Introduction
The populations of several bird species breeding in Europe have undergone marked, mostly negative, demographic changes during recent decades (Tucker and Heath Reference Tucker and Heath1994, BirdLife International 2004, Donald et al. Reference Donald, Sanderson, Burfield and van Bommel2006). The sign and steepness of these trends, however, are distributed non-randomly among taxa and according to species’ ecology and life-histories, as susceptibility to decline is associated with specific habitat preferences and major life-history traits such as e.g. migratory behaviour (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Møller et al. Reference Møller, Rubolini and Lehikoinen2008, Both et al. Reference Both, van Turnhout, Bijlsma, Siepel, Van Strien and Foppen2010).
Birds breeding in farmland have suffered steeper declines than forest or aquatic species, partly because of the direct impact of the changes in agricultural practices that took place in the course of the second half of the twentieth century. These changes have resulted in a rapid shift from the traditional agricultural mosaic that characterised farmland habitats for centuries, to large-scale, homogeneous, intensively cultivated agro-ecosystems with low biodiversity (Chamberlain et al. Reference Chamberlain, Fuller, Bunce, Duckworth and Shrubb2000, Chamberlain and Fuller Reference Chamberlain and Fuller2001, Donald et al. Reference Donald, Green and Heath2001, Reference Donald, Sanderson, Burfield and van Bommel2006). For example, it has been shown that population trends of farmland birds negatively covaried with cereal yield across European countries (Donald et al. Reference Donald, Green and Heath2001), suggesting that agricultural intensification, determining large-scale shifts in land management, may be causally related to farmland bird declines (Chamberlain et al. Reference Chamberlain, Fuller, Bunce, Duckworth and Shrubb2000).
Long-distance trans-Saharan migratory birds have declined more than short-distance migrants and residents (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Møller et al. Reference Møller, Rubolini and Lehikoinen2008, Both et al. Reference Both, van Turnhout, Bijlsma, Siepel, Van Strien and Foppen2010). This can have several concomitant causes. The varying rate of change in ecological conditions occurring in the areas where birds spend different parts of their annual life cycle may result in an ecological mismatch of species that are not able to track the optimal conditions for reproduction under a changing climate (Both and Visser Reference Both and Visser2001, Both et al. Reference Both, Bouwhuis, Lessells and Visser2006, Ambrosini et al. Reference Ambrosini, Saino, Rubolini and Møller2011, Saino et al. Reference Saino, Ambrosini, Rubolini, von Hardenberg, Provenzale, Hüppop, Hüppop, Lehikoinen, Lehikoinen, Rainio, Romano and Sokolov2011). Rapid changes in the ecological conditions of African wintering or stopover habitats may also negatively affect migrant survival and population trends (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Zwarts et al. Reference Zwarts, Bijlsma, van der Kamp and Wymenga2009). In particular, it has been observed that most declining species winter in open-dry habitats in Africa (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006), probably due to increasing habitat degradation and loss within African drylands, such as the Sahel region, a major wintering and staging area for Afro-Palearctic migrants (Zwarts et al. Reference Zwarts, Bijlsma, van der Kamp and Wymenga2009).
The Barn Swallow Hirundo rustica epitomises some of the risk factors that have been shown to predict demographic decline in comparative studies. It is a long-distance migrant that overwinters in open habitats south of the Sahara Desert (Cramp Reference Cramp1988, Møller Reference Møller1994, Turner Reference Turner2006). It is also a farmland bird, foraging mainly on open hayfields and pastures, and along hedgerows. In addition, it is strictly associated with traditional rural buildings for nesting. In fact, since breeding takes place most often in cowsheds and stables with cattle and horses, it can be markedly affected by rapid changes in livestock farming practices that have occurred widely in Europe during recent decades, resulting in the progressive abandonment of traditional cattle sheds in favour of modern, intensive sheds that are less suitable for Barn Swallow nesting (Møller Reference Møller1994, Reference Møller2001, Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002, Turner Reference Turner2006).
Barn Swallows breeding in farms where livestock is reared have larger reproductive success than those breeding in farms without it (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). This probably occurred because Barn Swallows benefit from warmer indoor temperatures when they nest in buildings with livestock (Ambrosini and Saino Reference Ambrosini and Saino2010). Warmer temperatures in turn allow for earlier reproduction and a larger number of pairs laying a second clutch (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). In addition, presence of livestock at a farm is usually correlated with higher food availability for the insectivorous Barn Swallow, both because manure enhances insect production, and because hayfields and pastures, which are the preferred foraging habitat of this species, are larger around farms with livestock (Møller Reference Møller2001, Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002, Evans et al. Reference Evans, Wilson and Bradbury2007, Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). All these benefits result in an overall larger nestling survival rate in farms with livestock, particularly of second broods (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010), and may explain the strong preference of nesting Barn Swallows for cowsheds, stables and, in general, for buildings where livestock is reared (Ambrosini and Saino Reference Ambrosini and Saino2010, Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). Cessation of livestock farming at a farm may therefore result in lower reproductive success (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010) and fewer yearlings recruited to the colony (Møller Reference Møller2001).
The Barn Swallow has declined in several parts of its European range, but the extent of this decline varies widely across geographical areas (Møller Reference Møller1989, Tucker and Heath Reference Tucker and Heath1994, Siriwardena et al. Reference Siriwardena, Baille, Buckland, Fewster, Marchant and Wilson1998, Robinson et al. Reference Robinson, Crick and Peach2003, BirdLife International 2004, PECBMS 2009). Several mechanisms operating in different parts of the annual life-cycle, and thus in different geographical regions, have been invoked as causes of the decline. Agricultural intensification and cessation of livestock farming at a farm may determine the decline of the local colony (Møller Reference Møller2001, Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002), whereas habitat degradation and loss on breeding and wintering grounds, or along migration routes probably act synergistically to determine the general decline of Barn Swallow populations (Saino et al. Reference Saino, Szép, Ambrosini, Romano and Møller2004, Robinson et al. Reference Robinson, Balmer and Marchant2008).
In the present study, we first report on the population dynamics of Barn Swallows breeding in three agricultural areas in northern Italy, which differ in general ecological conditions, such as altitude, major land use, and farming intensity, based on a very large sample of 190 farms where breeding pairs have been censused both in 2001 and 2010. Then, we analyse the effect of animal farming on local population trends to test the prediction that cessation of animal farming during the study period resulted in more negative population trends compared to conditions where animal farming did not change (i.e. it was present or absent both at the start and the end of the study period). By comparing population changes taking place at each of the three study areas, we could also explore whether the effects of animal farming differed according to general ecological conditions of the area where colonies were located.
Methods
Study areas and field methods
The study was carried out in Northern Italy, specifically in the Parco Regionale Adda Sud (‘AS’ hereafter; coordinates of the approximate centre: 45°19’N, 9°40’E, 24.260 ha), in the Parco Piemontese della Valle del Ticino (‘TP’, 45°33’N, 8°44’E, 6.561 ha), and in the Parco Regionale di Montevecchia e della Valle del Curone (‘MC’, 45°42’ N, 9°22’ E, 2.350 ha) (Figure 1). Maize fields (44%) and hayfields (32%), i.e. fields where grass or alfalfa Medicago sativa are not grazed but cut to produce dry feed for livestock during the winter, are the prevalent crop types in AS, which is located in the low Po Plain of Lombardy (height of monitored farms: 40–108 m asl). Woods (37%) and hayfields (25%) prevail in TP, which is in the high Po Plain in Piedmont (height of monitored farms: 99-281 m a.s.l.). MC is a hilly area (height of monitored farms: 258–442 m) in Lombardy where coppices (38%) and hayfields (24%) are the predominant land uses.

Figure 1. (a) Lombardy (dark grey) and Piedmont (light grey) in Italy and Europe. (b) The study areas in Lombardy and Piedmont: TP: Parco Piemontese della Valle del Ticino, MC: Parco Regionale di Montevecchia e della Valle del Curone, AS: Parco Regionale Adda Sud. (c-e) The monitored farms within each study area. Symbols represent the demographic trend of colonies between 2001 and 2010; squares: farms with constant demographic trend, upper triangles: farms with increasing populations; lower triangles: farms with decreasing populations; diamonds: farms where only presence-absence data were available.
The size of farms, as estimated during the 2010 census and expressed as the overall area of cowsheds, stables, barns and other buildings that are accessible to Barn Swallows at each farm (see below for a definition of ‘farm’), was much larger in AS (on average 3,053.3 ± 288.5 SE m2, n = 110) than in the other study areas (TP: 1,000.8 ± 195.0 SE m2, n = 50, this information was unavailable for 6 farms; MC: 238.1 ± 45.5 SE m2, n = 49, all farms censused in 2010 were included in this analysis). Conversely, farm density is larger in MC (3.4 farms km-2, total number of farms in the study area: n = 80) than in AS (1.3 farms km-2, n = 319) or in TP (1.2 farms km-2, n = 76), total number of farms in the study area: n = 80), as estimated by a complete census of all farms at each study area performed by means of detailed maps (scale 1:10,000), aerial photos, and Google Earth (Mountain View, CA).
As sample units we used groups of rural buildings (hereafter ‘farms’) that were separated by at least 100 m from other groups of buildings (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002). Albeit most buildings were originally farms, their use at the time of the censuses could have changed to e.g. houses, restaurants, or farm holiday centres. In AS, a long-term monitoring project of Barn Swallow populations is ongoing since 1999 in a random sample of the farms in the Park or in the surrounding area (see Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002 for details). In this study area, 108 farms were monitored both in 2001 and 2010 and 94 in all years in 1999-2010. In TP, 56 farms within the boundaries of the Park or in the surrounding area were monitored both in 2001 and 2010. The other farms in this study area could not be censused due to inaccessibility or unwillingness of the owners. For the same reasons, in 2010 we could not obtain reliable estimates of colony size at three farms in TP (though we could confirm that breeding took place). These farms were therefore excluded from the analyses of demographic trends and colony size, but not from those of colony extinction probability. In MC, 26 randomly chosen farms were monitored both in 2001 and 2010.
In 2010 all farms at each study area were monitored according to a standardised protocol reported in detail in Ambrosini et al. (Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002). Briefly, each farm was visited every 14 days and the content of all nests inspected. The number of pairs at a farm was then estimated as the maximum number of nests simultaneously active (i.e. with eggs or nestlings) during April–June. In 2001 farms in AS were monitored according to the same protocol as above, while farms in TP and in MC were visited monthly and all nests inspected. Colony size was estimated as above.
Data on the presence of livestock at each farm were collected during visits to the farms in each year in AS. In TP and in MC livestock data for 2010 were collected during the visits to the farms, while those for 2001 were obtained by interviewing the farmers (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002). This information was summarised as a dichotomous variable (‘livestock farming’) accounting for presence or absence of livestock on a farm in a given year. In addition, for each farm a three-level categorical variable (‘livestock category’) was generated, accounting for the presence of livestock in a farm both in 2001 and 2010 (‘Present’), in none of the two years (‘Absent’), or only in 2001 (‘Ceased’). Three farms in AS and three in TP where livestock was reared in 2010 but not in 2001 were discarded from the analyses where this latter variable was entered as predictor.
Statistical analyses
The total population size at each study area in 2001 and 2010 was calculated by multiplying the mean colony size at each farm by the total number of farms at each study area. Standard errors of total population sizes were calculated while accounting for the sampling fraction (Sutherland Reference Sutherland2006).
The annual growth rate of populations (r parameter) at each study area and at each farm was estimated as the slope of the Poisson regression, corrected for data overdispersion, of the number of breeding pairs on year (Pannekoek and van Strien Reference Pannekoek and van Strien2005). The annual colony extinction probability (hereafter termed E parameter) was calculated as above by regressing the number of colonies on year. Since most populations declined during the study period, in this paper we also refer to the ‘decline rate’ of a population as the opposite of the annual growth rate (-r) for simplicity.
Estimates of E from the sample of AS farms monitored in 2001 and 2010 were within the 95% confidence limits of the same parameters calculated on the AS farms sampled each year during 1999–2010 (E = 0.011, 95% CL: 0.005; 0.017; see Table 1 for estimates for 2001 and 2010). The value of r estimated from farms monitored in 2001 and 2010 was slightly lower than that estimated from farms monitored each year during 1999–2010 (r = -0.063, 95% CL: -0.092, -0.034). There was also no evidence of significant deviation from linearity of demographic trends estimated from Poisson regressions of annual censuses (significance of the quadratic term of year: |t|9 ≤ 1.0, P ≥ 0.34 in both cases). Hence, the analysis of data from 2001 and 2010 returned estimates of decline rates and extinction risk of colonies similar to those obtained by annual censuses. We therefore focused on parameter estimates obtained by the comparison of data collected in 2001 and 2010, as these data were available for all the study areas.
Table 1. Summary statistics of demographic trends recorded in the different study areas; n is the number of censused farms; E is the annual extinction probability, r is the annual population growth rate. Numbers in brackets represent standard errors. Estimates of total population sizes were obtained by the ratio estimator, and their standard errors were corrected for small populations.

* n = 53 farms
Decline rates were then compared among study areas and livestock categories in Generalized Linear Models (GLM) models or in Generalized Least Squares (GLS) models when variances were heterogeneous between study areas or cattle categories (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Colony extinction was coded as a dichotomous variable equal to 1 if a colony that existed in 2001 was extinct in 2010 and to 0 otherwise. This variable was then analysed in binomial GLMs to investigate whether the probability of colony extinction differed between study areas and cattle categories. Post-hoc tests (Tukey method) were also performed. We note that the calculation of decline rates of populations allows us to easily compare demographic trends calculated in all years in 1999–2010 and in years 2001 and 2010.
In order to investigate in detail the effect of livestock farming on the number of breeding Barn Swallow pairs per farm, and the effect of change in livestock farming on population trends and extinction probability, we used Generalized Linear Mixed Models (GLMMs). A Poisson error distribution was assumed in the models of number of breeding swallows at farms, a binomial error distribution in models of colony extinction and a Gaussian error distribution in models of population trend.
Year (2001 or 2010, entered as a dichotomous variable) was included as a fixed effect in the GLMMs of the number of breeding pairs at each study area. In developing these models, we first investigated the most appropriate structure of their random part. According to Zuur et al. (Reference Zuur, Ieno, Walker, Saveliev and Smith2009), we initially included year as a random slope at the farm level, and checked whether this improved the fit of the model. In all cases, random slope models fitted the data better than models only including farm as a random factor (likelihood ratio tests (LRT): χ22 ≥ 12.3, P ≤ 0.002). Inclusion of year as a random slope at the farm level allows the models to control for the between-farm variation in growth rates, and avoids inflating type-I error rate (Schielzeth and Forstmeier Reference Schielzeth and Forstmeier2009). However, inclusion of random slopes enlarges the number of random parameters that must be estimated by the models. In our GLMMs, in particular, the number of random parameters was twice the number of farms (one intercept and one slope per farm), thus equalling the total number of available observations (two years of data per farm). Hence, the inclusion of a random slope in a model where only two years of data were available per farm saturated the number of random effects that could be entered in the model, as it is not possible to estimate a number of random effects larger than the number of observations. This prevented extension of the GLMM to analyse the effect of livestock farming on colony size at all study areas simultaneously. Indeed, a model of this kind would have required entering study area as an additional random factor. To obtain an overall test of the effect of livestock farming and year on the number of breeding pairs at all study areas, we therefore had to rely on a different approach, whereby we summarised the results from models for each study area by the weighted Z-method, a procedure that allows combining information across multiple tests of the same null hypothesis (Whitlock Reference Whitlock2005).
Conversely, we used GLMMs with study area entered as a random factor and assuming a Gaussian or a binomial error distribution, to investigate the effect of livestock category on population trend or colony extinction probability, respectively, at all study areas. Since ‘livestock category’ seemed to differently affect decline rates and extinction probabilities in different study areas, we first included in the GLMMs this factor as a random slope within study area, besides as a fixed effect (Schielzeth and Forstmeier Reference Schielzeth and Forstmeier2009). However in all cases, models with a simpler random structure only, including the study area as a random factor, had a similar fit than random slope models (LRTs: χ25 ≤ 4.51, P ≥ 0.48), and were therefore preferred. Since models were underdispersed (dispersion parameter ≤ 0.70), we conservatively did not correct for overdispersion in the Poisson and binomial GLMMs (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009).
We used R 2.8.1 (R Development Core Team 2008) for statistical analyses, with the nlme procedure (Pinheiro et al. Reference Pinheiro, Bates, DebRoy and Sarkar2008) for GLS and Gaussian mixed models, the lme4 procedure (Bates et al. Reference Bates, Maechler and Dai2008) for Poisson and binomial GLMMs, and the multcomp procedure (Bretz et al. Reference Bretz, Genz and Horton2001) for post-hoc tests.
Results
Demographic trends in the three study areas
Decline rates and extinction probabilities calculated for the three study areas by comparing the number of breeding pairs and the number of colonies recorded in 2001 and 2010 are shown in Table 1. Combining the data from the three study areas it appeared that the size of the breeding population declined by 53.1% (i.e. by 8.4% per year), and the number of colonies by 19.6%, between 2001 and 2010.
The decline rate of the breeding pairs in colonies that existed both in 2001 and 2010 significantly differed between study areas (GLS model for inequality of variances: F 2,104 = 3.15, P < 0.001), with a significant difference between AS and MC, while decline rates in the other comparisons were similar (Figure 2a; details not shown).

Figure 2. (a) Average annual decline rates (-r parameters in a population growth model) of Barn Swallow colonies and (b) proportion of colonies that went extinct between 2001 and 2010 in the Parco Regionale Adda Sud (AS), Parco Piemontese della Valle del Ticino (TP), Parco Regionale di Montevecchia e della Valle del Curone (MC). Bars represent standard errors. Numbers represent sample sizes. Bars with different letters indicate significant (P < 0.05) differences between study areas at post-hoc tests.
Study area also significantly explained variation in the probability of colony extinction (binomial GLM: χ22 = 10.7, P = 0.005), that was significantly higher in MC than in AS. No other significant difference was observed (Figure 2b).
Livestock farming and colony size and presence
The mean number of breeding pairs per farm was significantly larger in farms with than without livestock, both in AS and TP, but not in MC (Table 2). The decline in the number of breeding pairs, that was statistically significant in all study areas (Table 2), occurred at a similar rate in farms with and without livestock, as indicated by the fact that the livestock by year interaction was never significant and was therefore removed from all models (AS: -0.43 ± 0.32 SE, z = 1.33, P = 0.185; TP: 0.57 ± 0.51, z =1.1, P = 0.27; MC: 0.61 ± 0.77 SE, z = 0.80, P = 0.43). These findings were confirmed by pooling results from the three study areas (effect of livestock: Zw = -8.700, P < 0.001; effect of census year: Zw = -7.928, P < 0.001).
Table 2. Fixed effects from Poisson GLMMs of the number of breeding pairs per farm according to presence of livestock farming and census year. The livestock per year interaction was never significant (all P ≥ 0.185), and was therefore removed from the models. In all models farm was entered as a random factor and year as a by-farm random slope.
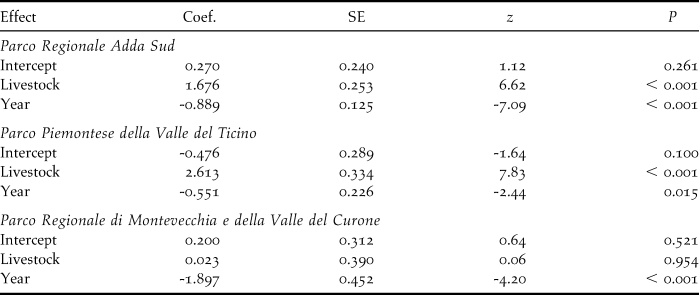
Livestock farming and demographic trends
Comparison of the decline rates of colonies on the farms censused both in 2001 and 2010 showed significant differences according to livestock category in TP (F 2,21 = 6.70, P = 0.006), with colonies on farms where livestock farming ceased declining more than those in the other livestock categories (post-hoc test: |t|21 ≥ 2.84, P ≤ 0.025, Figure 3a). Marginally non-significant differences were found in AS (F 2,67 = 2.79, P = 0.068), and MC (GLS model for inequality of variances: F 2,6 = 4.93, P = 0.054).
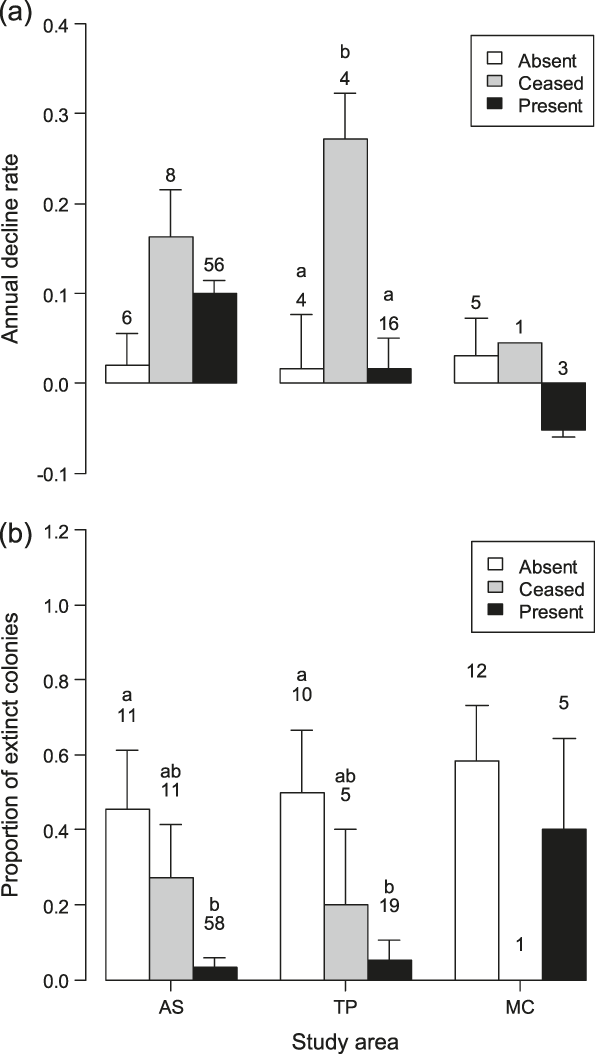
Figure 3. (a) Annual decline rates (-r parameter in a population growth model) of Barn Swallow colonies and (b) proportion of colonies that went extinct between 2001 and 2010 in the three livestock categories within each study area. Bars represent standard errors and numbers sample sizes. Bars with different letters indicate significant (P < 0.05) differences between the livestock categories within each study area at post-hoc tests.
The mixed model analysis combining data from the three study areas revealed a significant variation in the decline rates according to livestock category (F 2,98 = 6.90, P = 0.001). Specifically, Barn Swallow colonies in farms where livestock farming ceased were estimated to decline significantly more than in farms where livestock was always or never reared (|z| ≥ 3.29, P ≤ 0.003; Figure 4a).

Figure 4. (a) Annual decline rates (-r parameter in a population growth model) of Barn Swallow colonies and (b) proportion of colonies that went extinct between 2001 and 2010 in farms in different cattle categories in the three study areas. Bars represent standard errors and numbers sample sizes. Bars with different letters indicate significant (P < 0.05) differences between cattle categories at post-hoc tests.
Probability of colony extinction varied according to livestock category in AS (LRT: χ22 = 14.8, P < 0.001) and in TP (LRT: χ22 = 7.9, P = 0.020, Figure 3b), being significantly smaller in farms where livestock was reared in both years than in farms where livestock was always absent (|z| ≥ 2.40, P ≤ 0.043). No significant difference in the probability of colony extinction between farms in different livestock categories appeared in MC (LRT: χ22 = 1.9, P = 0.383).
The mixed model analysis of data from the three study areas revealed a highly significant difference in colony extinction probability between farms in different livestock categories (LRT: χ22 = 25.1, P < 0.001), with colonies on farms where livestock was reared in both years showing a significantly smaller estimated extinction probability than that on farms where livestock was never reared and where it ceased between 2001 and 2010 (Figure 4b). The difference in the probability of colony extinction between farms where livestock farming ceased and where livestock has never been reared was marginally non-significant (post-hoc test: z = -1.85, P = 0.064). Addition of colony size in 2001 as a covariate did not affect the results (LRT of livestock category: χ22 = 15.8, P < 0.001) and revealed that initially large colonies had a smaller extinction probability compared to small ones (coefficient: -0.170 ± 0.058 SE; LRT: χ21 = 15.1, P < 0.001). No interaction effect between livestock category and colony size in 2001 was observed (LRT of interaction: χ22 = 4.29, P = 0.12).
Discussion
In this study we gathered current (2010) and historical (2001) information on the size of breeding Barn Swallow populations in a very large sample of 190 farms in three study areas in Northern Italy where general ecological conditions differ. We also collected detailed information on colony size in each year between 1999 and 2010 in 94 farms in one study area, and we could therefore assess that demographic trends calculated on the basis of data from 2001 and 2010 reliably reflect those from annual censuses.
We documented a dramatic decline in the Barn Swallow population (8.4% per year), larger than that reported for the whole of Lombardy (4.3% per year) by Bani et al. (Reference Bani, Massimino, Orioli, Bottoni and Massa2009) by means of point counts, and much larger than the 9% estimated for Europe by the European Bird Census Council in the period 1990–2006, corresponding to an annual decline of 1% (EBCC 2008, PECBMS 2009). This decline is however similar to that documented in a Danish population during 1970–1999 (7.6% per year), for which time to extinction was estimated at 22 years by means of stochastic population models (Engen et al. Reference Engen, Sæther and Møller2001). Differences in demographic trends among Barn Swallow populations breeding in different parts of Europe are probably due to differences in farming practices (and in their change over time) among European regions (Báldi and Batáry Reference Báldi and Batáry2011). In addition, geographical populations of Barn Swallow segregate in different African regions during winter (Ambrosini et al. Reference Ambrosini, Møller and Saino2009, Reference Ambrosini, Saino, Rubolini and Møller2011), and therefore may be affected differently by changes in ecological conditions at their wintering grounds.
Barn Swallow populations are known to show large fluctuations at decadal scales (Siriwardena et al. Reference Siriwardena, Baille, Buckland, Fewster, Marchant and Wilson1998, Robinson et al. Reference Robinson, Crick and Peach2003). However, the decline of this population seems almost continuous during the 10 study years, at least in AS, as indicated by the fact that overall population trend in the sample of farms that were monitored in all years did not deviate from linearity (see Methods). In addition, the observed decline seems, unfortunately, to reflect the steep negative demographic trend of this species in the Po plain that took place in previous years. Indeed, Selmi and Checchi (Reference Selmi and Checchi2001) reported a decline of 54.6% in the number of Barn Swallow pairs breeding in the municipality of Spilamberto (Modena province) between 1990 and 1999. This information therefore suggests that Barn Swallows in Northern Italy may have declined to one quarter of their initial population size during the last 20 years.
Barn Swallows seem to have declined at different rates in the three study areas. Before further discussing these results, two main caveats deserve consideration. First, the number of farms that were censused both in 2001 and 2010 was much lower in MC than in the other study areas. The non-significant results in the analysis comparing population size and trend at farms in different cattle categories in MC may therefore be due to the low power of statistical tests. Second, sampling protocols in 2001 (but not in 2010) differed among study areas, as farms in TP and MC were visited monthly in that year, while farms in AS every second week. We checked whether different sampling rates may have biased our estimates of colony sizes in 2001 and, consequently, of decline rates. To this end, we reassessed the number of breeding pairs at 20 randomly chosen farms in AS in 2001 as if we had sampled them once a month, rather than every second week. This was simply done by considering only the data collected on each second visit to a farm. Halving the sampling rate implied a reduction of estimated decline rate at a colony by only 0.005, i.e. one order of magnitude lower than the significant difference in decline rates between AS and MC (0.09 ± 0.03 SE, Figure 2). Hence, we are confident that difference in sampling rates in different areas did not bias our general conclusions.
The decline was steeper in AS, an intensively cultivated area in the low Po plain where mean colony size was the largest, and lowest in MC, a hilly area that hosted the smallest colonies, being intermediate in TP, an intensively cultivated area in the high Po plain, where mean colony size is also intermediate. Intensification of farming practices, which have been invoked to explain negative trends of Barn Swallow populations in Switzerland (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010), may have occurred at different rates in the three study areas, and may therefore explain the observed differences in decline rates. Indeed, AS and TP, where swallows declined the most, are more intensively cultivated than MC. However, colony extinctions have occurred at a lower rate in AS and in TP than in MC, probably due to the larger average size of colonies in the former study areas. In addition, in MC half of the colonies went extinct between 2001 and 2010, probably because several farms were remodelled in these years (R. Ambrosini, pers. obs.), and therefore have probably become unsuitable for Barn Swallow reproduction. MC may therefore be an area where Barn Swallows breed at low densities due to reduced breeding site availability. Farms are smaller in this study area compared to the others, and farm density is higher. In addition, climate and general ecological conditions in this hilly area differ from those in the other intensively cultivated study areas on the plain. Habitats in MC may therefore be sub-optimal for this species, as suggested by the observation that in 2010 farms in MC hosted an average number of breeding pairs (1.18 ± 0.37 SE) similar to that of farms without livestock in the other study areas (AS: 1.70 ± 0.43; TP: 0.82 ± 0.34).
Presence of livestock farming at a farm positively influenced the size of Barn Swallow colonies, as expected (e.g. Møller Reference Møller1994, Reference Møller2001, Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002, Turner Reference Turner2006), in AS and TP, and in the analyses combining the results at the three study areas, but not in MC. This lack of association between livestock farming and Barn Swallow distribution at this area is difficult to explain, and may again be related to the different general ecological conditions in this area. Alternatively, it may simply be the by-product of the low power of statistical tests due to the small sample of farms at this study area.
The strong positive association between Barn Swallows and livestock explains the large negative impact that cessation of livestock farming had on their breeding populations. Indeed, colonies in the farms where livestock farming ceased declined more steeply than those in farms where livestock was always or never reared. This result is consistent with previous studies carried out in Denmark (Møller Reference Møller2001), which demonstrated that cessation of livestock farming determined a lower recruitment of yearlings, leading to a rapid decline of the colony (Møller Reference Møller2001). In addition, presence of livestock in the same room of the nest favours Barn Swallow reproduction (Ambrosini et al. Reference Ambrosini, Ferrari, Martinelli, Romano and Saino2006, Ambrosini and Saino Reference Ambrosini and Saino2010, Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). However, the number of farms with livestock declined by approximately 10% between 2001 and 2010 in all three study areas (AS: 10.8%. TP: 9.09%, MC: 10.0%), so the observed variation in the decline rate of populations between these areas could not be explained by differential variation in farming practices.
Probability of colony extinction also differed among livestock categories, being largest in farms that never hosted livestock, intermediate in farms where livestock farming ceased, and smallest in farms always with livestock. In our sample, farms where livestock farming ceased between 2001 and 2010 hosted a similar number of breeding pairs in 2001 than farms where it was maintained (as assessed by a Poisson mixed model with study area as a random factor: z = 1.75, P = 0.08). The intermediate extinction probability in farms where livestock farming ceased may therefore result from a steeper decline of originally large colonies.
Presence of livestock seems therefore to enhance the number of pairs breeding at a farm, probably by a combination of direct and indirect benefits to Barn Swallow reproduction. First, flying insects are more abundant in farms with than without livestock (Møller Reference Møller2001). Second, hayfields, which are the preferred foraging sites for Barn Swallows (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002, Evans et al. Reference Evans, Wilson and Bradbury2007), are wider around farms with livestock (+11.6% in 2010, our unpubl. data) within 400 m of the farm, corresponding to the foraging range of this species (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002). Third, rooms housing livestock are significantly warmer than rooms without, and this affects nestling phenotype (Ambrosini et al. Reference Ambrosini, Ferrari, Martinelli, Romano and Saino2006, Ambrosini and Saino Reference Ambrosini and Saino2010) and survival (Grüebler and Naef-Daenzer Reference Grüebler and Naef-Daenzer2006). All these benefits disappear after cessation of livestock farming, probably resulting in the steep decline in colony size we observed. In addition, buildings housing livestock are usually accessible to Barn Swallows for nesting, but, once farming ceases, may be rearranged and become inaccessible to swallows, thus determining nest site loss. Unfortunately, we have no detailed information on accessibility of buildings to breeding Barn Swallows in 2001 both in TP and MC. Conversely, in AS we could estimate the impact of nest site loss on breeding colonies. The rate of decline of colonies in farms where buildings that hosted breeding swallows in 2001 were remodelled or made otherwise unavailable for the swallows in 2010 (n = 54 farms), was not larger than that in farms where all nest sites were preserved (n = 16 farms) (t-test: t 68 = 1.00, P = 0.32). The effect of nest site reduction was also not significant when the effect of cattle category was taken into account (GLM: F1,66= 0.14, P = 0.71, other details not shown). Hence, nest site loss does not seem to have determined the decline of colonies in AS. However, the effect of nest site loss may have been more severe in TP and MC than in AS, as average farm size is much lower in the first two areas than in the latter (see Methods). Remodelling of buildings in a manner that denied access may therefore have contributed to the general decline of Barn Swallows in these areas.
Small colonies have also become extinct with a higher probability than large ones. These originally small colonies probably occurred in sub-optimal areas where recruitment of young individuals in their first breeding season was probably low, and became even smaller in a period of general population decline.
Three farms in AS and three in TP started livestock farming between 2001 and 2010. Colonies in two of these farms went extinct, while the other showed slightly positive demographic trends (r = +0.02 ± 0.04 SE on average, n = 4). The extinct colonies were very small in 2001 (one and two breeding pairs, respectively), and, at least in one case, extinction is probably the result of disturbance due to farm rearrangement (albeit the old cowshed was maintained at this farm). The positive trend in the other farms thus supports the hypothesis that livestock farming enhances the suitability of a farm to Barn Swallow reproduction, albeit the small sample size prevented formal statistical analyses.
Barn Swallow breeding colonies have declined at a similar rate in farms where livestock farming has either never been practised or where cattle occurred in all years. This suggests that the general decline of breeding populations is due not only to cessation of livestock farming, but also to other causes. Changes in general climatic conditions both in the breeding and the wintering grounds may be involved, as they seem to have determined a severe decline in the populations of several migratory birds (Møller et al. Reference Møller, Rubolini and Lehikoinen2008). In addition, changes in environmental conditions both in the wintering grounds and along migration routes have been shown to affect survival of migrant birds (Szép et al. Reference Szép, Møller, Piper, Nuttall, Szabo and Pap2006), mainly for those species inhabiting dry open habitats during winter (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006). This may have profound effects on population size of several species, as adult mortality of aerial insectivores like the Barn Swallow mainly occurs during these phases of the annual life cycle (Robinson et al. Reference Robinson, Balmer and Marchant2008).
Conservation implications
The Barn Swallow is currently listed as “Least Concern” by IUCN (2010), but the sharp decline we documented (about 50% in 10 years) calls for planning conservation strategies for this species in Italy and elsewhere (e.g. Denmark; Engen et al. Reference Engen, Sæther and Møller2001). The general negative trend observed in all three study areas, irrespective of changes in livestock farming practices, is probably due to factors acting over larger or even global geographical scales, such as climatic and environmental changes in the African wintering and passage areas, as is likely for many other African wintering species (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006, Møller et al. Reference Møller, Rubolini and Lehikoinen2008, Both et al. Reference Both, van Asch, Bijlsma, van den Burg and Visser2009, Jones and Cresswell Reference Jones and Cresswell2010, Saino et al. Reference Saino, Ambrosini, Rubolini, von Hardenberg, Provenzale, Hüppop, Hüppop, Lehikoinen, Lehikoinen, Rainio, Romano and Sokolov2011). However, changes in farming practices occurring in the breeding quarters, such as the cessation of livestock farming, appear to have additive negative effects on Barn Swallow populations. Conservation actions aiming at buffering the global negative trends of Barn Swallows should thus favour the maintenance of livestock farming, which appears pivotal in limiting the decline of the breeding populations of this species. Whether the buffering effect of livestock farming on Barn Swallow declines is due to livestock presence per se, possibly via its positive effects on seasonal reproductive success (Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010), or to habitat characteristics associated with livestock farming, such as pastures and hayfields, that represent preferential foraging sites for the species, remains to be elucidated. Agri-environment schemes (AES) specifically designed for Barn Swallow conservation should focus on the maintenance of livestock farming at farms, as swallow colonies were largest, declined the least, and had the lowest extinction risk on farms that always had livestock. However, to the best of our knowledge, we are unaware of any AES specifically designed for Barn Swallow conservation. Nevertheless, this species may benefit from implementation of AES aimed at maintaining hayfields and improving hedgerows, as these habitats are preferred foraging sites (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002, Evans et al. Reference Evans, Wilson and Bradbury2007, Grüebler et al. Reference Grüebler, Korner-Nievergelt and von Hirschheydt2010). A less intensive approach to farming and a more wildlife-friendly agriculture, which should be promoted by the future Common Agricultural Policy, may be beneficial for farmland birds in general (Báldi and Batáry Reference Báldi and Batáry2011) and, hopefully, also for the Barn Swallow. However, the benefit of an environmentally friendly approach to agriculture for Barn Swallow is not unequivocal, as this species was found to be more abundant in organic than in traditional farms in Denmark (Christensen et al. Reference Christensen, Jacobsen and Nøhr1996), but not in the Netherlands (Kragten and de Snoo Reference Kragten and de Snoo2008, Kragten et al. Reference Kragten, Reinstra and Gertenaar2009). In addition, Barn Swallows population levels were not correlated with agricultural intensification in Britain (Robinson et al. Reference Robinson, Crick and Peach2003).
At a local scale, conservation actions would be most effective in AS than in other study areas, both because the population decline was steepest in this area, and because it still supports the largest population. Unfortunately, AES specifically designed for improving Barn Swallow populations seem difficult to plan in this area, as they would imply large economic costs to farmers. Indeed, cessation of livestock farming and conversion of hayfields to arable fields for biomass production is currently economically advantageous to farmers (R. Ambrosini, unpubl. data).
In conclusion, our study suggests a careful assessment of the conservation status of the migratory Barn Swallow that has showed an alarming local population decline, and indicates that plans for Barn Swallow conservation should aim to maintain livestock farming, as this may contribute to limiting population declines.
Acknowledgements
We are grateful to all the farm owners who allowed us to carry out this study in their cowsheds and houses. The GEV group of the Parco Regionale di Montevecchia e della Valle del Curone kindly provided data from 2001 and also contributed to field work in 2010. Several field assistants greatly contributed to data collection in all years. We thank the Parco Regionale Adda Sud, the Parco Piemontese della Valle del Ticino and the Parco Regionale di Montevecchia e della Valle del Curone for logistic support. The study was carried out under the auspices of the “Programma di cooperazione transfrontaliera Italia-Svizzera 2007-2013 - Indagine naturalistica e variabilità ambientale” (INTERREG project ID 15 7624065 – Misura 1.2) and was partly funded by the Università degli Studi di Milano (grant 2009-ATE-0015 to D.R.) and the Parco Regionale Adda Sud.








