Introduction
Extinction risk is high for many Hawaiian bird species and this situation creates an urgent need for reliable assessment of their density and distribution. The Maui Parrotbill (hereafter parrotbill) Pseudonestor xanthophrys is a ‘Critically Endangered’ insectivorous forest bird endemic to the island of Maui (BirdLife International 2012, U.S. Fish and Wildlife Service 2006). Limited range, combined with small population size and low densities (U.S. Fish and Wildlife Service 2006) put the parrotbill at particular risk of extinction. Like most Hawaiian forest birds, parrotbills are currently limited to high elevation forests that are relatively free of mosquitoes and avian malaria Plasmodium sp. (Atkinson and LaPointe Reference Atkinson and LaPointe2009, Mountainspring Reference Mountainspring1987, Scott et al. Reference Scott, Mountainspring, Ramsey and Kepler1986), but these forests are otherwise likely suboptimal habitat (Becker et al. Reference Becker, Mounce, Rassmussen, Rauch-Sasseen, Swinnerton and Leonard2010, Simon et al. Reference Simon, Baker, Baker, Poole and Gill1997, U.S. Fish and Wildlife Service 2006). In addition, widespread habitat loss, especially of koa Acacia koa forests, the parrotbills’ preferred foraging habitat (Perkins Reference Perkins and Sharp1903), has contributed to their current limited distribution on the windward slopes of east Maui (U.S. Fish and Wildlife Service 2006). The depredation of nests, juveniles, and adults also may limit the parrotbill population and, while the cause and importance of depredation is unclear, rodents Rattus spp., feral cats Felis catus, and the invasive small Indian mongoose Herpestes auropunctatus are present throughout the area where the current population persists (Malcolm et al. Reference Malcolm, Swinnerton, Groombridge, Sparklin, Brosius, Vetter and Foster2008, Sugihara Reference Sugihara1997).
Based on the 1980 Hawaii Forest Bird Survey that used island-wide point counts, the Maui Parrotbill population was estimated at 502 ± 116 (Scott et al. Reference Scott, Mountainspring, Ramsey and Kepler1986). As a rare species with low detection rates, more recent surveys have been unable to confirm the stability of the parrotbill population (Gorreson et al. Reference Gorreson, Camp, Reynolds, Woodworth and Pratt2009). Indeed, Brinck et al. (Reference Brinck, Camp, Gorresen, Leonard, Mounce, Iknayan and Paxton2012) found that the repeated sampling frequencies and number of visits that would be necessary to increase the power of these surveys to detect trends in the parrotbill population would be particularly high and therefore logistically unfeasible. Due to the limitations of these survey efforts for estimating the population size of a rare species, demographic analysis is perhaps the only alternative means for providing a better insight into the population dynamics of the Maui Parrotbill. An understanding of population demography, coupled with an understanding of the factors limiting population growth, is essential for recovering populations of endangered species, designing effective conservation strategies and making informed management decisions (Anders and Marshall Reference Anders and Marshall2005). While studies of population dynamics depend heavily on mortality and recruitment rates (Lebreton et al. Reference Lebreton, Pradel and Clobert1993), such information is often lacking for endangered species but it is often these same species of conservation focus that would benefit the most from such studies (Beissinger and Westphal Reference Beissinger and Westphal1998).
Fundamental to population demography is an understanding of the variability in survival among individuals (Eberhardt Reference Eberhardt1985, Lack Reference Lack1954). Accurate measurements of population-specific survival are essential for estimating reliable rates of population change, as many models of population dynamics are sensitive to small deviations in estimates of demographic measures (Noon and Sauer Reference Noon, Sauer, McCullough and Barrett1992, Porneluzi and Faaborg Reference Porneluzi and Faaborg1999, Woodworth Reference Woodworth1999). Constant effort mist-netting and banding has historically been a common method used to estimate survival rates of passerines (DeSante and Burton Reference DeSante and Burton1994). This technique is limited by the fact that previously banded individuals are not always recaptured even though they may still be alive (Chase et al. Reference Chase, Nur and Geupel1997). Indeed, whether or not a banded bird is subsequently detected is a function of probabilities: survival, emigration and detection. Re-sighting and re-capturing marked individuals has since improved this method for generating the most accurate estimations of survival in forest bird species (Gardali and Nur Reference Gardali and Nur2006, Johnson et al. Reference Johnson, Sherry, Holmes and Marra2006, Sandercock et al. Reference Sandercock, Beissinger, Stoleson, Melland and Hughes2000). Furthermore, understanding variation in age-specific and sex-specific survival can provide valuable insights to inform the ecology and conservation of a species (Martin Reference Martin2002, Sandercock et al. Reference Sandercock, Beissinger, Stoleson, Melland and Hughes2000).
Considering the limitations of accurately estimating the population of rare species, accurate demographic data would provide managers with a yardstick to monitor the population trajectory of a species. However, the low densities and few individuals indicative of a rare and endangered species result in mark-recapture studies requiring large amounts of time and effort. In addition, parrotbills inhabit very rugged and remote terrain. Both these characteristics make collecting demographic data a challenge. Indeed, long-term demographic data for rare species inhabiting remote areas are rarely available for managers. Here we improve upon previous demographic estimates for this species (Vetter et. al Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012) by summarising survival probability of parrotbill in the core of their population range using 18 years of encounter data and we examine differences in age- and sex-specific survival probabilities.
Methods
Study Area
We conducted this study within the Hanawi Natural Area Reserve (NAR) on the windward slope of Haleakala volcano, Maui, Hawaii (Figure 1). Our 180 ha study site extended from 1,600 to 2,100 m in elevation. This study area is located within an 800 ha portion of the reserve, managed by the State of Hawaii and which has been fenced and free from invasive ungulates since 1997; it protects some of the most pristine native forest remaining in Hawaii. The area is mainly a montane wet forest characterised by rugged and steep terrain. Ohia Metrosideros polymorpha and olapa Cheirodendron trigynum are the dominant canopy species, although subalpine scrub and subalpine grassland occur at the highest elevations (Jacobi Reference Jacobi1989). The study site supports the highest known density of Maui Parrotbill (Pratt et al. Reference Pratt, Atkinson, Banko, Jacobi and Woodworth2009, Scott et al. Reference Scott, Mountainspring, Ramsey and Kepler1986).

Figure 1. Study area within the Hanawi Natural Area Reserve, east Maui, Hawaii.
Mark-Recapture
Mark-recapture was a combination of recapture and re-sight efforts which varied across years, beginning at the higher elevations in 1994–1997 and resuming in 2006–2011 (Simon Reference Simon1998, Simon et al. Reference Simon, Pratt, Berlin and Kowalsky2000, Reference Simon, Pratt, Berlin and Kowalsky2001, Reference Simon, Pratt, Berlin, Kowalsky, Fancy and Hatfield2002, Berlin et al. Reference Berlin, Simon, Pratt, Baker and Kowalsky2001a, Reference Berlin, Simon, Pratt, Kowalsky and Hotfieldb, Pratt et al. Reference Pratt, Simon, Farm, Berlin and Kowalsky2001) and beginning at the lower elevations in 1998 and continuing through 2011. Recapture and re-sight effort was consistent since banding of parrotbills began with some inter-year variation. Most recapture and re-sight effort has occurred from January to June during the peak of the breeding season.
Banding occurred across two field sites connected by an extensive trail system (Figure 1). Individuals were initially captured in mist-nets and banded with a unique combination of a U.S. Fish and Wildlife Service numbered band and three darvic plastic colored leg bands. To increase the capture rates above that of passive mist net efforts, playbacks were used in areas where unbanded individuals had been located. Once captured, parrotbills were aged and sexed using plumage and morphometric criteria (Berlin et al. Reference Berlin, Simon, Pratt, Baker and Kowalsky2001a). Both passive and targeted banding continued annually in different locations covering each study site. Re-sights were obtained by searching for banded individuals systematically across all trails, during each breeding season, as well as opportunistically in the same areas throughout the rest of the year. Subsequent re-sights were documented along with GPS locations.
Analyses
Based on capture, recapture, and re-sight histories from 1994 to 2011 for individually-marked Maui Parrotbill, we used Cormack-Jolly-Seber (CJS) models of live recaptures in program MARK, version 6.0, (White and Burnham Reference White and Burnham1999) to estimate apparent annual survival (ϕ) and encounter probability (ρ). While not explicitly designed for the combination of re-sight and recapture data, CJS is the most appropriate mark-recapture model for this type of data and has been widely used with similar datasets (Nur and Sydeman Reference Nur and Sydeman1999, Sandercock et al. Reference Sandercock, Beissinger, Stoleson, Melland and Hughes2000, Vanderwerf Reference Vanderwerf2009). We used an encounter period of one year given that (i) many individuals were only detected once a year and (ii) that subsequent encounters were often 10–12 months apart. Because of the rugged terrain, the fate of subsequently undetected individuals was unknown, thus the sampled population was defined as open and survival estimates represent apparent survival. Using dates for the initial capture, and all subsequent recaptures and re-sights, we compiled an encounter history for each individual across the 18-year period.
In separate analyses, Maui Parrotbills were grouped by sex (male or female) and age class (juvenile or adult). Hatch-year (HY, juvenile) parrotbills cannot be conclusively sexed and were excluded from the sex-specific analysis. For each analysis, we started with the simplest model in which ϕ and ρ were both constant. Using standard model notation, this model is represented as ϕ(.)ρ(.) for each model set (Lebreton et al. Reference Lebreton, Burnham, Clobert and Anderson1992).
Each model was compared with Akaike’s Information Criterion corrected for small sample size using the quasi-likelihood adjustment (QAICc), as calculated by program MARK. The model with the lowest QAICc value was considered to have the best fit (Burnham and Anderson Reference Burnham and Anderson2002). To test that the arrangement of our data met expectations based on the assumptions underlying the model, we evaluated goodness-of-fit of our global (highest parameterized) model using the program RELEASE GOF provided in program MARK. We adjusted both analyses to the goodness-of-fit calculated value of ĉ (variance inflation factor or lack of fit) from 1,000 simulations before model selection. For both age and sex, we present the most parsimonious model and all models with QAICc weight in addition to the null (ϕ(.)ρ(.)), global (ϕ(g*t)ρ(g*t)), and fully time dependent (ϕ(t)ρ(t)) models.
Results
Between 1994 and 2011, 146 individual Maui Parrotbill were banded in the study area (capture histories can be found in appendices S1 and S2 in the online supplemental materials) and included in the age-specific analysis. Of these, 136 (64 females, 72 males) were included in our sex-specific analysis. Ten were HY birds and were excluded from the sex analysis. The number of individuals recaptured and re-sighted varied each year, an average of 18.11 unique individuals were detected annually.
The best-fit age-specific and sex-specific models for Maui Parrotbill showed apparent survival that varied with age and sex but was constant across years and showed an encounter probability that varied with time (Table 1, Model 1; Table 2, Model 1; Figure 2). No other models were a reasonable fit for either group. We found strong support for sex-specific differences in survival with males showing higher survival rates than females (males 0.82 ± 0.03; females 0.72 ± 0.04). We also found juveniles to show lower survival rates than adults (juveniles 0.17 ± 0.15; adults 0.78 ± 0.02) (Figure 3). While our sex-specific model had good fit to the CJS model selected (GOF Test 2 + Test 3 χ2 = 51.320, df = 57, P-value = 0.687, ĉ = 0.900), our age-specific model did not (GOF Test 2 + Test 3 χ2 = 56.164, df = 35, P-value = 0.013, ĉ = 1.605) due to insufficient data in the HY group to calculate independent χ2 results.
Table 1. Apparent survival (ϕ) and encounter probability (ρ) models for Maui Parrotbill grouped by age (juvenile and adult). Subscripts indicate whether parameters differed among groups (e.g. ϕage) or time (ϕt) or were constant (ϕ.). Over-dispersion is corrected to 1.605 (ĉ) based on goodness of fit test on global model (ϕage*tρage*t). ΔQAICc is the difference from the best (lowest AICc) model. AICc weight is the relative likelihood of each model.
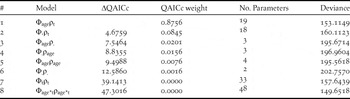
Table 2. Apparent survival (ϕ) and encounter probability (ρ) models for Maui Parrotbill grouped by sex (juvenile birds omitted). Subscripts indicate whether parameters differed among groups (e.g. ϕsex) or time (ϕt) or were constant (ϕ.). Data under-dispersed (ĉ = 0.900) based on goodness-of-fit test on global model (ϕsex*tρsex* t), ĉ left at 1.00. ΔQAICc is the difference from the best (lowest AICc) model. AICc weight is the relative likelihood of each model.


Figure 2. Encounter probability variation in Maui Parrotbill survival analyses by age (a) and sex (b). Error bars indicate standard error for each year 1995–2010.

Figure 3. Apparent survival probability in Maui Parrotbill by sex (a) and age (b). Error bars indicate standard error for each individual group.
Discussion
As with most survival studies of an open population, mortality, and emigration cannot be separated and thus survival is likely to be underestimated (Cilimburg et al. Reference Cilimburg, Lindberg, Tewksbury, Hejl and Thompson2002). This effect is especially true in rugged terrain which limits detectability. Even so, adult apparent survival was similar to that of other Hawaiian avifauna; Akohekoke Palmeria dolei show the highest annual survival of any Hawaiian passerine at 0.95 (Simon et al. Reference Simon, Pratt, Berlin and Kowalsky2001) but the average annual adult survival of 16 Hawaiian passerines averaged 0.78 ± 0.03 (Pratt et al. Reference Pratt, Atkinson, Banko, Jacobi and Woodworth2009, Woodworth and Pratt Reference Woodworth and Pratt2009). Conversely, juvenile apparent survival was lower than expected. Although the greater dispersal of young can contribute to differences in adult and juvenile survival (Greenwood and Harvey Reference Greenwood and Harvey1982), juvenile parrotbill survival was lower than that demonstrated for other Hawaiian birds. Woodworth and Pratt (Reference Woodworth and Pratt2009) reported that the average annual juvenile survival of 13 other Hawaiian passerines was 0.32 ± 0.03. However, the juvenile parrotbill survival estimate was based on only 10 individuals, which contributes to the large standard error and only a moderate fit to our CJS model. While acknowledging that our estimate lacks precision, juvenile parrotbill survival is certainly lower than that of adults and it would not be surprising if juvenile survival of this species is particularly low. Unpublished data on territory occupancy in the Hanawi study site indicates that most available habitat is occupied. Young birds may be forced into poorer quality habitat, limiting their chances of survival. Although, we currently have little information on juvenile dispersal, and a larger sample size is necessary for a more precise survival estimate, it should be noted that the juvenile individuals included in Vetter et al. (Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012) were never re-sighted in the subsequent years of this study. The lack of detection for any of these juvenile individuals in the years that followed, combined with high estimates of juvenile survival during years of low detection probability early on in this study, accounts for the large difference in juvenile survival estimates between the two datasets (Figure 4). Several re-sights of juvenile individuals during years of low overall detection probabilities artificially inflated the early juvenile survival estimates used in Vetter et al. (Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012). We appreciate that both datasets are still sparse in their data on juvenile individuals but given that none of the juveniles marked during the years of high detection probabilities were seen again, we believe that this brought the average juvenile survival down to a more representative value.
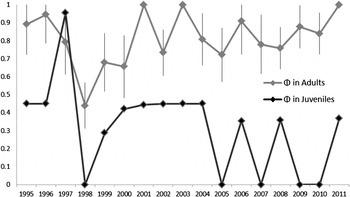
Figure 4. Apparent survival for juvenile Maui Parrotbill varied much more through time than did adult apparent survival illustrating the limitations of using a smaller dataset may distort the results to suggest higher juvenile survival.
Vetter et al. (Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012) also did not detect strong differences in apparent survival between male and female parrotbills. As both analyses were conducted using similar methods, the increased detection probability in the survey years 2008–2011 in this study provided a more robust sample with which to demonstrate the sex-specific apparent survival. We found that Maui Parrotbill maintain strong pair bonds throughout the year and are often seen together. We found little bias for sex-specific encounter probabilities and re-sights were nearly evenly distributed between the sexes (females 410, males 465). Additionally, if we consider the Φsex.ρsex model (QAICc weight = 0.000), and assume that sex-specific encounter rates had a larger effect than the models suggested, males had a detection probability of 0.457 (± 0.043) and females 0.666 (± 0.060). As with many species, the difference in apparent survival between males and females is more likely a result of higher reproductive costs for females, a higher rate of depredation, and/or higher emigration rates. While we can only speculate on the latter due to a lack of data, females do incur high energetic demands associated with egg production and incubation and have a higher risk of being depredated on the nest (Fontaine and Martin Reference Fontaine and Martin2006, Ghalambor and Martin Reference Ghalambor and Martin2001, Nur Reference Nur1998). Although female parrotbills alone incubate eggs and brood nestlings, the cost of reproduction for females may be similar to the cost to males of establishing and defending territories as well as provisioning females and offspring. Owens and Bennett (Reference Owens and Bennett1994) found that provisioning chicks can have a higher direct mortality cost to adults than nest building and incubation. In parrotbills, higher female mortality is more likely the result of higher rates of depredation. Rodents Rattus spp. are predators of native island birds and have been documented depredating incubating and brooding females (Atkinson Reference Atkinson1977, Moors et al. Reference Moors, Atkinson and Sherley1992, Robertson et al. Reference Robertson, Hay, Saul and McCormack1994). In Hawaii, rats are responsible for the high female mortality in the Oahu Elepaio Chasiempis ibidis (Vanderwerf and Smith Reference Vanderwerf and Smith2002) and may account for the sex-specific survival difference noted in the parrotbill.
Our highest selected models all incorporated a detection probability that varied considerably through time. Annual survey effort was influenced by the remoteness of our field sites and the rugged terrain. Access to the study area (by helicopter and on foot) was typically influenced by weather. Poor weather further influenced the probability of detecting individuals as re-sighting individuals in rain or mist was difficult. Despite uneven detection probabilities, the differences in survival estimates between this study and Vetter et al. (Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012) illustrates the importance of long-term datasets for rare and cryptic species as well as those which may provide a scarcity of data within any period of years. We analysed just the 2003–2011 datasets using the same methodologies to better understand the differences between these two studies. While the first portion of this dataset used in Vetter et al. (Reference Vetter, Swinnerton, Vanderwerf, Garvin, Mounce, Breniser, Leonard and Fretz2012) may have overestimated juvenile survival and was not able to resolve sex-based differences in apparent survival, the same is true of the latter half of the dataset when considered independently. Although these years had very high detection probabilities associated with them, and found similarly low juvenile survival, this subset of data had unresolved model rankings.
Our results suggest that conservation management focused on increasing female (and possibly juvenile) survival would likely benefit the recovery of the Maui Parrotbill population. Male survival would appear to be high for a small passerine, but although not necessarily so for a tropical species (see Vanderwerf Reference Vanderwerf2009). Landscape-scale rodent control would likely benefit the parrotbill and other native forest birds on Maui. Female survival of the Oahu Elepaio has been shown to increase following rodent control (Vanderwerf and Smith Reference Vanderwerf and Smith2002). Other strategies could include intensive management such as supplementary feeding. Food supplementation has been a successful strategy in recovering endangered birds including the San Clemente Loggerhead Shrike Lanius ludovicianus mearnsi (Heath et al. Reference Heath, Kershner, Cooper, Lynn, Turner, Warnock, Farabaugh, Brock and Garcelon2008), Florida Scrub Jay Aphelocoma coerulescens (Schoech et al. Reference Schoech, Bridge, Boughton and Reynolds2008), Hihi Notiomystis cincta (Castro et al. Reference Castro, Brunton, Mason, Ebert and Griffiths2003), and Kakapo Strigops habroptila (Clout et al. Reference Clout, Elliott and Robertson2002) and has served as a short-term measure to support populations while longer-term habitat restoration occurs.
Finally, restoring high elevation forests that are buffered from extreme weather and that have a high abundance of koa trees may provide the greatest opportunity to increase the parrotbill population size (Becker et al. Reference Becker, Mounce, Rassmussen, Rauch-Sasseen, Swinnerton and Leonard2010, Simon et al. Reference Simon, Baker, Baker, Poole and Gill1997, U.S. Fish and Wildlife Service 2006). More data may be able to resolve our juvenile survival estimates for this model. If juvenile survival is indeed low as a result of all suitable habitats being already occupied, the addition of new high quality habitat may be the only management strategy capable of increasing juvenile survival. Currently 1,100 ha of mesic koa forest is being restored on leeward east Maui, possibly the single most significant conservation action taken for the parrotbill since the exclusion of feral ungulates from Hanawi in 1997. Experimental releases of Maui Parrotbill into this habitat are scheduled to occur in the next five years.
Supplementary Material
The supplementary materials for this article can be found at journals.cambridge.org/bci.
Acknowledgements
This study was conducted by the Maui Forest Bird Recovery Project, a project administered and funded by the Pacific Cooperative Studies Unit, University of Hawaii, the State of Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife, and the U.S. Fish and Wildlife Service. The Division of Forestry and Wildlife, and the Pacific Island Office of the U.S. Fish and Wildlife Service provided scientific input, access into the Hanawi Natural Area Reserve and the required state and federal permits. We would like to thank the following partners for logistical support and access to their properties: the Hawaii Natural Area Reserves System Commission, Haleakala National Park, Haleakala Ranch, The Nature Conservancy, Tri-Isle RC and D, Pacific Helicopters, and Windward Aviation. We would like to thank Thane Pratt, John Simon, Paul Baker, and Stuart Pimm for use of their banding and re-sight data. We are grateful to Rick Camp and the Hawaii Forest Bird Interagency Database Project for providing population estimates and densities from historical and recent bird surveys. This manuscript was greatly improved by the help of Eben Paxton and one anonymous reviewer. Finally, we thank all of the seasonal biologists who assisted in the strenuous data collection for this study.








