LEARNING OBJECTIVES
After reading this article you will be able to:
• better understand the clinical presentation, progression and risk factors for neuroleptic malignant syndrome
• understand the principles of management of neuroleptic malignant syndrome
• confidently reinstate antipsychotic treatment in a patient with a history of neuroleptic malignant syndrome.
Neuroleptic malignant syndrome (NMS) is a rare idiosyncratic adverse reaction to drugs. NMS can be of varying severity, ranging from mild to life-threatening. Subtle forms are difficult to recognise because of symptom overlap with other conditions. It is mainly associated with antipsychotic drugs, originally known as neuroleptic drugs. It was first described by Delay and colleagues, who called it ‘akinetic hypertonic syndrome’ (Delay Reference Delay, Pichot and Lempiere1960). With the use of first-generation antipsychotics (FGAs), the incidence of NMS in the 1980s and 1990s was reported to be 0.2% (Caroff Reference Caroff and Mann1993). More recent studies have reported the incidence with second-generation antipsychotics (SGAs) to be 0.01–0.03% (Belvederi Murri Reference Belvederi Murri, Guaglianone and Bugliani2015; Lally Reference Lally, Mccaffrey and O'Murchu2019).
Even though the increasingly widespread use of SGAs instead of FGAs over the years might lead us think that the incidence of NMS is probably declining, no clear trend was found in a meta-analysis of 26 studies on the topic (Gurrera Reference Gurrera, Simpson and Tsuang2007). This might be due to several factors, including a probable increase in the overall (and especially off-label) use of antipsychotics (Procyshyn Reference Procyshyn, Su and Elbe2014), increased pharmacovigilance (Tse Reference Tse, Barr and Scarapicchia2015) and several systematic biases in the published studies (Gurrera Reference Gurrera, Simpson and Tsuang2007).
Early diagnosis is paramount in reducing mortality and relies on high clinical suspicion for diagnosis and treatment. The most important and critical intervention in the management of NMS in psychiatric settings is discontinuation of the antipsychotic medication (the offending agent). NMS is best considered a medical emergency to be managed in an acute hospital (Box 1). Supportive care is the mainstay of treatment.
BOX 1 Royal College of Psychiatrists and Royal College of Physicians statement on neuroleptic malignant syndrome
‘The Colleges make the following joint recommendations for the diagnosis and management of neuroleptic malignant syndrome (NMS):
1 NMS is a rare and serious complication of antipsychotic therapy about which there is much uncertainty over definitions, cause, course and outcome. Nonetheless, all psychiatrists practising without immediate on-site supervision should be able to diagnose NMS.
2 NMS is best considered a medical emergency and is properly managed in an acute hospital. All medical staff in acute hospitals with responsibility for taking emergency referrals should know this and act accordingly. Acute clinicians should be prepared to accept cases of diagnosed NMS without reference to the current clinical state of the patient. Any debate over whether a patient should be transferred to the acute hospital should be about the issue of the diagnosis only. Liaison psychiatry services within acute hospitals can manage the mental health needs of such patients, so these needs should never influence the decision to transfer.’
Clinical features of neuroleptic malignant syndrome
The classic presentation of NMS is described in Box 2 and it comprises:
• hyperpyrexia
• muscle rigidity
• mental state changes
• autonomic instability.
Subtle forms are not uncommon and may be difficult to recognise because symptoms overlap with other conditions. Indeed, most symptoms are typically mild and go unrecognised (Velamoor Reference Velamoor2017). In particular, hyperpyrexia can be replaced by a febricula and may be falsely attributed to a co-occurring infection. Rigidity can be mild and interpreted as pseudo-Parkinsonism associated with the use of antipsychotics. As mental state is commonly fluctuating in NMS, a cross-sectional assessment might miss important diagnostic changes. Similarly, increased heart rate can be mild and falsely attributed to agitation, anxiety or to the anticholinergic effects of psychotropic medication. Moreover, when patients are acutely disturbed, the baseline vital signs might have been impossible to obtain or might have been unreliable owing to agitation or psychomotor activation.
BOX 2 Classic presentation of neuroleptic malignant syndrome (NMS)
• Hyperpyrexia (>38.0 °C or >100.4 °F, on at least two occasions measured orally); in certain cases, hyperpyrexia can be severe and be associated with dehydration.
• Muscle rigidity that is generally generalised and severe. In its most severe forms, it is typically described as ‘lead-pipe’ rigidity and can lead to rhabdomyolysis, myoglobinuria and acute renal failure. This rigidity might not respond well to anti-Parkinsonian medication.
• Mental state changes, ranging from stupor to coma. Fluctuating mental state is also common.
• Autonomic instability, as evidenced by:
• profuse diaphoresis
• tachypnoea
• tachycardia
• increased or fluctuating blood pressure
• urinary incontinence
• pallor.
Typically, blood pressure elevation in NMS is defined as systolic or diastolic increase ≥25% above baseline. Blood pressure fluctuation is generally defined as ≥20 mmHg diastolic change or ≥25 mmHg systolic change within 24 h. Hypermetabolism is commonly defined as increased heart rate (≥25% above baseline) and respiratory rate (≥50% above baseline).
A systematic review found that hyperthermia was present in 88% and muscle rigidity in 86% of reported cases of NMS (Lang Reference Lang, Lang and Becker2015). There have been reports of SGA-associated NMS often being less severe, and thus possibly more challenging to diagnose, than FGA-associated NMS. Indeed, it has been reported that SGA-associated NMS showed less muscle rigidity and much less severe creatinine kinase abnormalities, possibly resulting in a less severe, atypical presentation. With the common use of SGAs nowadays, it is possible that such presentations are becoming more and more common. It is important not to miss or misdiagnose these forms of NMS, since supportive management and medication adjustment are still needed in these cases (Belvederi Murri 2015; Tse Reference Tse, Barr and Scarapicchia2015).
The order in which symptoms develop in NMS is variable. However, initial symptoms commonly take the form of mental state changes, followed by muscle rigidity, autonomic instability and then hyperthermia (Tormoehlen Reference Tormoehlen and Rusyniak2018).
Paraclinical findings
Laboratory evaluation is essential to exclude other causes of hyperthermia (mainly infections, metabolic and endocrine abnormalities, as well as drug-induced syndromes) and to detect medical complications of NMS. Laboratory findings may also help with the positive diagnosis of NMS. However, no single abnormality is specific to the diagnosis.
The most commonly observed abnormality is elevated creatine kinase. The threshold that is considered to be ‘suggestive of NMS’ varies from study to study: at least four times the upper limit of normal (Gurrera Reference Gurrera, Caroff and Cohen2011) or >1000 U/L (Levenson Reference Levenson1985). However, even though creatine kinase elevation is often considered as the most important biological finding in favour of NMS, such an elevation may be observed in up to 70% of patients who develop fever while on antipsychotics (without actually having NMS), and even in up to 30% of patients who develop fever while not on any psychotropics (O'Dwyer Reference O'Dwyer and Sheppard1993).
Other observed findings include: elevations in catecholamines, lactate dehydrogenase and aspartate transaminase; leucocytosis; low serum iron levels; metabolic acidosis; and hypoxia (Velamoor Reference Velamoor2017; Ware Reference Ware, Feller and Hall2018). Cerebrospinal fluid analysis and neuroimaging studies are generally normal and may help in differential diagnosis. Electroencephalography may show generalised slowing (Caroff Reference Caroff and Mann1993).
Diagnosis
NMS should always be considered in patients exposed to antipsychotics who present with fever and rigidity, especially if the antipsychotic has been recently commenced or the dose increased. It is important to note, however, that the majority of patients on antipsychotics who develop fever will not be suffering from NMS and it is therefore important to consider other diagnoses. By definition, NMS is a diagnosis of exclusion. Therefore, a thorough review of the case by obtaining an accurate symptom and medication history (especially any temporal relationship), physical examination and laboratory investigations can help in diagnosis and further management (Velamoor Reference Velamoor2017; Rowland Reference Rowland, Banga and Ayadurai2018; Ware Reference Ware, Feller and Hall2018).
The most commonly used diagnostic criteria are the DSM-5 criteria (American Psychiatric Association 2013) and Levenson's criteria (Levenson Reference Levenson1985). An international multispecialty consensus group published diagnostic criteria (Gurrera Reference Gurrera, Caroff and Cohen2011) that are based on positive clinical and laboratory findings as well as the exclusion of alternative causes. However, no threshold score has been defined and validated for making a diagnosis of NMS.
These diagnostic criteria help improve diagnostic agreement, as well as diagnostic reliability and validity, thus allowing for more robust research about NMS. However, the diagnosis of NMS remains clinical, since strict use of the criteria might make clinicians miss atypical (less severe) forms of NMS (Tse Reference Tse, Barr and Scarapicchia2015).
Differential diagnosis
NMS is a diagnosis of exclusion. Hence, it is important to rule out other diagnoses that may present similarly (Table 1). These include neurological and medical conditions, substance- or medication-induced syndromes, as well as psychiatric conditions. Neurological or medical conditions that need to be ruled out include central nervous system infections, inflammatory or autoimmune conditions, status epilepticus, subcortical structural lesions and systemic conditions (e.g. pheochromocytoma, thyrotoxicosis, tetanus, heat stroke) (Velamoor Reference Velamoor2017; Ware Reference Ware, Feller and Hall2018).
TABLE 1 Differential diagnoses for neuroleptic malignant syndrome (NMS)
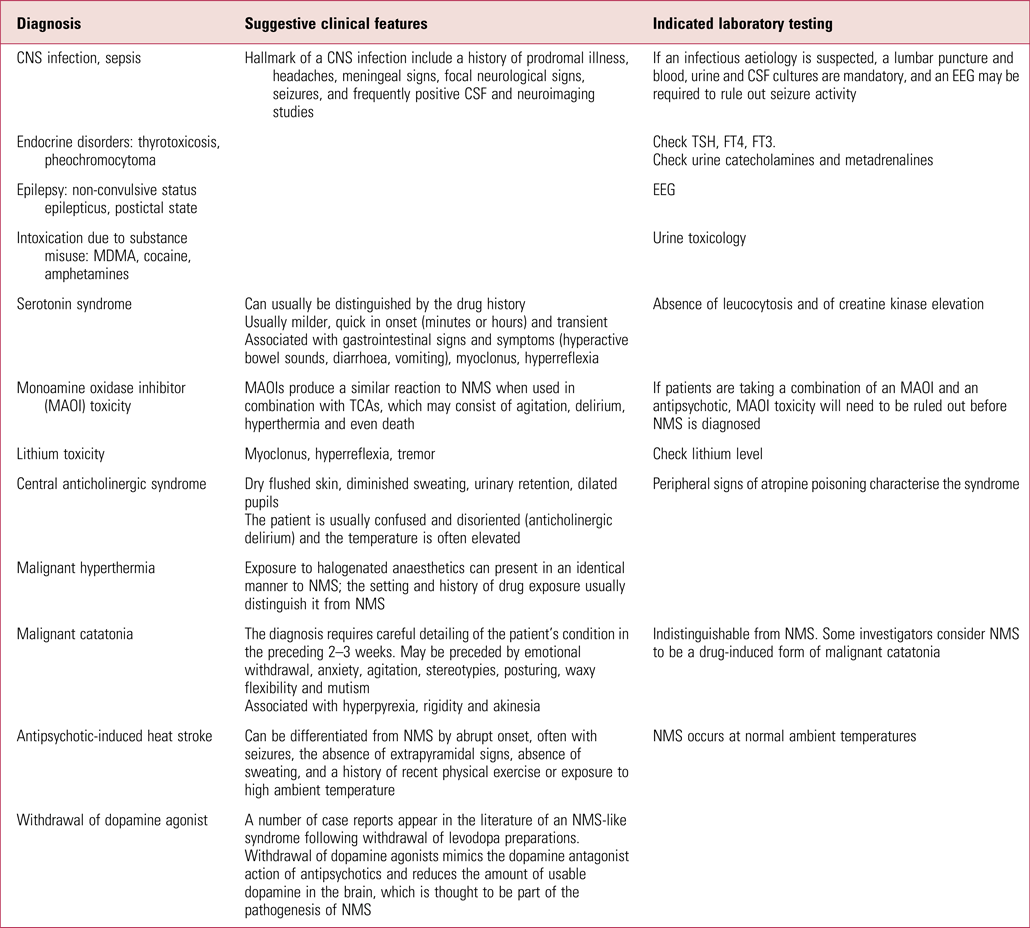
CNS, central nervous system; CSF, cerebrospinal fluid; EEG, electroencephalogram; TSH, thyroid-stimulating hormone; FT, free thyroxine; MDMA, 3,4-methylenedioxymethamphetamine; TCA, tricyclic antidepressant.
Source: adapted from Guzofski & Peralta (Reference Guzofski and Peralta2006).
Similar syndromes resulting from the use of other substances or medications include serotonin syndrome, Parkinsonian hyperthermia syndrome following abrupt discontinuation of dopamine agonists, alcohol or sedative withdrawal, malignant hyperthermia occurring during anaesthesia, hyperthermia associated with misuse of stimulants and hallucinogens, as well as atropine poisoning from anticholinergics.
Psychiatric differential diagnoses are primarily represented by malignant catatonia associated with mood or psychotic illness (Sethi Reference Sethi2004). Indeed, individuals with schizophrenia or a mood disorder may present with malignant catatonia, which may be indistinguishable from NMS. Some investigators consider NMS to be a drug-induced form of malignant catatonia (Velamoor Reference Velamoor2017; Ware Reference Ware, Feller and Hall2018).
Serotonin syndromeFootnote 1
Serotonin syndrome is an important differential diagnosis, but it is hard to differentiate from NMS when it comes to clinical presentation owing to overlap of symptoms. Serotonin syndrome is described as a clinical triad of mental status changes, autonomic hyperactivity and neuromuscular changes. However, these may not all be present in every patient with the syndrome. Serotonin syndrome is an adverse reaction caused by therapeutic drug use, intentional overdose or drug interactions leading to excessive stimulation of serotonergic receptors in the peripheral and central nervous system. Serotonin syndrome is believed to be due to excess precursors of 5-hydroxytryptamine (5-HT) and its agonists, increased release of 5-HT, decreased uptake or lower metabolism in the nervous system.
Differentiating NMS and serotonin syndrome can be a challenge, but clinical course, signs and laboratory findings may help. Important distinguishing clinical features pointing to the diagnosis of serotonin syndrome include gastrointestinal symptoms, hyper-reflexia (often in the form of clonus, more marked in the lower extremities), ocular clonus and tremors. In comparison, NMS is a bradykinetic syndrome characterised by uniform ‘lead-pipe’ rigidity and hyporeflexia.
Symptoms of serotonin syndrome are also frequently seen within the first 24 h of starting serotonergic agents and resolve within a few days of omitting the offending agent and starting the treatment of the serotonin syndrome. In contrast, NMS is often slower in onset and usually takes 9–14 days to remit in spite of appropriate treatment.
Aetiology and pathophysiology
NMS is caused by exposure to dopamine antagonists. It can occur at any time during antipsychotic therapy, but the risk is highest immediately after starting the medication or following a dose increase. NMS has been noted to occur within the first 4 weeks in 96% of cases (Sethi Reference Sethi2004; Berman Reference Berman2011).
Most antipsychotics have been implicated, and NMS usually occurs within the therapeutic dose range. First-generation antipsychotics (FGAs) have been reported in the literature to cause NMS more frequently than second-generation (SGAs), which may reflect their long history of use (Nakamura Reference Nakamura, Yasunaga and Miyata2012). Dopamine antagonists used in medical settings (e.g. metoclopramide, prochlorperazine) can also induce NMS (American Psychiatric Association 2013).
The pathophysiology of NMS is not well understood and it involves a complex interaction between antipsychotic medication and a susceptible individual. It is triggered by antipsychotic blockade of dopamine D2 receptors in the key neural pathways that affect thermoregulation (hypothalamus), motor tone (nigrostriatal pathway and brain-stem) and mental status (reticular activating system). However, central thermoregulation is mediated by noradrenergic, serotonergic and cholinergic pathways, as well as the dopaminergic pathways, and it is therefore unlikely that NMS is due to central dopamine blockade alone. To date, however, none of the theories put forth as the underlying cause of NMS has been able to explain why only a small fraction of patients exposed to antipsychotics develop this condition. Furthermore, it remains unknown why patients who develop NMS are usually able to continue being treated with similar medications and, at times, even the same offending agent (Khaldi Reference Khaldi, Kornreich and Choubani2008; Berman Reference Berman2011).
Risk factors
Both age and gender distributions of NMS correspond with the distribution of exposure to antipsychotic agents. Hence, age and gender are not in themselves risk factors (Caroff Reference Caroff and Mann1993).
A history of NMS may increase the risk of reoccurrence. Indeed, about 15–20% of individuals with NMS will have experienced a similar episode before (Ouyang Reference Ouyang and Chu2013). There is limited evidence to suggest genetic susceptibility, possibly through a reduction in the D2 dopamine receptor function (Berman Reference Berman2011).
Other medical risk factors include catatonia, organic brain syndrome or previous brain injury, Parkinson's disease, hyperthyroidism, alcoholism, use of restraints, iron deficiency, exhaustion, dehydration and agitation (Berman Reference Berman2011; Stroup Reference Stroup and Gray2018).
Medication-related factors include antipsychotic polypharmacy, high-potency or high-dose antipsychotics, adjunctive psychotropic medications (e.g. lithium), rapid titration of antipsychotics, abrupt discontinuation of antipsychotics, poorly controlled or treatment-resistant antipsychotic-induced extrapyramidal symptoms, and withdrawal of anti-Parkinsonian medications (Stroup Reference Stroup and Gray2018).
Second- versus first-generation antipsychotics
NMS was originally described with first-generation (‘conventional’ or ‘typical’) antipsychotics. Although second-generation (‘atypical’) antipsychotics are overall less likely to induce severe hyperthermia or rigidity, NMS has been reported with virtually all SGAs (Velamoor Reference Velamoor2017; Anzai Reference Anzai, Takahashi and Watanabe2019), including those with weak D2 antagonist effects such as quetiapine and clozapine (Teo Reference Teo, Wong and Tan2018) and those with partial D2 agonist effects (e.g. aripiprazole) (Agrawal Reference Agrawal, Bajaj and Bajaj2019). Yet, apart from possibly blonanserin and perospirone, SGAs appear to be significantly less associated with NMS than haloperidol, with clozapine being possibly the safest in this regard, followed by quetiapine (Anzai Reference Anzai, Takahashi and Watanabe2019).
Course and complications
There is substantial variation in clinical presentation of NMS, ranging from mild to life-threatening.
NMS presents a challenge as the outcome depends on its prompt recognition and treatment. Although relatively uncommon, NMS can be fatal. However, in most cases, if the offending agent is discontinued, NMS is self-limited. Indeed, following antipsychotic discontinuation, patients recover within an average of 7 to 10 days. Nonetheless, the duration may be prolonged when long-acting antipsychotics are implicated (Caroff Reference Caroff and Mann1988).
Complications of NMS are often due to physiological consequences of severe rigidity and immobilisation, such as dehydration, deep vein thrombosis, pulmonary embolism, aspiration pneumonia and disseminated intravascular coagulation (Berman Reference Berman2011). Myoglobinuria, renal failure and rhabdomyolysis are serious complications of NMS. These are strong predictors of death, with a mortality rate of approximately 50% if renal failure is present (Shalev Reference Shalev, Hermesh and Munitz1989; Chandran Reference Chandran, Mikler and Keegan2003). Overall mortality rates that were reported in the 1970s and 1980s were high: 27.7% before 1980, dropping to 22.6% between 1980 and 1983, and then to 11.6% between 1984 and 1987 (Shalev Reference Shalev, Hermesh and Munitz1989). Data from a Japanese database between 2004 and 2008 revealed mortality rates of 7.6% in FGA-associated NMS and 3.3% in SGA-associated NMS (Nakamura Reference Nakamura, Yasunaga and Miyata2012). In a study including 1346 in-patients from a US nationwide sample for the years 2002–2011, the NMS mortality rate was 5.6%, with a trend of decreased mortality over the years (Modi Reference Modi, Dharaiya and Schultz2016). This improvement in NMS outcome is probably due to better recognition of the syndrome, early intervention and the availability of intensive supportive care (Modi Reference Modi, Dharaiya and Schultz2016).
Management
NMS is a medical emergency to be managed in an acute hospital (Box 1).
There are no published randomised controlled trials on the management of NMS, and there are no treatments specifically approved for NMS. The most important and critical intervention remains discontinuation of the antipsychotic medication (Berman Reference Berman2011).
Immediate management in psychiatric wards
Successful treatment of NMS depends on early clinical recognition and prompt withdrawal of the antipsychotic agents. Antipsychotics cannot be removed from the body by dialysis, and blood concentrations decline only slowly. If NMS is suspected, all antipsychotic medication should be immediately discontinued (Friedman Reference Friedman2015). This includes drugs with weak dopamine-blocking properties such as promethazine, which has been incriminated in cases of NMS and should be immediately withdrawn if NMS is suspected (Chandran Reference Chandran, Mikler and Keegan2003; Velamoor Reference Velamoor2017).
A thorough medical work-up should be initiated. Laboratory evaluation is essential to exclude other causes of hyperthermia and detect any medical complications. Supportive care is the mainstay of treatment for NMS and it should occur in a setting where blood pressure, cardiac rhythm and pulse oximetry can be continuously monitored. In the UK this usually means transfer to a medical assessment unit (MAU), since psychiatric units do not have such facilities or training. In moderate to severe cases, the need for continuous monitoring makes it imperative to transfer patients to an intensive care setting (Berman Reference Berman2011). There is generally lack of clear guidelines for transfer to intensive care units (ICUs) or treatment options within ICUs. However, some recent publications have tried to classify NMS into various stages (Schönfeldt-Lecuona Reference Schönfeldt-Lecuona, Cronemeyer and Hiesener2020) and also laid out treatment options (van Rensburg Reference van Rensburg and Decloedt2019; Schönfeldt-Lecuona Reference Schönfeldt-Lecuona, Cronemeyer and Hiesener2020) (Table 2). A recent review of NMS treatments reported only 14 guidelines thematically related to its management, 8 of which were in English, 6 in German and 1 in French (Schönfeldt-Lecuona Reference Schönfeldt-Lecuona, Cronemeyer and Hiesener2020). None of the guidelines were from the UK.
TABLE 2 Neuroleptic malignant syndrome (NMS) stages and treatment recommendations
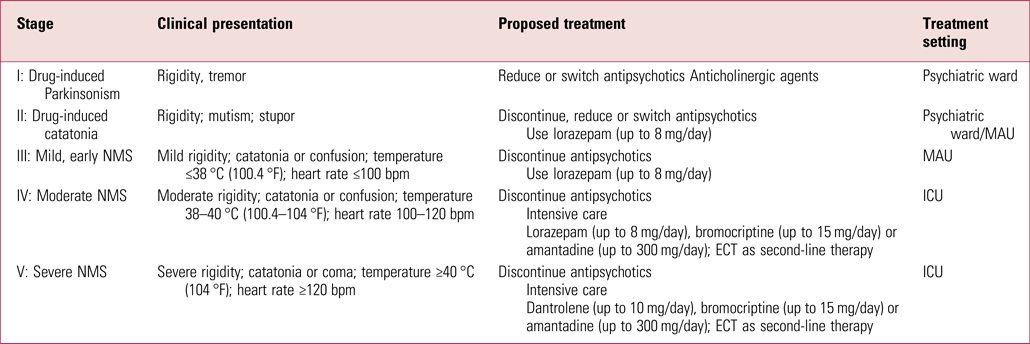
MAU, medical assessment unit; ICU, intensive care unit; ECT, electroconvulsive therapy.
Source: adapted from Schönfeldt-Lecuona et al (Reference Schönfeldt-Lecuona, Cronemeyer and Hiesener2020) and van Rensburg & Decloedt (Reference van Rensburg and Decloedt2019).
As shown in Table 2, mild forms of NMS can be treated in a psychiatric unit or medical setting (on an MAU) and do not necessarily need ICU care unless there is further deterioration. Again, it may not be possible always to manage mild cases of NMS on psychiatric units owing to the lack of the necessary facilities, such as access to laboratories, equipment and medically trained staff (Schönfeldt-Lecuona Reference Schönfeldt-Lecuona, Cronemeyer and Hiesener2020a).
Overview of management in an intensive care facility
Supportive care is the mainstay of treatment. Pharmacological intervention with dopamine agonists produces mixed results and there have been no prospective randomised controlled trials comparing treatment regimens in people with NMS. Benzodiazepines, bromocriptine (a centrally acting dopamine agonist), amantadine (a dopamine agonist) and dantrolene (a muscle relaxant) can be used.
Again referring to Table 2, pharmacological management can be guided by the severity of symptoms and treatment response. Oral or intravenous benzodiazepines are the mainstay of early treatment of catatonia in NMS, may reduce fever and rigidity, and treat agitation (Chandran Reference Chandran, Mikler and Keegan2003; Chung Reference Chung and Lee2018). For early or mild forms of NMS, benzodiazepines along with withdrawal of the causative agent can be initiated in a psychiatric ward before transfer to a medical ward and they do not necessarily need ICU care. Several clinical reports suggest that lorazepam and other benzodiazepines may reduce the recovery time and hence improve the outcome (Yacoub Reference Yacoub and Francis2006; Tural Reference Tural and Onder2010). A lorazepam dose of 1–2 mg orally or intravenously every 4–6 h remains the first-line pharmacological intervention (Wijdicks Reference Wijdicks2018). Intramuscular administration should be avoided if possible to avoid diagnostic confusion due to potential elevation in creatine kinase (CK) levels (Konikoff Reference Konikoff, Halevy and Theodor1985).
NMS of moderate severity requires ICU care, and use of benzodiazepines and/or bromocriptine has been shown to be effective in improving clinical response compared with supportive care alone (Rosenberg Reference Rosenberg and Green1989). Use of benzodiazepines and/or bromocriptine has also been shown to reduce mortality in NMS compared with supportive care alone (Sakkas Reference Sakkas, Davis and Janicak1991). Bromocriptine must be administered orally or through a nasogastric tube as there is no parenteral preparation available. A starting dose of 2.5 mg administered 2–3 times daily, to be increased by 2.5 mg every 24 h until a response is obtained or until a maximum dose of 45 mg/day is reached can be used. It is recommended that bromocriptine to be continued for 10 days after symptoms are controlled and then tapered slowly to minimise the likelihood of recurrence of NMS (Bhanushali Reference Bhanushali and Tuite2004; Strawn Reference Strawn, Keck and Caroff2007).
Amantadine can be used as an alternative to bromocriptine but there is limited evidence about its efficacy compared with the other medications mentioned.
Dantrolene is recommended for severe forms of NMS, administered alone or together with benzodiazepines and bromocriptine. There are mixed reports about its efficacy, with some meta-analyses suggesting improvement in approximately 80% of patients receiving dantrolene monotherapy (Sakkas Reference Sakkas, Davis and Janicak1991; Mann Reference Mann2003); however, a more recent review suggest higher mortality with monotherapy and longer recovery times in combination treatments (Reulbach Reference Reulbach, Dütsch and Biermann2007). Dantrolene is administered intravenously starting with an initial bolus dose of 1–2.5 mg/kg, followed by 1 mg/kg every 6 h up to a maximum dose of 10 mg/kg/day (Tsutsumi Reference Tsutsumi, Yamamoto and Matsuura1998; Bhanushali Reference Bhanushali and Tuite2004; Strawn Reference Strawn, Keck and Caroff2007). Response is noted within minutes of administration. Owing to risk of hepatotoxicity, dantrolene should be typically discontinued once symptoms begin to resolve. However, to minimise relapse some recommend continuing for 10 days, followed by a slow taper with doses of oral dantrolene that range from 50 to 200 mg daily (Bhanushali Reference Bhanushali and Tuite2004).
As discussed, symptoms of NMS sometimes return if treatment is discontinued before complete clearance of the offending medication. We therefore recommend that if bromocriptine, dantrolene or both are used, treatment should continue for 10 days beyond the resolution of symptoms, or for 2–3 weeks if the offending agent is an extended-release depot antipsychotic.
General symptomatic treatment, such as hydration, nutrition and reduction of fever, is essential. Secondary complications, such as hypoxia, acidosis and renal failure, must be treated aggressively. Low-dose heparin seems to be indicated to prevent venous thrombosis in an immobilised patient. Other dopamine antagonists, such as metoclopramide, should be avoided (Berman Reference Berman2011; Velamoor Reference Velamoor2017).
Role of ECT in the management
The treatment of NMS with pharmacological agents in combination with electroconvulsive therapy (ECT) is still debatable, owing to the lack of large-scale randomised controlled trials, and most evidence is based on case reports. Electroconvulsive therapy alone may be an option, although again the evidence is mostly based on case reports and case series. Clinical response to ECT is presumably by increasing central dopaminergic transmission. ECT seems to be relatively safe in people with NMS, with a response onset usually observed within few sessions (Nisijima Reference Nisijima and Ishiguro1999). ECT is known to reduce mortality in NMS compared with supportive care alone (Davis Reference Davis, Janicak and Sakkas1991).
ECT may be considered as a second-line treatment for patients who have not improved after 48 h of pharmacological treatment. Besides, ECT may be the preferred first-line treatment (a) where it is not clear whether the cause of the symptoms is NMS or malignant catatonia, (b) when the underlying psychiatric diagnosis is psychotic depression or (c) when catatonic features are prominent in the NMS clinical picture (Trollor Reference Trollor and Sachdev1999; Hashim Reference Hashim, Zeb Un and Alrukn2014).
Prevention of neuroleptic malignant syndrome
Prevention of NMS in the first place is probably the most important aspect in the management of the syndrome.
Primary prevention
An obvious preventive action is to reduce unjustified prescription of antipsychotics. This is particularly important, as up to 75% of antipsychotic prescription is off-label (Carton Reference Carton, Cottencin and Lapeyre-Mestre2015). Even low-dose antipsychotics used for indications other than psychiatric illness can still expose to the risk of NMS, so avoiding unnecessary prescriptions may help decrease the incidence of NMS. In psychiatry, the optimisation of antidepressant, anxiolytic and mood-stabilising treatment can also help avoid augmentation with antipsychotics or, in certain cases, allow the use of lower doses of antipsychotics.
Antipsychotic polypharmacy increases the risk for NMS and avoiding it is always recommended. Antipsychotic polypharmacy is a widespread practice worldwide that has not been associated with any better efficacy when it comes to treating psychosis. Instead, it has been linked to higher morbidity and mortality, in particular increased risk of QTc prolongation, extrapyramidal side-effects and metabolic side-effects (Gallego Reference Gallego, Bonetti and Zhang2012). A higher incidence of NMS is possibly one of the explanations of this reported increase in mortality.
Where possible, clinicians should avoid parenteral administration, rapid titration and high doses of antipsychotics, as all are associated with a higher risk for NMS.
Antipsychotics that are less commonly associated with NMS, in particular those with lower D2 blocking effects (i.e. the SGAs, and clozapine in particular), should be chosen whenever clinically possible. High-potency antipsychotics (FGAs such as the butyrophenones and thioxanthines) have the highest propensity to cause NMS and might be better avoided as first-line interventions if there are ‘safer’ options.
Secondary prevention
Secondary prevention of NMS involves timely detection through monitoring of vital signs, mental state and extrapyramidal signs, especially in patients requiring high doses, rapid titration or parenteral antipsychotics. In addition, educating patients and their families about the signs that may indicate NMS could help.
Tertiary prevention
Tertiary prevention might involve immediate discontinuation of all antipsychotics in the case of the slightest suspicion of NMS. Antipsychotic medication could be resumed once NMS has been ruled out (Velamoor Reference Velamoor2017).
Antipsychotic rechallenge following neuroleptic malignant syndrome
Patients with a history of NMS are likely to require future antipsychotic treatment, depending on the diagnosis that led to antipsychotic medication in the first place. Prevention of NMS on rechallenge awaits a better understanding of the underlying pathophysiology. Not all patients will experience a recurrence. The estimated risk of developing NMS again with repeated exposure is 30%. It is also debated whether the potency and dose of the rechallenge drug are an independent predictor of recurrence. The evidence supporting recommendations for reinstating treatment after NMS is limited (Rosebush Reference Rosebush, Stewart and Gelenberg1989; Stroup Reference Stroup and Gray2018).
In practice, rechallenge should not be tried until at least 2 weeks after full recovery from NMS and ideally it should take place in an in-patient setting. It is indeed likely that the risk of recurrence is more linked to the wash-out period than to the actual antipsychotic agent that is reintroduced (Pileggi Reference Pileggi and Cook2016). The wash-out period should take into account the severity of the NMS, the presence of any sequelae, the severity of the underlying psychiatric disorder and the pharmacokinetic properties of the offending agent (Pileggi Reference Pileggi and Cook2016).
When rechallenging after NMS, it is generally advised that a different antipsychotic is used, although one case series found that recurrence rates were similar regardless of whether the same or a different antipsychotic was used (Wells Reference Wells, Sommi and Crismon1988). The clinician's caution and the patient's preference generally favour a change of antipsychotic. When selecting a new agent, it is prudent to choose one with low nigrostriatal D2 affinity (Pileggi Reference Pileggi and Cook2016). Given its low D2 effects, clozapine is an option. A systematic review found that the outcome of rechallenge using clozapine following non-clozapine antipsychotic-associated NMS was favourable in 79% of cases. In patients who had developed NMS on clozapine, the outcome of clozapine rechallenge was favourable in 92% of cases, with no death reported even in the ‘unfavourable outcome’ group who had an NMS recurrence (Lally Reference Lally, Mccaffrey and O'Murchu2019).
When rechallenging after NMS, it is recommended to begin with a low dose and to advance slowly towards the target dose. Careful monitoring for fever, autonomic instability, mental state change, extrapyramidal symptoms and dehydration is indicated. Antipsychotics should be discontinued if fever, muscular rigidity and/or labile blood pressure are noted (Box 3). Serial measurements of white blood cell count and creatine kinase are also warranted. Agitation should be treated aggressively with benzodiazepines, since agitation increases the risk for NMS. Adjunctive treatment with a mood stabiliser, antidepressant or both for the affective symptoms may minimise the required dose of antipsychotic (Velamoor Reference Velamoor2017).
BOX 3 Reinstating antipsychotic treatment after neuroleptic malignant syndrome (NMS): checklist
• Recheck the accuracy of the diagnosis of the NMS episode
• Document indications for antipsychotic medications
• Consider alternative pharmacological agents
• Discuss risks and benefits, including the risk of recurrence, with patient and family
• Minimise risk factors
• Prescribe an initial test dose
• Monitor vital signs and neurological status
• Titrate doses gradually
Conclusions
Neuroleptic malignant syndrome is rare idiosyncratic reaction to antipsychotics, which is associated with substantially high morbidity and mortality. Early recognition remains paramount to avoid complications and death, with careful monitoring of vital signs and mental state. Prompt discontinuation of antipsychotics is essential with any suspicion of NMS. Management requires admission to an acute medical unit, for supportive care. One of the main difficulties with treating NMS is that it is very hard to predict who will develop it and when. Further studies may help provide a deeper insight into the pathophysiology of NMS and help clinicians to predict it better. In the meantime, emphasising conservative prescribing guidelines and providing proper education to patients and families are vital in early recognition and management of NMS.
Author contributions
All three authors were involved in the conception of the article and the literature review. All contributed to the drafting and critical review and to the revision following peer review. All approved the final draft.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2020.71.
MCQs
Select the single best option for each question stem.
1 Diagnosis of neuroleptic malignant syndrome requires the presence of:
a severe hyperthermia
b tachycardia and hypotension
c elevated creatine kinase
d myoglobinuria
e none of the above.
2 Neuroleptic malignant syndrome can be differentiated from serotoninergic syndrome by:
a the presence of hyperthermia
b the presence of tachycardia and hypertension
c the presence of muscle rigidity
d the absence of gastrointestinal symptoms
e the presence of delirium.
3 A higher risk for neuroleptic malignant syndrome has been associated with:
a young age
b male gender
c the use of electroconvulsive therapy
d antipsychotic polypharmacy
e combination of benzodiazepines with antipsychotics.
4 The single most important therapeutic intervention for neuroleptic malignant syndrome is:
a rehydration
b discontinuing all antipsychotic drugs
c timely administration of dopamine agonists, e.g. bromocriptine
d use of benzodiazepines and anticholinergics
e electroconvulsive therapy.
5 As regards antipsychotic rechallenge following an episode of neuroleptic malignant syndrome:
a antipsychotic rechallenge should occur as soon as possible, especially when the patient is agitated
b the use of depot antipsychotics for the rechallenge can be a good option, thanks to more regular pharmacokinetics
c alternatives to antipsychotics, whenever possible, are warranted
d creatine kinase monitoring following rechallenge is useless
e if the patient does not develop neuroleptic malignant syndrome within 1 month of the rechallenge, then there is no longer any risk of recurrence.
MCQ answers
1 e 2 d 3 d 4 b 5 c






eLetters
No eLetters have been published for this article.