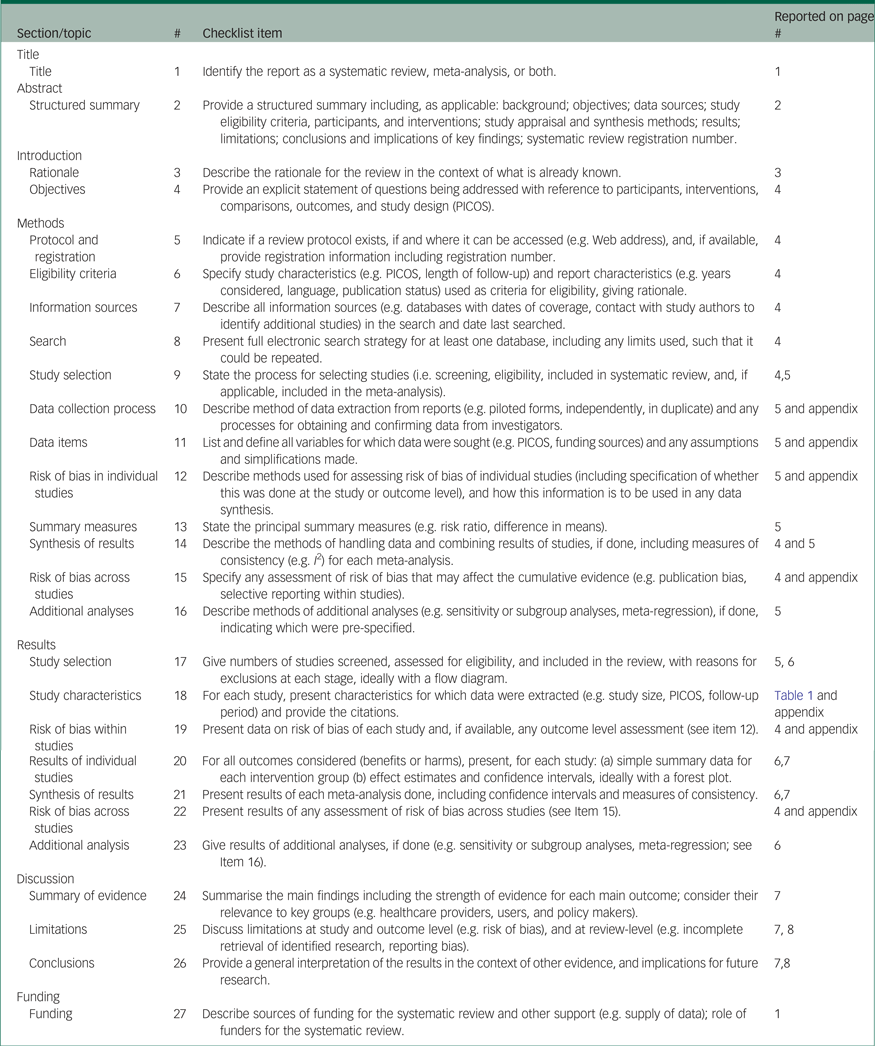
Appendix 2 MOOSE checklist

Appendix 3 Study protocol
Working title of the project
Magnesium and disorders of mood: a systematic review with meta-analyses
Review question(s)
1. Does mood disorder prevalence or incidence vary by dietary Mg2+ intake.
2. Do Mg2+ levels in bodily fluids vary by mood disorder status and severity.
3. Does Mg2+ supplementation have an effect on mood.
Searches
We conducted comprehensive searches in three major databases: PubMed, Web of Science, and Embase through December 2017. We used the following terms: (Magnesium OR Mg*) AND (depression OR depress* OR affect* OR mood OR mania OR bipolar).
The reference-lists of identified articles were scrutinised, as were the references that were made to the 2 seminal papers on the topic (Nielsen, 1964 and Malleson, Frizel, and Marks, 1968) to which, at the date of our latest search, 65 and 5 references were made respectively).
Nielsen J. Serum and erythrocyte magnesium in patients with manic states during lithium treatment. Acta Psychiatr Scand 1964; 40(2): 190–6.
Malleson A, Frizel D, Marks V. Ionized and total plasma calcium and magnesium before and after modified ECT. Br J Psychiatry 1968; 114(510); 631–33.
Types of study to be included
1. Cross-sectional or prospective studies or randomised controlled trials on the relation between dietary Mg2+ intake and the prevalence or incidence of a mood disorder (unipolar or bipolar depression of any kind).
2. Cross-sectional or prospective studies or randomised controlled trials on Mg2+ levels in bodily fluids as a function of mood disorder status and severity.
3. Open- or blinded trials (random and non-random, including one-group pre-post designs) reporting on the effects of Mg2+ supplementation on any type of mood outcome (e.g. self- and clinician rated questionnaires, diagnosis).
Condition or domain being studied
Psychiatry; mood disorders (unipolar or bipolar depression of any kind).
Participants/population
No restrictions
Intervention(s), exposure(s)
1. Dietary Mg2+ intake as measured by a food frequency questionnaire, recall, or diary.
2. Mood disorder status versus healthy control status including gradations in this defined by severity.
3. Mg2+ supplementation on any type and any dose.
Comparator(s)/ control
1. High versus low Dietary Mg2+ intake of any kind (e.g. continuous, highest quartile versus lowest quartile).
2. Healthy control condition.
3. Placebo (blinded and non-blinded), active control condition (blinded and non-blinded), pre-post measurement in a single group.
Outcome(s)
Primary outcomes (ABS)
Question 1. Prevalence and incidence of mood disorders.
Question 2. Blood levels (in any blood component/bodily fluid) of Mg2+.
Question 3. Changes in mood of any type.
Secondary outcomes
Not applicable
Data extraction
Two of the authors (Danny Phelan and Marc Molendijk) independently screened titles and abstracts of potentially eligible articles. When indicated, this was followed by a review of the full texts of potentially candidate papers. Any type of disagreement with regard to inclusion was resolved by consensus after discussion with a third author.
Risk of bias (quality) assessment
The Newcastle-Ottawa Scale (NOS) cohort version (Wells et al, 2016) was used to assess the methodological quality of the included cross-sectional studies on the association between dietary Mg2+ intake and the prevalence of mood disorders.
The prospective cohort studies on the relation between dietary Mg2+ intake and the incidence of mood disorders were assessed regarding their methodological quality by using the method proposed by Lievense et al (2002).
The NOS case-control version (Wells et al, 2016) was used to assess the methodological quality of the included cross-sectional studies on the association between abnormalities in Mg2+ levels in blood components/bodily fluids as a function of mood disorder status.
Methodological quality of treatment trials on changes in mood over the course of Mg2+ supplementation was assessed by means of the method of evaluation of (randomised) trials provided by the US Department of Health and Human services (2016).
Lievense AM, Bierma-Zeinstra SMA, Verhagen AP, Van Baar ME, Verhaar JAN, Koes BW. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatol, 2000; 41(10): 1155–62.
The US Department of Health and Human services, National Heart, Lung and Blood Institute http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/rct.
Wells, G. A., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2013). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2009. Epub Available from: http://www.ohri.ca.
Strategy for data synthesis
Quantitative synthesis will be performed by means of random-effects meta-analyses performed in STATA version 13 (2013).
StataCorp LP. (2013). Stata Statistical Software: Release 13-statistical software. College Station, TX.
Analysis of subgroups or subsets
To examine the potential source of heterogeneity across studies, the following sensitivity analyses (per question) were conducted:
Question 1. Analyses by study type (cross-sectional / prospective studies / randomised controlled trials)
Question 2. Analyses by disorder (major depressive disorder / depressive symptoms / bipolar disorder / mania), treatment status (antidepressants / electroconvulsive therapy / untreated / not known), blood component / bodily fluid (plasma / serum / urine / cerebrospinal fluid).
Question 3. Analyses by disorder (major depressive disorder / depressive symptoms / bipolar disorder / mania), control condition (yes / no).
Sources of heterogeneity were also investigated by means of meta-regression analyses with sample size, average age of the sample, female percentage of the sample and methodological quality of the study as predictor. For the third question we also regressed number of weeks of treatment and Mg2+ on outcome.
Organisational affiliation of the review
None
Anticipated or actual start date
July 2016
Anticipated completion date
December 2017
Funding sources/sponsors
The review and meta-analyses were supported by a Leiden University research appointment (Marc Molendijk).
Language
English
Country
The Netherlands
Subject index terms
Depression, mood, bipolar disorder, mania, trace-elements, magnesium, Mg2+, diet, review, meta-analysis







eLetters
No eLetters have been published for this article.