LEARNING OBJECTIVES
After reading this article you will be able to:
• demonstrate up-to-date understanding of the current use of nitrous oxide
• demonstrate improved understanding of the neuropsychiatric effects of nitrous oxide use, low vitamin B12 and high homocysteine
• demonstrate increased understanding of the role of vitamin B12 and the impact of functionally inactive vitamin B12.
Medical use of nitrous oxide
Nitrous oxide (N2O) can be commonly found as a component in prescribed medication. Alongside this, it is used as a recreational substance. In its prescribed form, N2O is a gas indicated for use in combination with other anaesthetic agents and for short-term analgesia when used in a 50:50 combination with oxygen as Entonox®. This is beneficial where rapid onset and clearance are needed (Savage Reference Savage and Ma2014; BOC 2015; Heads of Medicines Agencies 2019). The analgesic, sedative and cognitive effects of N2O are dose-dependent (Heads of Medicines Agencies 2019). Analgesic effects are obtained at a 25% concentration of N2O and so continuous inhalation is not needed; rather, it is given through a demand valve operated as the patient inhales (BOC 2015). Contraindications for medical use of N2O include respiratory or cardiac risk factors such as pneumothorax and persistent signs of confusion; caution should also be used if there is a risk of exacerbating folate or vitamin B12 deficiency (Heads of Medicines Agencies 2019).
Interestingly, there is ongoing investigation into the potential role of N2O in reducing withdrawal symptoms for those reducing use of other substances such as alcohol or cocaine. Furthermore, a role in depression treatment is being considered (Amsterdam Reference Amsterdam, Nabben and van den Brink2015).
Recreational use of nitrous oxide
Randhawa & Bodenham (Reference Randhawa and Bodenham2016) described an increasing trend for use of N2O as a recreational drug, in part because it is comparatively simple and inexpensive to obtain, owing for example to its common use in the catering events industry. N2O is often bought for recreational use in the form of small pressurised canisters, which are then released into balloons for inhalation (Sheldon Reference Sheldon, Reid and Schon2020). These cannisters contain variable percentages of N2O (Randhawa Reference Randhawa and Bodenham2016; Fidalgo Reference Fidalgo, Prud'homme and Allio2019).
The effect of this is rapid onset (within 10 s) of euphoria, disinhibition, analgesia and in some cases a hallucinogenic effect which is short lasting without substantial hangover effects. Although this is usually well tolerated, in those with pre-existing risks such as epilepsy or cardiac disease, or if combined with centrally acting arrhythmogenic drugs, the potential for seizures, arrhythmias and respiratory or cardiac arrest is raised. Furthermore, any vomiting may present an aspiration risk (Amsterdam Reference Amsterdam, Nabben and van den Brink2015; Randhawa Reference Randhawa and Bodenham2016). In the event of N2O overdose, potential consequences include hypoxaemia, unconsciousness and ischaemia (Heads of Medicines Agencies 2019). Treatment for N2O overdose is focused on stopping administration and supportive oxygenation (BOC 2015).
When inhaled from a balloon there is a similar safety net as with the valve in medicinal use, as if the user becomes overly sedated, they will not be able to maintain administration. However, if administered as a continuous flow, this will not apply, significantly increasing the risk of adverse effects and overdose (Randhawa Reference Randhawa and Bodenham2016). Furthermore, in obtaining this drug for recreational use, there is an increased risk that manufacturing safeguards present for medical products will not be present and also that users might administer together with other drugs or alcohol (Randhawa Reference Randhawa and Bodenham2016). When N2O is used, it is often as multiple administrations over a few hours. In heavy use, balloons may be filled from larger tanks rather than small canisters (Amsterdam Reference Amsterdam, Nabben and van den Brink2015).
Extent of recreational use
In 2016, 9% of schoolchildren in England reported being offered N2O, with 2.4% of 16- to 59-year-olds reporting use in the previous year (2016–2017) and 2.3% in 2017–2018. In children and young adults, N2O was found to be the second most used drug after cannabis, with 9% of 16- to 24-year-olds reporting use in 2016–2017 and 8.8% in 2017–2018, with this age group forming the majority of users (Home Office 2018). In 2016 there were 8 deaths recorded due to the use of N2O in England and Wales, notably an increase from 0–5 deaths recorded each year between 2001 and 2015; however, whether the deaths resulted from recreational or medical use is not specified (Office for National Statistics 2018a, 2018b). After combining data from multiple studies, Sheldon et al (Reference Sheldon, Reid and Schon2020) concluded that use was highest amongst young males and in high income areas such as the UK.
The most recent Global Drug Survey was reported in 2020 and collects data from over 25 countries. In their population, use of N2O was reported by 13.1% of respondents in 2020, with 11.9% reporting use in 2019 and 6.9% in 2018. Comparatively, lysergic acid diethylamide (LSD) use was reported by 21% and ketamine by 15.9% in 2020. Although this is a large data-set, the data are collected using non-probability sampling methods and so it is difficult to generalise from their findings (Winstock Reference Winstock, Timmerman and Davies2020).
The majority of nitrous oxide users take less than 10 balloons during one episode and 80% of users have less than 10 episodes of use per year. Around 1% of users take more than 50 cannisters in a session, with a small subgroup of heavy users requiring the equivalent of 75–125 cannisters to remain under the influence. Overall, quantity of use is highly variable (Amsterdam Reference Amsterdam, Nabben and van den Brink2015; Garakani Reference Garakani, Jaffe and Savla2016).
Nitrous oxide and UK law
In the UK, nitrous oxide is a psychoactive drug covered by the Psychoactive Substances Act 2016, meaning that it is illegal to supply and produce for its psychoactive effect, with those convicted facing up to 7 years in prison and an unlimited fine. There is currently no penalty for possession (unless already in prison) but you could receive a fine, driving ban or prison sentence if found using N2O while driving (Crown Prosecution Service 2017; FRANK 2020). Within the first 12 months following the introduction of the 2016 Act, there were 202 records of police across 32 forces in England and Wales seizing N2O (Home Office 2018).
Addictive potential
Addiction is a potential risk where there has been repeated administration of N2O, with a frequency that is not yet clear in the drug safety data (BOC 2015; Heads of Medicines Agencies 2019). Alhough there is not felt to be a physiological addiction, use may be habit forming, with associated financial and social implications (Randhawa Reference Randhawa and Bodenham2016).
Fidalgo et al (Reference Fidalgo, Prud'homme and Allio2019) recently conducted a systematic review considering misuse or dependence on N2O and EMONO (equimolar mixture of oxygen and nitrous oxide – a premixed combination of 50% oxygen and 50% N2O) defined using DSM-5 criteria. They reported higher misuse in men, adolescents and young adults. Interestingly, they reported that alongside recreational use, there were cases in which people taking N2O combinations for initially medical purposes later developed a substance use disorder. In some cases, this occurred when EMONO was given as a medical intervention; when it was stopped, withdrawal symptoms and tension with the medical team were reported. Overall, they concluded that there was the potential for substance use disorder to develop.
Mechanism of action
Nitrous oxide modulates central nervous system neurotransmitters, including noradrenergic transmission, gamma-aminobutyric acid (GABA) receptor systems, N-methyl-d-aspartate (NMDA) antagonism and endogenous opioid systems, leading to anxiolytic and anaesthetic effects (Savage Reference Savage and Ma2014; Heads of Medicines Agencies 2019; Sheldon Reference Sheldon, Reid and Schon2020). The TREK-1 potassium channel has also been implicated in the anaesthetic effects of N2O and in pain perception (Savage Reference Savage and Ma2014). Furthermore, there is evidence of influence on dopaminergic transmission in the nucleus accumbens reward system and of cross-tolerance with morphine (Fidalgo Reference Fidalgo, Prud'homme and Allio2019; Sheldon Reference Sheldon, Reid and Schon2020).
N2O reportedly causes functional vitamin B12 deficiency (Sheldon Reference Sheldon, Reid and Schon2020), where vitamin B12 remains present, but in an inactivated form and thus unable to function via its normal role. N2O inactivation of B12 is via oxidation of the cobalt ion, preventing the conversion of homocysteine to methionine (which plays a role in myelination), along with the conversion of 5-methyltetrahydrofolate to tetrahydrofolate (role in DNA synthesis), and finally, helping convert L-methylmalonyl-Co-A to succinyl-Co-A (involved in the citric acid cycle) (Sheldon Reference Sheldon, Reid and Schon2020).
N2O is excreted unchanged primarily through alveolar ventilation and exhalation (BOC 2015; Heads of Medicines Agencies 2019). Following a brief inhalation, adverse psychomotor effects are rarely evident after 20 min although neuropsychiatric effects such as those mediated through vitamin B12 inactivation may persist, particularly with prolonged use (Heads of Medicines Agencies 2019).
Manifestations of persistent nitrous oxide use
Persistent nitrous oxide misuse can lead to a range of neuropsychiatric and medical presentations. These are summarised in Table 1.
TABLE 1 Manifestations of nitrous oxide misuse

Sources: Amsterdam et al (Reference Amsterdam, Nabben and van den Brink2015); Garakani et al (Reference Garakani, Jaffe and Savla2016); Kaar et al (Reference Kaar, Ferris and Waldron2016); Shoults (Reference Shoults2016); Heads of Medicines Agencies (2019); Chien et al (Reference Chien, Huang and Chen2020); Samia et al (Reference Samia, Nenow and Price2020).
A systematic review by Garakani et al (Reference Garakani, Jaffe and Savla2016) investigated the neuropsychiatric and medical consequences of nitrous oxide misuse. Their data searches combined information from 91 cases of misuse resulting in neuropsychiatric or medical problems across 77 publications. Of the 91 cases reported, 72 recorded neurological symptoms, including myeloneuropathy and subacute combined degeneration of the spinal cord; 11 cases reported psychiatric symptoms such as psychosis and 8 included medical effects such as pneumomediastinum and frostbite. Across these 91 cases, 52 of the patients had low or low-normal vitamin B12 levels and some had normal B12 levels even in the presence of significant symptoms. Where the data were available, the reviewers commented that quantity and duration of use was linked to sequelae. More often, when use was daily and multiple whippets/cartridges were used (they defined the N2O content of one whippet/cartridge as 8 g of 100% N2O) more sequelae were observed. Alongside this they recorded 11 publications reporting 29 deaths resulting from nitrous oxide misuse. Death was often associated with significantly increased self-administration directly from a larger tank and was as a result of cardiac arrhythmia and hypoxia.
Kaar et al (Reference Kaar, Ferris and Waldron2016) reported in large a cohort of 4883 nitrous oxide users that common experiences included hallucinations (27.8%), confusion (23.9%) and persistent numbness (4.3%), alongside accidental injury (1.2%). Accidental injury in particular was shown to be a dose-dependent effect. Amsterdam et al (Reference Amsterdam, Nabben and van den Brink2015) reported that accidental injury in nitrous oxide use was linked with dizziness, disorientation, loss of balance and cognitive changes following use, meaning falls were more likely to occur. They further report presentations of hypothermic skin trauma when N2O is used in larger quantities while being dispensed directly from a larger tank tap.
Chien et al (Reference Chien, Huang and Chen2020) combined data from seven cases of people with a history of severe nitrous oxide use disorder presenting for treatment of psychiatric manifestations. Five of these patients described daily use of nitrous oxide and the other two respectively reported use 2–3 times a week and weekly. Duration of use was between 4 months and 10 years. Primary symptoms included suicide attempts or suicidal thoughts, violent behaviours or irritability and psychosis symptoms such as hallucinations and delusions. Three of the seven people required a period of in-patient treatment in view of symptom severity. However, six of the seven had a history of mood disorders, including major depressive disorder and bipolar disorder. Furthermore, all were reported to have a history of substance misuse often including ketamine and 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), making it more difficult to establish the role of nitrous oxide use alone.
Indeed, combining the available evidence there appears to be a common pattern linking severity of symptoms to heavy and prolonged use or ‘abuse’ of nitrous oxide.
Vitamin B12 metabolism
Vitamin B12 is found in meat, fish, eggs and milk and the average daily diet contains approximately 5–30 μg, of which 2–3 μg is absorbed. The average adult will store 2–3 mg in the liver and so it may take up to 2 years before a deficiency becomes evident, as daily losses are small (Kumar Reference Kumar and Clark2020; Mukku Reference Mukku, Suhas and Thippeswamy2018). Vitamin B12 is liberated from proteins by gastric enzymes, where it then becomes attached to a vitamin B12 binding protein (R-binder) and later is released by pancreatic enzymes, to instead become bound to intrinsic factor. When bound to intrinsic factor it is then transported to specific mucosal cells in the ileum. Here the intrinsic factor remains in the lumen of the ileum and vitamin B12 is transported to the bone marrow and other tissues by glycoprotein transcobalamin II (TC II). Approximately 1% of an oral dose of vitamin B12 is absorbed passively through the duodenum and ileum without the need for intrinsic factor (Kumar Reference Kumar and Clark2020). This process is summarised in Fig. 1.
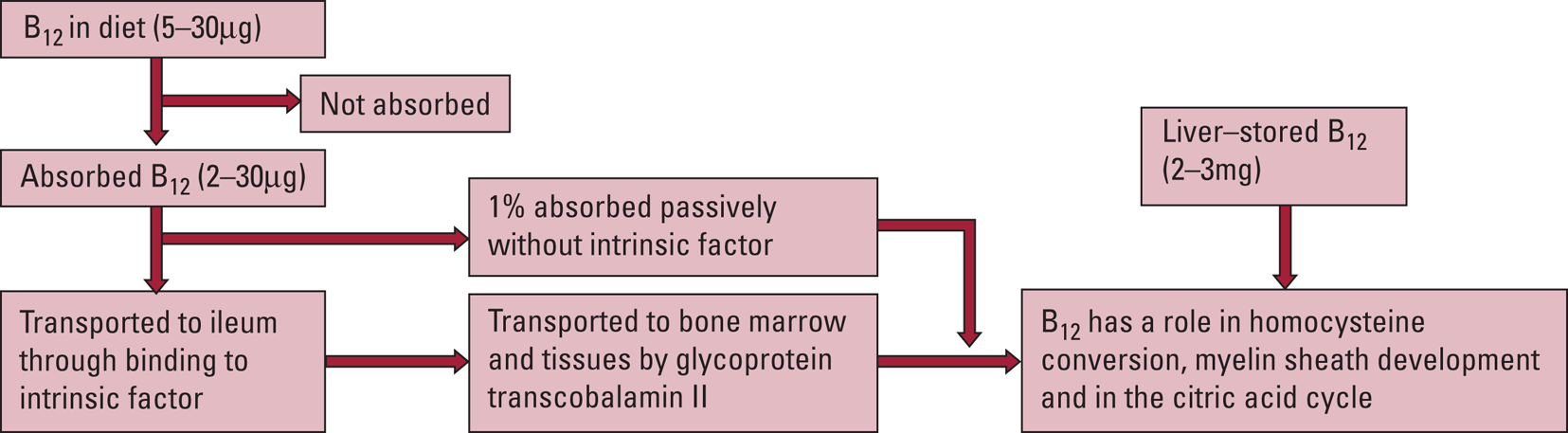
FIG 1 Summary of B12 metabolism (Kumar Reference Kumar and Clark2020).
Vitamin B12 deficiency or inactivation
The most common cause of vitamin B12 deficiency is pernicious anaemia. This is an autoimmune disorder with loss of parietal cells and subsequent reduction in intrinsic factor production, which leads to vitamin B12 malabsorption. Comparatively, nitrous oxide use is a recognised cause of vitamin B12 inactivation (Kumar Reference Kumar and Clark2020). Those at increased risk of vitamin B12 deficiency also include older adults and those who are pregnant or follow strict vegetarian diets (Mukku Reference Mukku, Suhas and Thippeswamy2018).
Vitamin B12 ordinarily plays a role in converting homocysteine to methionine in order to support myelin sheath development and has further roles within the citric acid cycle. In view of this, when levels are low or it is inactivated by compounds such as N2O, homocysteine and methylmalonic acid accumulate. It is possible to measure both homocysteine and methylmalonic acid levels to assess the extent of inactivation, as measurement of vitamin B12 level alone will not demonstrate the proportion of vitamin B12 able to complete its intended functions (Sheldon Reference Sheldon, Reid and Schon2020). The lowest bracket of the vitamin B12 normal range in blood plasma is 160 ng/L and significant neurological changes can occur when levels drop below 60 ng/L (Kumar Reference Kumar and Clark2020). More recently, the National Institute for Health and Care Excellence (NICE) guidance stated that a serum level of under 200 ng/L (148 picomol/L) will diagnose 97% of people with a vitamin B12 deficiency (NICE 2019).
High or prolonged Entonox use has the potential to result in neurological and haematological effects similar to those of other causes of low vitamin B12 such as impaired dietary intake or pernicious anaemia (BOC 2015). However, neurological toxicity may occur without anaemia or macrocytosis and with vitamin B12 laboratory levels appearing to be in the normal range, as Entonox can cause existing B12 to become inactive (GOV.UK 2014; BOC 2015; Heads of Medicines Agencies 2019; Sheldon Reference Sheldon, Reid and Schon2020). Therefore, clinicians should interpret B12 levels with caution where there has been nitrous oxide use.
Neuropsychiatric presentations of low functional vitamin B12 levels
Neuropsychiatric changes linked to low vitamin B12 include neuropathy, myelopathy and psychiatric disturbances such as symptoms of depression, psychosis or cognitive dysfunction, and these may occur prior to haematological changes such as anaemia (Hutto Reference Hutto1997; Lerner Reference Lerner and Kanevsky2002; Kumar Reference Kumar and Clark2020; Bar-Shai Reference Bar-Shai, Gott and Marmor2011; Mukku Reference Mukku, Suhas and Thippeswamy2018; NICE 2019; NHS 2020). It is not always clear whether the vitamin deficiency caused the psychiatric presentation or was a consequence of it, given the potential for psychiatric illness to disrupt appetite and dietary intake, making analysis of case reports challenging (Hutto Reference Hutto1997).
A systematic review and meta-analysis by Petridou et al (Reference Petridou, Kousoulis and Michelakos2016) identified 11 studies that explored the relationship between folate and depression in older adults, of which 9 also considered vitamin B12. The 9 studies with data on vitamin B12 included 6308 participants across multiple countries. Depression was linked to low vitamin B12, with an overall odds ratio of 1.20 (95% CI 1.02–1.42).
A recent cross-sectional study of 303 participants within the Bhil indigenous population of India reported that those with depression and anxiety did not have a different distribution of folate and B12 status when compared with those without these disorders. However, it did demonstrate that high homocysteine, mediated by vitamin B12 deficiency, was linked to an increased risk of depression and anxiety disorders. In this study the cut-off used for vitamin B12 deficiency was higher than the NICE guidance levels above as they used <220 picomol/L and in this case the cause of the vitamin deficiencies was felt to be linked to difficulties in obtaining adequate nutrition linked to the lower economic resources of the studied group (Saraswathy Reference Saraswathy, Ansari and Kaur2019). Similarly, in a smaller study of 66 individuals in a Greek population who were aged 60 years or more Dimopoulos et al (Reference Dimopoulos, Piperi and Salonicioti2007) reported a correlation between depression and low folate and/or B12 as well as higher plasma homocysteine. They noted that this is important as there is the potential for homocysteine accumulation to cause neurotoxic effects.
Sun et al (Reference Sun, Li and Lai2019) described a patient who presented with slowed reactions, apathy and communication difficulties who was initially treated for depression without success. He later developed weakness in his lower limbs, urinary incontinence and cognitive impairment, alongside difficulty in movements and gait and was demonstrated to have changes in his cerebellum, dorsal and lateral columns of the spinal cord and hydrocephalus. Further blood test analysis identified anaemia, low vitamin B12, elevated homocysteine and he was positive for anti-intrinsic factor antibodies. Once treatment for his hydrocephalus and low B12 had taken place, both his neurological and psychiatric symptoms improved. This highlights the importance of assessing for B12 levels in those presenting with depression, although in this case the cause of low B12 was linked to autoimmune abnormalities in relation to intrinsic factor rather than nitrous oxide use. Similarly, a further case report by Bram et al (Reference Bram, Bubrovsky and Durand2015) focused on B12 deficiency caused by pernicious anaemia reported that the patient's first presentation was with symptoms of insomnia, general slowing and anxiety, with later examination demonstrating symptoms of catatonia. In this case they were successfully treated with increased cyanocobalamin (vitamin B12) administration and lorazepam (Bram Reference Bram, Bubrovsky and Durand2015).
A presentation of psychosis and depression without other neurological signs was reported by Bar-Shai et al (Reference Bar-Shai, Gott and Marmor2011). In this case the 64-year-old male patient experienced auditory hallucinations, delusional content, core depressive symptoms such as low mood and anhedonia, and difficulties with short-term memory. Delusional content included thoughts of being terminally ill, fear of being watched by police and the tax department, and a fear of being poisoned. Importantly, this person had a history of chemotherapy for lymphoma and so was extensively investigated to ensure there was no recurrence of malignancy. He had also had a resection of the terminal ileum during this period, the primary site of B12 absorption, and had not received prophylactic supplements of vitamin B12. He was not found to be anaemic, but he did have low B12 levels. He was started on antipsychotics and antidepressants for 2 weeks without success and then on hydroxocobalamin once the B12 level was available. Within 1 week of commencing hydroxocobalamin, there was a complete resolution of symptoms. Although it is possible that this could be linked to the effects of antipsychotics and antidepressants becoming more visible, these were subsequently reduced with a goal of complete discontinuation and there was not an associated relapse in symptoms (Bar-Shai Reference Bar-Shai, Gott and Marmor2011).
A similar rapid symptom resolution was described in a case reported by Lerner & Kanevsky (Reference Lerner and Kanevsky2002). This 52-year-old man was admitted to a psychiatric ward for disturbance in memory, depressive mood, apathy and disorientation and later developed distressing hallucinations and delirium. He had experienced poor nutrition prior to this episode and had lost 10 kg in 6 months; this was thought to be linked to significant life stressors. Laboratory blood test analysis demonstrated low B12 and raised homocysteine levels, and treatment with vitamin B12 replacement resulted in symptom resolution within 7 days.
Sheldon et al (Reference Sheldon, Reid and Schon2020) described the case of a patient developing tactile, auditory and visual hallucinations alongside delusional content. This later developed into myeloneuropathy and symptoms were thought to be resulting from inactivated B12 following inhalation of a high quantity of N2O. Overall, they suggested that for weakness, numbness, paraesthesia or psychiatric effects in the context of known nitrous oxide use, tests should be conducted to measure not only B12 but also homocysteine and methylmalonic acid, which accumulate when there is B12 inactivation.
Seizures
M.K. reports a case of recurrent seizures in the context of B12 deficiency (Kumar Reference Kumar2004). This person responded well to B12 replacement. He reports that the exact mechanism by which seizure phenomena are caused is less established compared with other neurological effects; however, he felt it was likely to be linked to the potential for low B12 to cause myelin sheath damage to cerebral neurons and the altered susceptibility to glutamate effects. More recently Silva et al (Reference Silva, Velosa and Barahona-Corrêa2019) described functional deterioration, psychosis and seizures in the context of B12 deficiency in pernicious anaemia. With B12 correction their patient was able to make a significant recovery.
Similarly, Lee et al (Reference Lee, Chang and Wu2005) report the case of a patient who presented with generalised tonic–clonic seizures who was given a diagnosis of epilepsy of unknown etiology and anti-epileptic treatment. This person later developed some loss of sensation, cognitive impairment and altered reflexes and was found to have a very low B12 level and high homocysteine, with normocytic anaemia. Following B12 treatment, neuropsychiatric symptoms normalised with no further seizures reported and gait normalising after 6 months. They concluded that B12 deficiency should be considered when attempting to identify the cause of seizures.
Alongside the risk of seizures linked to low B12, seizures are listed as a side-effect in the summary of product characteristics data for N2O, although the frequency of this effect is as yet unclear (Heads of Medicines Agencies 2019). This ambiguity is noted by Zier & Doescher (Reference Zier and Doescher2010), who reported three cases of clinical seizure activity in children which were temporally related to procedural sedation with N2O, but they were unable to fully establish causality.
Mechanisms of neurotoxicity
There are multiple mechanisms proposed to account for the neurotoxicity associated with both nitrous oxide use and low vitamin B12. Savage & Ma (Reference Savage and Ma2014) reported that the brain is most vulnerable to N2O-associated neurotoxicity during early development (specifically, the perinatal period) and in older adulthood; the majority of this evidence came from studies in rat populations. Neurotoxicity is linked to NMDA antagonism, altered cerebral blood flow and enzyme inhibition, with each process being modulated by existing brain conditions (Savage Reference Savage and Ma2014).
NMDA receptors are excitatory receptors that respond to endogenous glutamate. NMDA antagonists can have both protective and harmful effects, depending on their activation. Prolonged exposure can result in neuronal cell death and some NDMA antagonists have demonstrated the ability to alter hippocampal synapses and pathways involved in learning and memory (Savage Reference Savage and Ma2014).
N2O binds to the cobalt atom in vitamin B12 and causes its inactivation. B12 is a cofactor for methionine synthase and so there is disruption in the metabolic pathways converting homocysteine to methionine. Homocysteine itself does not appear to have a role outside of this process, although accumulation can lead to neurotoxicity, atherosclerosis and cardiovascular dysfunction, with its primary mechanisms of cellular damage being oxidative stress, mitochondrial dysfunction and depletion of the myelin layer (Lerner Reference Lerner and Kanevsky2002; Savage Reference Savage and Ma2014; Mukku Reference Mukku, Suhas and Thippeswamy2018).
Homocysteine levels can take up to a week to normalise after N2O administration despite the N2O itself being rapidly cleared. Notably, methionine synthase inactivation can begin as early as 40 min into administration of 50% N2O compounds (Savage Reference Savage and Ma2014). Folate functioning is also altered when B12 is low or inactive, and this in itself can have neuropsychiatric complications, with both folate and B12 implicated in monoamine synthesis alongside methionine cycles (Hutto Reference Hutto1997; Lerner Reference Lerner and Kanevsky2002; Bar-Shai Reference Bar-Shai, Gott and Marmor2011; Mukku Reference Mukku, Suhas and Thippeswamy2018; Allott Reference Allott, McGory and Yuen2019). Increased synthesis of tetrahydrobiopterin (BH4) has further been proposed as a potential mechanism through which nitrous oxide use can be linked to experiences of psychosis, as this is also involved in synthesis of monoamines such as dopamine (Garakani Reference Garakani, Jaffe and Savla2016).
Risk factors for high homocysteine notably include Alzheimer's disease, vitamin B12 deficiency and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene (Savage Reference Savage and Ma2014; Allott Reference Allott, McGory and Yuen2019). Interestingly, high maternal homocysteine in the perinatal period is linked to increased risk for later development of schizophrenia in the offspring. Furthermore, single nucleotide polymorphisms (SNPs) altering homocysteine levels are one of many genetic factors linked to increased psychosis risk (Allott Reference Allott, McGory and Yuen2019; Romain Reference Romain, Eriksson and Onyon2019). High homocysteine has been observed from illness onset in first-episode psychosis and has been linked to higher negative symptom scores (Allott Reference Allott, McGory and Yuen2019). The MTHFR gene is involved in the regulation of folate, and polymorphisms here have been linked to schizophrenia, particularly negative symptoms (Allott Reference Allott, McGory and Yuen2019).
In view of the concerns regarding N2O and functional vitamin B12 deficiency, a drug safety update from the UK's Medicines and Healthcare products Regulatory Agency (MHRA) reports that vitamin B12 levels should be considered prior to administration of N2O where possible, particularly for those with risk factors for vitamin B12 deficiency. It suggests that N2O should not be given continuously for longer than 24 h or more frequently than every 4 days without close supervision and blood test monitoring, although it recognises that symptoms may occur in the absence of haematological changes (GOV.UK 2014).
Use in pregnancy
Nitrous oxide has been used as a simple inhalant anaesthetic agent during obstetric care of women in labour and is present on most UK labour wards as part of normal care. From available research, there appears little in the way of evidence showing negative effects when used as a short-term anaesthetic option during labour. More research, however, exists into the effects of repeated environmental exposure to N2O of healthcare workers involved in the obstetric teams caring for these patients (Rowland Reference Rowland, Baird and Weinberg1992; Ahlborg Reference Ahlborg, Axelsson and Bodin1996). Ahlborg et al suggest that there is an increased rate of infertility and association with longer time to conception among these cohorts, thought to be linked to increased exposure to N2O.
Utility of vitamin supplementation
When discussing the neuropsychiatric presentations of low vitamin B12, many case reports described a positive symptom response to supplementation (Lerner Reference Lerner and Kanevsky2002; Bar-Shai Reference Bar-Shai, Gott and Marmor2011; Bram Reference Bram, Bubrovsky and Durand2015; Sun Reference Sun, Li and Lai2019).
Similarly, Lan et al (Reference Lan, Kuo and Chou2019) identified nine patients presenting with symptoms of subacute combined degeneration of the spinal cord (SCD) in the context of nitrous oxide misuse. Of these, four patients were found to have low serum vitamin B12 and eight were shown to have raised homocysteine. When treated, they observed full recovery of muscle power, but persistence of sensory deficit symptoms was noted in five patients and sensory ataxia in one. They therefore suggested prompt treatment with vitamin replacement in patients with chronic nitrous oxide misuse to avoid permanent neurological damage. Samia et al (Reference Samia, Nenow and Price2020) followed a patient case of N2O-induced SCD, identifying low vitamin B12 levels. Following a treatment regime of 7 days of daily vitamin B12 injections and subsequent once weekly injections, the patient reported overall symptomatic improvement. However, their results were less positive for some neurological symptoms, as ataxia and neuropathy associated with loss of vibratory sense and proprioception persisted.
Allott et al (Reference Allott, McGory and Yuen2019) conducted a randomised controlled trial (RCT) in a first-episode psychosis out-patient cohort in which they randomised 120 individuals with psychosis to either the placebo control group or the group who were given adjunctive supplements of B12, B6 and B9 (folate) for 12 weeks with the aim of assessing whether reducing homocysteine levels could be therapeutic. Only 14% of their total cohort had raised homocysteine at baseline. They measured change in homocysteine level, psychosis symptoms and neurocognition. During baseline testing they also assessed for genotypes, including the MTHFR gene, but did not find any interactive effects in this case. This study demonstrated reduced homocysteine in the supplemented group but did not demonstrate any benefit over placebo with regard to symptom management. Although there is the possibility that reducing homocysteine may be neuroprotective, they were not able to demonstrate symptom change in this study and concluded that vitamin supplementation should be considered on an individual needs basis. When they compared their results with studies showing more positive outcomes, it is apparent that higher supplement doses were used in the latter. This study notably had a heterogeneous participant group in that it included participants with a variety of different diagnoses, including schizophrenia, schizoaffective disorder, bipolar disorder with psychosis features and delusional disorder (Allott Reference Allott, McGory and Yuen2019).
Firth et al (Reference Firth, Stubbs and Rosenbaum2017, Reference Firth, Stubbs and Rosenbaum2018) combined data from 18 RCTs involving a total of 832 patients for their systematic review and meta-analysis. This work focused on trials in which over 90% had a diagnosis of non-affective psychosis and demonstrated a moderate benefit in psychosis symptom reduction with supplements of B6, B9 and B12, with no benefit over placebo shown for antioxidant vitamins or minerals. However, there was significant heterogeneity noted in the included trials and often vitamins were co-administered, making it difficult to determine which was responsible for outcomes or whether the combination was important. Trials using higher doses or combined vitamins were often more successful in reducing symptoms, as were trials in which participants were selected on the basis of nutritional deficiencies such as low folate or high homocysteine at baseline and those targeting participants at earlier stages of illness. They reported a potential role of genetic variance where outcomes differed, and this will be an important consideration in future research.
When considering cognition, Behrens et al (Reference Behrens, Graessel and Pendergrass2020) in their systematic review and meta-analysis of 20 studies found no benefit from supplementation of oral vitamin B compounds (B1, B6, folic acid and B12) to prevent cognitive decline in the absence of existing cognitive impairment. On the other hand, they reported no evidence that vitamin B oral supplementation harmed cognitive function and suggested that further work could consider more specific cognitive domains rather than global function. Similarly, despite raised homocysteine being linked to cognitive impairment, no cognitive benefit was found when supplementing vitamin B with an aim of lowering homocysteine levels in the systematic review and meta-analysis completed by Ford & Almeida (Reference Ford and Almeida2019) of 10 RCTs.
Health Quality Ontario (2013) combined data from 7 systematic reviews and 11 observational studies evaluating vitamin B12 and cognition and assessed the data quality and limitations. Key findings included that treatment with vitamin B12 and folate for those with mild cognitive impairment appeared to slow the rate of brain atrophy. However, this finding was based on low to moderate-quality evidence and it was not clear whether this would translate to clinical benefit. They also found an association between elevated plasma homocysteine levels and the onset of dementia but reported that this conclusion was on the basis of very low-quality evidence, limiting its utility and generalisability. Further data are therefore needed to investigate these two hypotheses.
Zhang et al (Reference Zhang, Luo and Yuan2020) also completed a systematic review and meta-analysis and identified 21 cross-sectional and longitudinal studies investigating whether B vitamins could help prevent cognitive decline. They reported that better cognition was linked with higher B12 levels in their cross-sectional studies, but this was not further demonstrated during stratified analysis or within the prospective studies that investigated this either on the basis of vitamin B12 intake or blood concentration. They therefore concluded that vitamin B12 may not be a modifiable risk factor when trying to slow cognitive decline.
Thus, overall there is little evidence for prophylactic supplementation at present. However, for cases where low vitamin B12 is present or vitamin B12 is inactivated through nitrous oxide use, correcting this deficiency has been shown to have significant benefits in reducing neuropsychiatric symptoms.
Conclusions
There is still knowledge to be gained in understanding the links between low vitamin B12, high homocysteine, nitrous oxide use and their respective neuropsychiatric effects. It is important that clinicians consider assessment of vitamin B12 levels and nitrous oxide use for those presenting with neuropsychiatric symptoms (key practice points are summarised in Box 1).
BOX 1 Key practice points
• For patients using N2O-containing compounds, consider the impact on B12 levels
• B12 and folate should be routinely checked when establishing a diagnosis in patients presenting with psychiatric symptoms
• Functional deficiency of B12 should be considered for patients with neurological or psychiatric symptoms and a history of nitrous oxide use
• Investigations for suspected functional deficiency of B12 should include blood tests for B12; if there is diagnostic uncertainty, homocysteine and methylmalonic acid levels could be considered
• Where possible, do not give N2O compounds continuously for longer than 24 h or more frequently than every 4 days without close monitoring
Author contributions
All authors contributed significantly to this work, with J.F. leading the project, K.R. and M.I. contributing to the writing, and M.K. and W.Y.M. developing and editing the manuscript.
Funding
This work has received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
MCQs
Select the single best option for each question stem
1 Which of the following neurotransmitters does not have a key role in the mechanism of action of nitrous oxide?
a GABA
b NMDA
c serotonin
d noradrenaline
e endogenous opioid systems.
2 NICE defines vitamin B12 deficiency as lower than:
a 300 ng/L
b 250 ng/L
c 200 ng/L
d 150 ng/L
e 100 ng/L.
3 Entonox contains a mix of nitrous oxide and oxygen in the ratio:
a 10:90
b 20:80
c 30:70
d 40:60
e 50:50.
4 Close haematological monitoring should be carried out if N2O-containing compounds are used more frequently than every:
a 2 days
b 4 days
c 6 days
d 8 days
e 10 days.
5 The immediate euphoric effects of N2O usually wear off within:
a 10 min
b 20 min
c 30 min
d 45 min
e 60 min.
MCQ answers
1 c 2 c 3 e 4 b 5 b






eLetters
No eLetters have been published for this article.