Implications
Hyperketonemia (HYK) is a complex metabolic status described by high levels of ketone bodies in blood, the most common being β-hydroxybutyrate (BHB). In general, HYK increases the risk of health problems during early lactation, with consequent negative effects on herd profitability. This review shows controversial results in the relationship between BHB and milk production and composition, which could be related to the use of a single cut-off to discriminate between healthy and hyperketonemic animals. More research is still needed to identify and validate trustworthy methods to discriminate between cows affected by HYK and healthy animals in field conditions.
Introduction
Ketosis is one of the most detrimental metabolic diseases in dairy cows. It occurs in early lactation when animals experience a negative energy balance (NEB), defined as the lack of trade-off between energy intake (input) and demands for increased milk production (output) (Herdt, Reference Herdt2000). During this period, cows use alternative energy sources to supply the decrease of available glucose that comes from gluconeogenesis; glucose is a fundamental nutrient strictly linked to the maintenance of normal functions in most of body tissues and lactogenesis. The inability of cows to cope with NEB and glucose drop due to an inadequate metabolic adaptation leads to an excessive mobilisation of adipose reserves, releasing abnormal concentrations of non-esterified fatty acids (NEFA) and ketone bodies (acetone, acetoacetate and BHB) in the blood. Thus, biochemical analyses are fundamental to help with the diagnosis of this complex disease. Elevated concentrations of ketone bodies in blood, defined as HYK, negatively affect immune function, health and milk production (McArt et al., Reference McArt, Nydam, Oetzel, Overton and Ospina2013). The BHB is the most common ketone body used to diagnose HYK (Oetzel, Reference Oetzel2004) because it is the predominant and more stable circulating ketone body in cow fluids (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009). However, BHB measurements vary for several reasons such as diurnal variation in body fluids (Nielsen et al., Reference Nielsen, Ingvartsen and Larsen2003), and methods of sampling and analysis (Krogh et al., Reference Krogh, Toft and Enevoldsen2011; Bach et al., Reference Bach, Heuwieser and McArt2016).
Hyperketonemia can result in subclinical ketosis (SCK) or clinical ketosis (CK). Some signs described in literature in animals suffering from CK are ketone smell in breath, reduced activity and appetite, excessive loss of body condition, weakness and apparent blindness (Berge and Vertenten, Reference Berge and Vertenten2014), but those signs are unspecific and/or difficult to be detected; this increases the error of diagnosis and makes arduous to precisely discriminate between CK and SCK. Both CK and SCK affect milk production, reproduction performance and health of dairy cattle (McArt et al., Reference McArt, Nydam, Oetzel, Overton and Ospina2013; Raboisson et al., Reference Raboisson, Mounié and Maigné2014), and thus, they are responsible of increased culling rate (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011) and costs at herd level (Liang et al., Reference Liang, Arnold, Stowe, Harmon and Bewley2017; Mostert et al., Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018). Given this background, the reinforcement of farmers’ awareness of HYK effects, the search for new opportunities of genetic improvement (Pryce et al., Reference Pryce, Parker Gaddis, Koeck, Bastin, Abdelsayed, Gengler, Miglior, Heringstad, Egger-Danner, Stock, Bradley and Cole2016) and the implementation of herd management strategies against HYK have recently gained more and more importance in the scientific community and dairy sector. The aim of this review is to summarise results on the associations of BHB concentration in blood and milk with cow health, milk production and composition, and reproductive performance, and to describe its genetic aspects and economic implications in dairy cattle.
Criteria and methodology of the review
Papers included in the present review were retrieved from Scopus (www.scopus.com) and ISI Web of Science (www.webofknowledge.com) databases for the period January 2007 to January 2018. The keywords used in the literature search were: ketosis, dairy cows, cattle, bovine, beta-hydroxybutyrate, β-hydroxybutyrate, BHB, milk beta-hydroxybutyrate, milk β-hydroxybutyrate, milk BHB, milk composition, milk yield, milk production, genetic, genomic, economic, cost, hyperketonemia, subclinical ketosis, performance, health, reproduction, fertility, ketone body and metabolic disease. Even if ketosis is a complex disease, only papers that focussed on the effects of blood and milk BHB concentrations on milk production, milk composition, reproduction and health performance, and on economic and genetic aspects of this ketone body and HYK or ketosis were considered. The determination of the most accurate blood BHB cut-off to diagnose HYK was not an aim of this review because it has been previously discussed in McArt et al. (Reference McArt, Nydam, Oetzel, Overton and Ospina2013).
In the reviewed papers ketosis and HYK have been often used as synonyms (McArt et al., Reference McArt, Nydam and Oetzel2012; Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015; Rutherford et al., Reference Rutherford, Oikonomou and Smith2016), and thus, we decided to use the term HYK because we consider it more adequate when investigating BHB concentration in blood or milk. The terms SCK and CK have been used when referring to studies that made clear reference to SCK and CK in the text. The unification of terminology such as HYK, CK and SCK, or frequency defined as prevalence or incidence, was sometimes very problematic and made difficult the comparison among studies. Data quality when analysing health events differed among the reviewed studies because some of them were based on voluntary declaration from farmers (Ospina et al., Reference Ospina, Nydam, Stokol and Overton2010a; Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Parker Gaddis et al., Reference Parker Gaddis, Megonigal, Clay and Wolfe2018) and others were from veterinarians (Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013). Moreover, although efforts have been made to standardise the clinical diagnosis, the participation of several observers leads to an unquantifiable error of diagnosis, involving a difficult interpretation of the results.
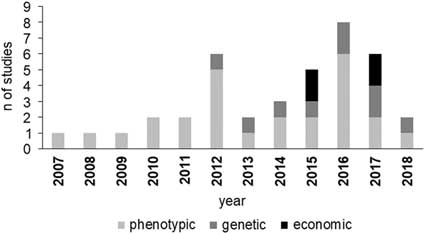
Studies on BHB effects on cow production have increased since 2012 (Figure 1), with an increasing incidence of papers dealing with genetic parameters and economic aspects. The increment of BHB studies underlines that metabolic disorders have been assuming more and more relevance in dairy industry and scientific community. Moreover, the increase of papers on genetic and economic aspects of HYK is likely related to the more recent availability of large data including BHB concentration as a routinely recorded trait in some production systems, which is necessary, for example, to estimate its genetic parameters.
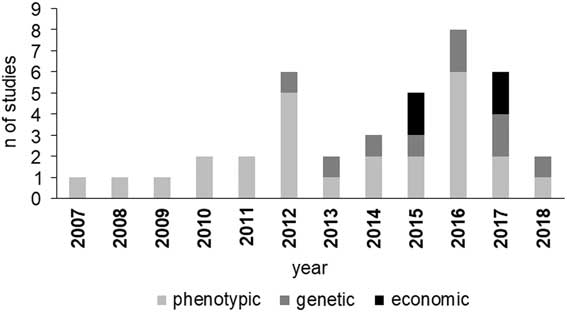
Figure 1 Number of reviewed studies in dairy cattle per area of interest and year of publication. Phenotypic area includes milk production and composition, reproduction and health performance.
Table 1 reports some information retrieved from the reviewed studies on HYK in dairy cattle, namely the country where the study was conducted, number of herds and cows, cow breed and parity, and observation period. Papers (n=4) that dealt with economic aspects were not included in Table 1 because they were based on literature values and data simulations. The papers mainly dealt with Holstein, as it is the most popular and productive cosmopolitan dairy cattle breed, and only few studies focussed on other breeds such as Jersey, Brown Swiss, Norwegian Red and local populations. The studies were conducted in 20 countries, with United States and Canada being the most represented with 15 and 10 papers, respectively (Table 1). As only few studies dealt with an observation period that started 1 to 3 weeks before calving, this review focussed more on the postpartum period. Table 1 also shows that in recent years several papers have dealt with BHB concentration in milk, which is a more practical matrix than blood.
Table 1 Description of reviewed studies on hyperketonemia in Holstein cows detected from blood or milk analysis
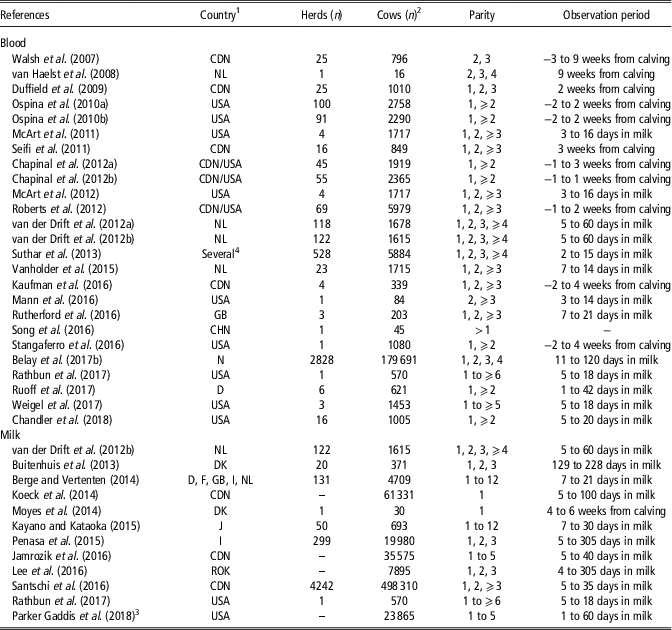
1 CDN =Canada; CHN=China; D=Germany; DK=Denmark; F=France; GB=United Kingdom; I=Italy; J=Japan; NL=The Netherlands; N=Norway; ROK=South Korea; USA=United States.
2 Berge and Vertenten (Reference Berge and Vertenten2014) (German Black Pied and other breeds); Suthar et al. (Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013) (Holstein-Friesian crossbreds, Jersey and Brown Swiss); Belay et al. (Reference Belay, Svendsen, Kowalski and Ådnøy2017b) (Norwegian Red); Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) (Jersey); Parker Gaddis et al. (Reference Parker Gaddis, Megonigal, Clay and Wolfe2018) (Jersey).
3 Producer-recorded cases.
4 Denmark, Spain, Croatia, Hungary, Italy, Poland, Portugal, Slovenia, Serbia, Turkey.
The keywords reported in each paper by their authors were extracted by adapting the Bibliometrix package (Aria and Cuccurullo, Reference Aria and Cuccurullo2017) for R v. 3.4 (R Core Team, 2017), which produced the network of all recurrent keywords weighted by their frequency (Figure 2). The closer two terms are located in the map, the stronger the relationship between the terms, and the bigger the nucleus, more frequently the word has been used. The total number of keywords retrieved after editing of synonyms was 75 and ‘dairy cows’, ‘β-hydroxybutyrate’, ‘ketosis’, ‘non-esterified fatty acids’ and ‘hyperketonemia’ were the most recurrent keywords.
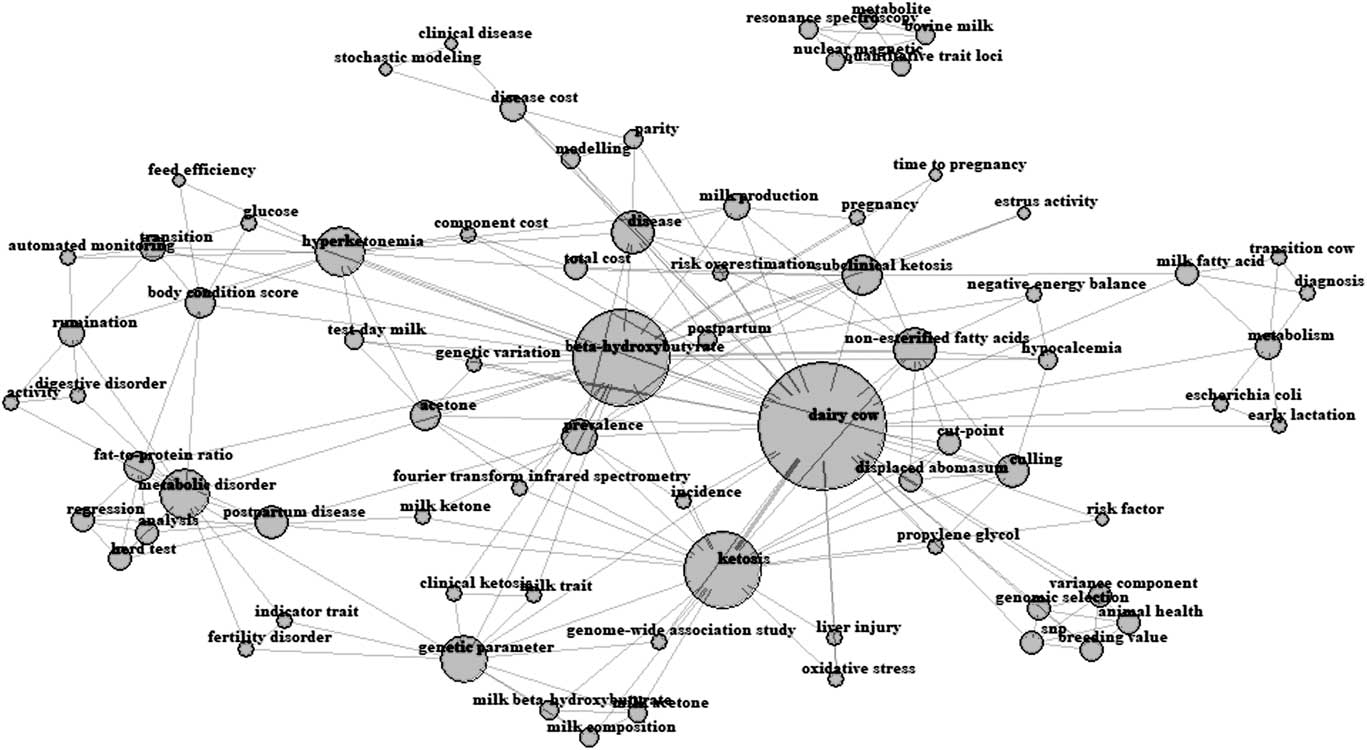
Figure 2 Author’s keywords occurrence map for reviewed studies in dairy cattle. The closer two terms are located in the map, the stronger the relation between the terms and the bigger the nucleus, the more frequent the word has been used.
Methods and thresholds used to define hyperketonemia
The tested matrix, method of determination and diagnostic threshold affect the results about relationships between BHB concentration and HYK and, consequently, the impact on cow performance. However, universal method and threshold for defining HYK and linking it to ketosis have not been established yet, probably because of the difficulty of accurately diagnose CK, and therefore discriminate between CK and SCK. Moreover, the cut-offs used in the reviewed papers could increase the error of HYK detection because BHB concentration in blood and milk fluctuates during the day (Nielsen et al., Reference Nielsen, Ingvartsen and Larsen2003). Most reviewed studies did not specify sampling times, which could lead to unpredictable differences between outcomes. However, a clear pattern of the diurnal variation of BHB concentration in blood and milk has not been described yet, even if a relationship with the energy content of the diet has been reported by Nielsen et al. (Reference Nielsen, Ingvartsen and Larsen2003). In particular, cows fed a total mixed ration with low-energy content showed a decrease of blood and milk BHB concentration in the evening and lower variation among cows, whereas cows fed a total mixed ration with high-energy content showed an increase of blood and milk BHB concentration in the evening and greater variation among cows (Nielsen et al., Reference Nielsen, Ingvartsen and Larsen2003). In the same study (Nielsen et al., Reference Nielsen, Ingvartsen and Larsen2003), BHB concentrations in blood of a cow fed a total mixed ration with high-energy content exceeded the most common threshold used to define HYK in blood (1.2 mmol/l) for 37.5% of the day, which could lead to a misclassification of positive HYK. Therefore, the tested matrix, method of determination and threshold need to be taken into account to correctly interpret the results. Table 2 summarises the methods of determination of BHB concentration and the cut-offs of BHB used to establish HYK, or SCK and CK, in the reviewed papers.
Table 2 Thresholds of β-hydroxybutyrate (BHB) concentration in blood and milk (mmol/l) used in the literature to determine hyperketonemia (HYK), also defined as subclinical ketosis (SCK) and clinical ketosis (CK) in some cases, in dairy cattle
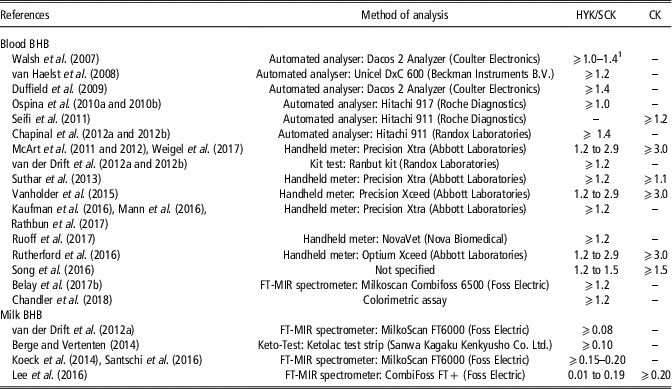
1 Thresholds for 1st and 2nd week of lactation, respectively.
The BHB can be detected in blood and milk. In particular, blood BHB concentration is the most common indicator to diagnose HYK and this explains why the majority of reviewed papers dealt with blood sampling (Table 2). The laboratory determination of BHB concentration is based on a colorimetric enzymatic reaction followed by a spectrophotometric analysis. Moreover, handheld blood ketone meters have been developed and validated in order to provide a more practical tool for on-field data collection. These on-farm testing systems include handheld devices and test strips based on an electrochemical reaction with a small amount of blood to determine the ketone concentration with acceptable specificity and sensitivity for HYK diagnosis (Bach et al., Reference Bach, Heuwieser and McArt2016; Sailer et al., Reference Sailer, Pralle, Oliveira, Erb, Oetzel and White2018). However, it should be taken in consideration that blood BHB concentration is usually based on a single blood sample and it represents the status of the animal at that sampling point of the day.
Blood sampling is a labourious and time-consuming procedure, and it is stressful for the animals. The possibility of using milk BHB concentration to diagnose HYK has been investigated (Table 2) because milk recording is already a routine and non-invasive procedure, and it facilitates monitoring at herd level. Moreover, it differs from blood measurement of BHB status of the animal as a milk sample represents a period of time. Although laboratory analyses such as photometrical method or enzymatic assays (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) and cowside strip tests (Keto-Test; Berge and Vertenten, Reference Berge and Vertenten2014) exist in this field, mid-IR spectroscopy (MIRS) is the most promising tool for the determination of milk BHB (Grelet et al., Reference Grelet, Bastin, Gelè, Davière, Johan, Werner, Reding, Fernandez Pierna, Colinet, Dardenne, Gengler, Soyeurt and Dehareng2016). Indeed, the use of chemical analysis or handheld meter to measure BHB in routine milk recording is not feasible because it is too expensive and time consuming, whereas MIRS allows to collect data at population level cheaply and provide routine reports to farmers for monitoring their herd. Currently, the accuracy of the developed prediction equations for BHB concentration in milk is not high enough to quantify the exact content but it has proved to be useful for screening purposes to detect cows with elevated BHB concentrations in milk (Grelet et al., Reference Grelet, Bastin, Gelè, Davière, Johan, Werner, Reding, Fernandez Pierna, Colinet, Dardenne, Gengler, Soyeurt and Dehareng2016; Lee et al., Reference Lee, Cho, Park, Choi, Kim and Do2016; Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). Moreover, predicted milk acetone and BHB have been used to develop models for the prediction of blood BHB from test-day milk and performance traits (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018), and the use of MIRS milk spectra has been proposed to directly predict blood BHB concentration (Belay et al., Reference Belay, Dagnachew, Kowalski and Ådnøy2017a and Reference Belay, Svendsen, Kowalski and Ådnøy2017b). Nevertheless, for this approach, we have to consider that usually blood BHB concentration is determined using a single blood sample that represents the status of the animal at the time of blood sampling, whereas a milk sample represents the BHB concentration of a period of time, which could interfere with the accuracy of the developed calibration model. To increase the accuracy of the prediction models using milk samples, several blood samples during the same period of time that the milk sample would represent could be collected.
For both blood and milk, different thresholds have been used (Table 2). In general, the optimum thresholds of BHB concentration for postpartum diseases occurrence (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009; Ospina et al., Reference Ospina, Nydam, Stokol and Overton2010a; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013), fertility indicators (Chapinal et al., Reference Chapinal, Carson, LeBlanc, Leslie, Godden, Capel, Santos, Overton and Duffield2012a), and change in milk production and composition traits (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009) have been identified based on the highest combination of sensitivity and specificity of the analysis performed. However, most of reviewed studies have defined the cut-offs before starting the experiment (Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015; Rutherford et al., Reference Rutherford, Oikonomou and Smith2016). The main difficulty was to unify terminology as some authors clearly specified a distinction between SCK and CK, whereas others considered only HYK. In general, regarding BHB cut-offs reported in the papers, HYK and SCK were defined from the same threshold. In blood, BHB ⩾1.2 mmol/l is generally used as cut-off to identify cows with HYK, or affected by SCK. A deeper analysis of the adequacy of this cut-off in blood has been discussed in McArt et al. (Reference McArt, Nydam, Oetzel, Overton and Ospina2013). A less uniform BHB cut-off for CK has been proposed in literature. Although most papers have reported a direct relationship between blood BHB concentrations and CK incidence and established the threshold at blood BHB ⩾3.0 mmol/l, some authors observed that CK occurrence can be associated to lower BHB concentration (e.g. BHB ⩾1.1 mmol/l) (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Song et al., Reference Song, Li, Gu, Fu, Peng, Zhao, Zhang, Li, Wang, Li and Liu2016).
Only few papers have used milk BHB concentration to detect HYK (Table 2) and, considering the limitations of strip tests and MIRS prediction models, there is not a clear cut-off point. Ranges of milk BHB concentrations to classify cows with suspect HYK (0.15 to 0.19 mmol/l) or positive HYK (⩾0.20 mmol/l) have been recently proposed by Koeck et al. (Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014) and Santschi et al. (Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). On the other hand, Lee et al. (Reference Lee, Cho, Park, Choi, Kim and Do2016) considered cows as affected by SCK with milk BHB concentration between 0.01 and 0.20 mmol/l, and affected by CK with milk BHB concentration ⩾0.20 mmol/l. However, even with elevated concentrations of milk BHB, a blood test and/or a veterinary visit is necessary to help on the diagnosis of HYK.
Frequency and risk factors of hyperketonemia
A disease frequency can be described through two different measures, prevalence and incidence, usually expressed as a percentage. Prevalence is defined as the number of existing cases of a specific disorder divided by the number of sampled animals at a given time, that is, a single test is required. Incidence is calculated as the number of new cases of a specific disease divided by the number of animals at risk during a defined period of time. Thus, to estimate incidence is necessary to test animals frequently enough to ensure that all cows that develop the disease during the observation period will be correctly identified. Moreover, incidence can be expressed as cumulative incidence or as incidence rate. Cumulative incidence is the most common one and provides a measure of risk in a given period of time. Incidence rate is the proportion of new cases calculated per unit of time and the result should be expressed per unit of time. Therefore, the ease of prevalence calculation explains why there is a lower number of papers that computed incidence (Table 3). Even if not clearly expressed in most of the cases, incidence values reported in reviewed studies corresponded to cumulative incidence, meaning that some misleading use of terminology to express frequency exists in literature. For instance, cumulative incidence was wrongly referred as rate in Weigel et al. (Reference Weigel, Pralle, Adams, Cho, Do and White2017), whereas prevalence and incidence rate were used as synonyms in Lee et al. (Reference Lee, Cho, Park, Choi, Kim and Do2016), leading to a difficult interpretation of the results.
Table 3 Prevalence and incidence (%) of hyperketonemia (HYK), also defined as subclinical ketosis (SCK) and clinical ketosis (CK) in some cases, in dairy cattle
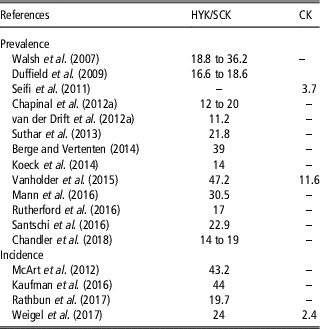
Prevalence and incidence of HYK reported in reviewed papers are displayed in Table 3. Prevalence ranged from 11.2% (van der Drift et al., Reference van der Drift, van Hulzen, Teweldemedhn, Jorritsma, Nielen and Heuven2012b) to 47.2% (Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015) when papers considered HYK or SCK. On the other hand, the percentage dropped off considerably when CK was considered, ranging from 3.7% (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011) to 11.6% (Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015). A similar situation was observed in the studies reporting incidence, showing a range between 19.7% (McArt et al., Reference McArt, Nydam and Oetzel2012) and 44% (Kaufman et al., Reference Kaufman, LeBlanc, McBride, Duffield and DeVries2016) for HYK or SCK and a value of 2.4% for CK (Weigel et al., Reference Weigel, Pralle, Adams, Cho, Do and White2017).
As reported above, to interpret and compare correctly the measures of frequency, several factors such as method of detection, threshold used and biological fluid analysed should be taken into account. Moreover, these frequency expressions greatly depend on stage of lactation (observation period) and parity (Table 1). As HYK is mainly the consequence of NEB experienced by the cow after calving, the highest HYK prevalence has been detected in the first 2 weeks of lactation, declining greatly thereafter (van der Drift et al., Reference van der Drift, Jorritsma, Schonewille, Knijn and Stegeman2012a; Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). For example, a decrease of prevalence by 60% (from 18% to 7%) between the 1st and the 2nd month of lactation has been reported by van der Drift et al. (Reference van der Drift, Jorritsma, Schonewille, Knijn and Stegeman2012a). Moreover, both HYK peak incidence (22.3% of cows with their first positive test) and prevalence (28.9% of cows with a positive test) have been detected at 5 days in milk in McArt et al. (Reference McArt, Nydam and Oetzel2012). Considering these high frequencies in 1st days of lactation, the calculation of the risk of disease occurrence through multiple tests during early lactation would be an appropriate approach to measure HYK frequency.
Several authors observed a higher frequency of HYK in multiparous than primiparous cows (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016; Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017; Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018), and suggested a direct relationship between increasing parity and HYK occurrence. Cumulative incidence from 8.6% to 26.2% (Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017) and prevalence from 18.8% to 27.6% (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) have been detected moving from first to third or greater lactation. The increase of HYK occurrence with parity may be due to the concurrent needs of gestation and lactation, as indicated by Berge and Vertenten (Reference Berge and Vertenten2014). For this reason, it could be appropriate to record separately HYK risk-estimates for different parity orders and then compute a herd level incidence risk by standardising parity-specific percentages. Conversely, the same pattern has not been observed in Jersey breed, as HYK was more prevalent in primiparous than multiparous cows (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018).
Other factors that should be considered for interpreting HYK occurrence are season of calving, breed and herd management. Authors generally agreed to identify spring as the season with greater prevalence of HYK, whereas contrasting results have been reported for late autumn and winter (Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015; Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) or summer (van der Drift et al., Reference van der Drift, Jorritsma, Schonewille, Knijn and Stegeman2012a; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013). However, in most cases, no biological reason or evidence has been reported to justify the greater HYK prevalence in spring (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). It has been suggested for Dutch farmers that the lower quality of the silage used during the first half of the year could explain the greater HYK prevalence in spring (Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015). Concerning breed, higher overall HYK prevalence in Jersey (19%) than Holstein cows (14%), with values that ranged from 11.4% to 25% in Jersey herds and 0% to 28% in Holstein herds, has been observed by Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018). Management and feeding of gestating heifers, dry cows and cows in early lactation, as well as on-farm prevention approaches and incidence of other diseases contribute to HYK prevalence (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). A negative association between the increase of herd size and HYK prevalence has been reported (Berge and Vertenten, Reference Berge and Vertenten2014) as bigger herds usually implemented strategies such as grouping cows based on milk production to better meet nutritional requirements. Berge and Vertenten (Reference Berge and Vertenten2014) also observed a lower prevalence of HYK in systems with cubicles, cubicles and yards, or tie-up bars than in systems with straw yards, and a slightly greater frequency in systems in which cows were on pastures rather than housed indoor. Moreover, a lower prevalence of HYK was reported in herds feeding forage and concentrate separately or total mixed ration compared with herds using partial mixed ration. Mixed ration refers to cows fed a total mixed ration between grazing periods. These differences in prevalence might be due to the fact that farmers cannot easily control animals in terms of nutritional level and health status when they are on pasture or housed in straw yards.
Associations of β -hydroxybutyrate with dairy cow performance
Health
Cows with BHB concentration ⩾1.2 or 1.1 mmol/l in blood (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013) or ⩾0.10 mmol/l in milk (Berge and Vertenten, Reference Berge and Vertenten2014) are from 4.7 to 14.7 times more likely to manifest clinical signs of HYK. In these studies, CK was diagnosed by veterinarians (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Berge and Vertenten, Reference Berge and Vertenten2014) or herd managers (Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013) according to the following definitions: decreased feed intake or appetite, decreased milk production, a positive urine or milk ketone test, low rumen fill, reduced activity or demeanour, excessive loss of body condition, constipation or hard/dry faeces, ketone odour in breath/milk and nervous signs. However, in the reviewed papers, it is not clear how authors used all those variables to establish CK. Moreover, it is commonly agreed that HYK increases the risk of the onset of other early lactation diseases, such as displaced abomasum (odds ratio (OR)=1.6 to 19.3; Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011; McArt et al., Reference McArt, Nydam, Ospina and Oetzel2011 and Reference McArt, Nydam and Oetzel2012; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Berge and Vertenten, Reference Berge and Vertenten2014), metritis (OR=1.5 to 1.7; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Berge and Vertenten, Reference Berge and Vertenten2014) and lameness (OR=1.7 to 1.8; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Berge and Vertenten, Reference Berge and Vertenten2014), and the risk increased with blood BHB concentration (Ospina et al., Reference Ospina, Nydam, Stokol and Overton2010a; McArt et al., Reference McArt, Nydam and Oetzel2012; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013). Overall, reviewed studies supported the hypothesis of Roberts et al. (Reference Roberts, Chapinal, LeBlanc, Kelton, Dubuc and Duffield2012) that cows with high blood BHB concentrations, especially multiparous animals, had greater probability of being removed from the herd in early lactation. In particular, in McArt et al. (Reference McArt, Nydam and Oetzel2012) cows diagnosed with HYK were three times more likely to die or be culled than non-hyperketonemic cows, observing also that each 0.1 mmol/l increment of blood BHB concentration during the 1st month of lactation increased the risk of culling by 1.4 times. Although most studies have focussed on the very early lactation (⩽21 days in milk), consequences of elevated BHB levels on health can be observed until 60 days in milk. Some authors noted that a greater disease risk occurred for cows diagnosed with HYK in the 1st week after calving (Seifi et al., Reference Seifi, LeBlanc, Leslie and Duffield2011; McArt et al., Reference McArt, Nydam and Oetzel2012). For instance, in McArt et al. (Reference McArt, Nydam and Oetzel2012) cows diagnosed with HYK from 3 to 5 days in milk were 6.1 times more likely to develop displaced abomasum than cows diagnosed with HYK after the 1st week. Furthermore, several authors highlighted that the risk of HYK occurrence (Berge and Vertenten, Reference Berge and Vertenten2014; Kaufman et al., Reference Kaufman, LeBlanc, McBride, Duffield and DeVries2016) or being culled after its detection (Roberts et al., Reference Roberts, Chapinal, LeBlanc, Kelton, Dubuc and Duffield2012) was more likely for multiparous cows, probably due to the greater milk yield and to possible problems experienced during the previous lactation and dry period.
The relationships between early lactation disorders are complex. From the outcomes of reviewed papers, displaced abomasum appears as a result of HYK. Despite this, in the studies of McArt et al. (Reference McArt, Nydam, Ospina and Oetzel2011 and Reference McArt, Nydam and Oetzel2012) some cows developed displaced abomasum before being diagnosed positive for HYK. Displaced abomasum and HYK are both generated by a poor adaptive response to early lactation requirements. Indeed, after calving cows (especially high-producing animals) do not assume the appropriate amount of energy to face the requirements of high production, mainly because the maximum intake capacity is reached 7 to 8 weeks postpartum. In addition, since HYK leads to hypoglycaemia in multiparous cows (Ruoff et al., Reference Ruoff, Borchardt and Heuwieser2017), and to reduced rumination time and activity (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009; Kaufman et al., Reference Kaufman, LeBlanc, McBride, Duffield and DeVries2016; Stangaferro et al., Reference Stangaferro, Wijma, Caixeta, Al-Abri and Giordano2016), HYK can be considered as a cause of displaced abomasum.
In a recent study, high levels of blood BHB have been described to be significantly correlated to oxidative stress and liver apoptosis damage (Song et al., Reference Song, Li, Gu, Fu, Peng, Zhao, Zhang, Li, Wang, Li and Liu2016). Thus, it is reasonable to conclude that the NEB status in early lactation and the physiological stress occurring during HYK have a role in the depression of the immune system. As a consequence, cows with HYK are more likely to be affected by metritis and lameness during the early lactation (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009; Ospina et al., Reference Ospina, Nydam, Stokol and Overton2010a; Suthar et al., Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013; Berge and Vertenten, Reference Berge and Vertenten2014). Regarding mastitis, controversial results have been reported. Although Berge and Vertenten (Reference Berge and Vertenten2014) found that cows diagnosed with HYK were almost twice as likely to have a mastitis event in the 1st month of lactation compared with healthy cows and Moyes et al. (Reference Moyes, Larsen, Sørensen and Ingvartsen2014) observed that udder inflammation caused an increase of milk BHB concentration, Duffield et al. (Reference Duffield, Lissemore, McBride and Leslie2009) and Suthar et al. (Reference Suthar, Canelas-Raposo, Deniz and Heuwieser2013) did not detect any association between mastitis and HYK.
Milk production
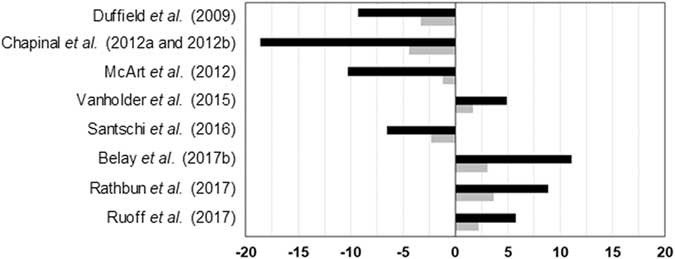
The effects of elevated blood or milk BHB levels on milk yield are controversial. While a decrease of daily milk production between 1% and 18% has been observed in several studies, an increase of daily milk yield from 5% to 11% in hyperketonemic cows has been reported by other authors (Figure 3). Besides, van der Drift et al. (Reference van der Drift, Jorritsma, Schonewille, Knijn and Stegeman2012a) and Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) did not report significant differences between cows with or without HYK. Generally, HYK affects more negatively milk production of multiparous than primiparous cows (Ospina et al., Reference Ospina, Nydam, Stokol and Overton2010b; Chapinal et al., Reference Chapinal, LeBlanc, Carson, Leslie, Godden, Capel, Santos, Overton and Duffield2012b; Kayano and Kataoka, Reference Kayano and Kataoka2015; Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016), which is reasonable because first lactation cows do not have NEB status of the previous lactation as potential risk factor, and on average they have better body condition and yield less milk than multiparous animals. However, Rathbun et al. (Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017) reported that the onset of HYK is not related to milk yield in previous lactation or to genetic potential for milk production. They suggested that HYK in high-producing cows is indeed due to energy requirements of current lactation. Further research is needed to confirm this hypothesis which seems contradictory to the results observed for primiparous and multiparous cows. The negative impact of HYK on milk yield is more pronounced when detected in the 1st week rather than in the 2nd week of lactation, even if cows show the same blood BHB concentration (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009; Chapinal et al., Reference Chapinal, Carson, LeBlanc, Leslie, Godden, Capel, Santos, Overton and Duffield2012a; McArt et al., Reference McArt, Nydam and Oetzel2012). The difference of milk yield between cows with HYK and without HYK increases during lactation (Kayano and Kataoka, Reference Kayano and Kataoka2015; Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016), probably due to the cumulative NEB in hyperketonemic cows.
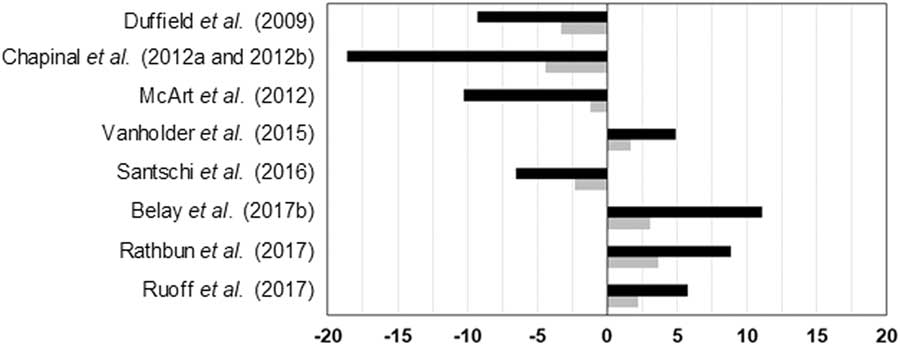
Figure 3 Greatest significant differences between normal and hyperketonemic cows for daily milk yield. Black bars express milk in %/day per cow and grey bars express milk in kg/day per cow. Negative and positive values indicate lower and higher values in hyperketonemic cows, respectively.
Milk composition
Hyperketonemia has been associated with greater milk fat content, fat-to-protein ratio (F : P) and energy-corrected milk and lower protein, lactose and urea nitrogen in milk (Table 4; Kayano and Katatoka, Reference Kayano and Kataoka2015). An increment in fat content between 2.4% (Vanholder et al., Reference Vanholder, Papen, Bemers, Vertenten and Berge2015) and 23.9% (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) has been reported in hyperketonemic compared with healthy cows. Generally, greater differences of fat content between hyperketonemic and healthy cows have been observed in very early lactation (Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017). However, a greater increment of fat percentage for cows with HYK in the second rather than in the 1st week of lactation was reported in the study of Duffield et al. (Reference Duffield, Lissemore, McBride and Leslie2009). This discrepancy could be related to the fact that Duffield et al. (Reference Duffield, Lissemore, McBride and Leslie2009) defined a greater BHB concentration threshold for the 2nd week (2 mmol/l) than for the 1st week of lactation (1.2 mmol/l). Moreover, while Santschi et al. (Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) reported that differences in milk fat content between hyperketonemic and healthy cows increased with parity, no significant differences were observed by Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018). Hyperketonemia negatively affects milk protein content; hyperketonemic animals produced milk with 0.3% (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) to 11.6% (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) less protein compared with healthy animals. Moreover, the greatest differences between hyperketonemic and healthy cows were detected in primiparous animals (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016; Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) and, when the week effect was considered, in the 2nd week of lactation (Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017).
Table 4 Significant differences between normal and hyperketonemic cows for milk compositionFootnote 1
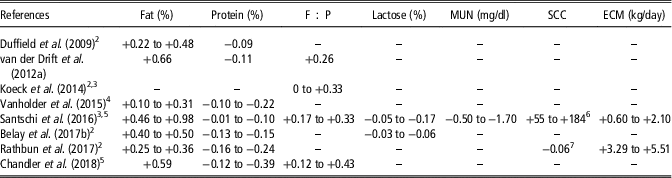
Negative and positive values indicate lower and higher values in hyperketonemic cows, respectively. Values are mean, or minimum and maximum.
1 F : P=fat-to-protein ratio; MUN=milk urea nitrogen; SCC=somatic cell count; ECM=energy-corrected milk calculated as in National Research Council (2001).
2 Values represent differences between days or weeks of lactation.
3 Values represent differences between suspect or positive cows for hyperketonemia.
4 Values represent differences between subclinical and clinical ketosis.
5 Values represent differences between parities.
6 SCC expressed as SCC×103/ml.
7 SCC expressed as log10 of SCC.
The F : P has been reported to be 10% to 32.8% higher in hyperketonemic than in healthy animals (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018). As it has been indicated for protein, those differences were greater for primiparous than multiparous cows. In addition, F : P and fatty acids (FA) have been proposed as indicators of HYK in early lactation (van Haelst et al., Reference van Haelst, Beeckman, Van Knegsel and Fievez2008; Mann et al., Reference Mann, Nydam, Lock, Overton and McArt2016). A significant decrease of several de novo (C6:0, C8:0, C10:0, C12:0, C14:0) and a medium-chain (C15:0) FA has been observed in milk of cows with HYK (Mann et al., Reference Mann, Nydam, Lock, Overton and McArt2016). Moreover, Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018) reported increased concentrations of long chain as well as total unsaturated and trans-FA in hyperketonemic Jersey cows. In both studies, monounsaturated FA increased with HYK. The decrease of the synthesis of de novo FA in milk might suggest a less metabolically active mammary gland, while the increment of long-chain FA and total unsaturated FA could be related to a greater acidogenic ruminal fermentation due to lower dry matter intake and higher passage rate. Overall, the association between milk FA and elevated concentrations of BHB needs further investigation.
High-producing cows suffer a pronounced lipomobilisation in early lactation, concurrently with low serum concentrations of glucose, total proteins and urea which could explain the results of Table 4. Hyperketonemic cows had from 4.6% to 16.6% less milk urea nitrogen compared with healthy animals. The greatest decrease between hyperketonemic and healthy cows has been observed in multiparous cows (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). The decrease of milk urea nitrogen could be related to the reduced feed intake, the oxidative stress and the liver apoptosis damage in cows affected by HYK, which leads to a low dietary protein availability and a lower protein biosynthesis in the liver, respectively (Duffield et al., Reference Duffield, Lissemore, McBride and Leslie2009; Song et al., Reference Song, Li, Gu, Fu, Peng, Zhao, Zhang, Li, Wang, Li and Liu2016). Energy-corrected milk has been reported to increase from 2% (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) to 12.6% (Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017) in hyperketonemic compared with healthy cows. Moreover, the greatest differences in energy-corrected milk between hyperketonemic and healthy cows were observed in the 1st week of lactation (Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017) and in pluriparous cows (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). The increase of energy-corrected milk in cows with HYK within the 1st month of lactation is mostly influenced by elevated fat percentage (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016; Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017) and in some cases by greater milk yield (Rathbun et al., Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017). Regarding lactose content, the inverse relationship between circulating BHB and glucose at metabolic level reduces the availability of this fundamental precursor for lactose synthesis in epithelial cells of the mammary gland. Thus, for lactose content a reduction between 0.6% (Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b) and 3.7% (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016) has been reported in hyperketonemic compared with healthy cows. As indicated for the other traits, the difference in lactose concentration between hyperketonemic and healthy cows was greater in primiparous than pluriparous cows (Santschi et al., Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016). As hyperketonemic cows have higher incidence of clinical mastitis compared with healthy cows (Berge and Vertenten, Reference Berge and Vertenten2014), greater somatic cell count is expected in hyperketonemic animals. Although results of Santschi et al. (Reference Santschi, Lacroix, Durocher, Duplessis, Moore and Lefebvre2016), who reported a 61.3% increment of mastitis incidence in hyperketonemic multiparous cows compared with healthy animals, supported this hypothesis, HYK and somatic cell count were uncorrelated in Vanholder et al. (Reference Vanholder, Papen, Bemers, Vertenten and Berge2015) and Chandler et al. (Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018), and were negatively associated in Rathbun et al. (Reference Rathbun, Pralle, Bertics, Armentano, Cho, Do, Weigel and White2017), who observed that the incidence of mastitis decreased by 3.2% in hyperketonemic compared with healthy cows. In addition, milk acetone concentration has shown a coefficient of correlation with blood BHB between 0.50 and 0.79, and thus it has been proposed as an additional milk indicator of HYK, similarly to milk BHB (van der Drift et al., Reference van der Drift, Jorritsma, Schonewille, Knijn and Stegeman2012a and Reference van der Drift, van Hulzen, Teweldemedhn, Jorritsma, Nielen and Heuven2012b; Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018).
Reproductive performance
The cow has to be in positive energy balance to fully express oestrus behaviour and become pregnant (Rutherford et al., Reference Rutherford, Oikonomou and Smith2016). All reviewed studies on the effect of HYK on reproductive performance dealt with HYK diagnosed with the analysis of blood BHB (Table 5). Animals with elevated blood BHB in the first 2 weeks after calving had lower pregnancy success at first artificial insemination than healthy cows (OR=0.47, P=0.003; Walsh et al., Reference Walsh, Walton, Kelton, LeBlanc, Leslie and Duffield2007), whereas no effects were observed by Chapinal et al. (Reference Chapinal, Carson, LeBlanc, Leslie, Godden, Capel, Santos, Overton and Duffield2012a) and McArt et al. (Reference McArt, Nydam and Oetzel2012). However, a decrease of pregnancy success within 70 days post-voluntary waiting period with a hazard ratio of 0.87 (P=0.10) was reported by Ospina et al. (Reference Ospina, Nydam, Stokol and Overton2010b). Moreover, greater number of inseminations per pregnancy (2.8 v. 2.0, respectively; P<0.05), lower peak activity (35% less activity), shorter activity at oestrus (14% less hours) and longer interval from calving to first observed oestrus in HYK than healthy cows were observed by Rutherford et al. (Reference Rutherford, Oikonomou and Smith2016), who reported also prolonged days open for multiparous cows.
Table 5 Associations between hyperketonemia and reproductive performance in dairy cattle

Genetic aspects
In recent years, genetic investigations on BHB have become more relevant, leading to an increased number of papers on this topic (Figure 1). Several authors described blood and milk BHB as heritable traits, with estimates that ranged from 0.09 to 0.37 and 0.04 to 0.29, respectively (Table 6). On average, estimates of heritability of both traits increased during lactation (Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Lee et al., Reference Lee, Cho, Park, Choi, Kim and Do2016; Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b), probably because the environmental and residual factors play a stronger role in early rather than in mid or late lactation. Some authors (Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016) observed that heritability of milk BHB decreased with increasing parity. On the other hand, an increase of heritability from first to second parity, and a slight decrease in third parity have been reported by Lee et al. (Reference Lee, Cho, Park, Choi, Kim and Do2016).
Table 6 Heritability of blood and milk β-hydroxybutyrate (BHB) in dairy cattle
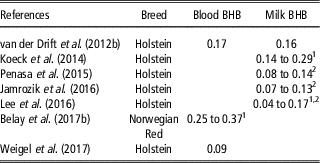
1 Values in different stages of lactation.
2 Values in different parities.
Heritability estimates of blood and milk BHB were greater than estimates of CK assessed by Koeck et al. (Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014), Jamrozik et al. (Reference Jamrozik, Koeck, Kistemaker and Miglior2016) and Belay et al. (Reference Belay, Svendsen, Kowalski and Ådnøy2017b) using linear animal models (Table 7); this could depend not only on the less variability of CK, which is a dichotomous variable (presence/absence of disease), but also on the possible discrepancy of health data, which were recorded by more than one observer in the studies of Koeck et al. (Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014), Jamrozik et al. (Reference Jamrozik, Koeck, Kistemaker and Miglior2016) and Belay et al. (Reference Belay, Svendsen, Kowalski and Ådnøy2017b). The greater heritability of blood and milk BHB compared with CK, coupled with positive moderate to strong genetic correlations between these traits suggest that BHB is a useful indicator to select against ketosis (Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016; Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b). Although BHB has been reported as a relatively good genetic indicator for metabolic disorders, it did not exhibit any potential as a predictor of fertility problems (Table 7; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016).
Table 7 Heritability and genetic correlations (r g ) of early lactation diseases, milk yield, fat, protein and lactose percentages, fat-to-protein ratio (F : P), and acetone with blood and milk β-hydroxybutyrate (BHB) in dairy cattle
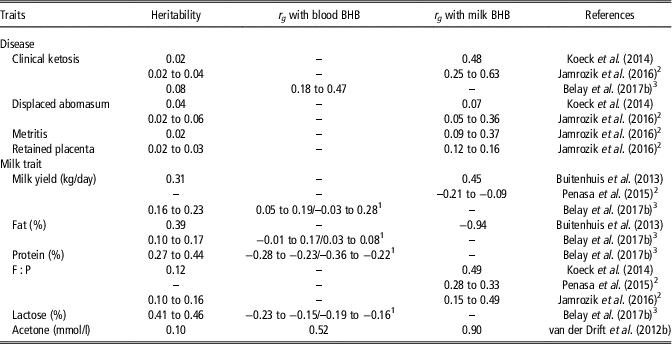
1 Correlations within and across stages of lactation.
2 Values represent differences between parities.
3 Values represent differences between stages of lactation.
Considering genetic correlations of BHB with milk yield and composition traits (Table 7), some controversial results have been reported in studies that considered the early (van der Drift et al., Reference van der Drift, van Hulzen, Teweldemedhn, Jorritsma, Nielen and Heuven2012b; Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016) or entire lactation (Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015) rather than mid lactation (Buitenhuis et al., Reference Buitenhuis, Sundekilde, Poulsen, Bertram, Larsen and Sørensen2013). Weak to moderate positive (Buitenhuis et al., Reference Buitenhuis, Sundekilde, Poulsen, Bertram, Larsen and Sørensen2013; Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b) and negative (Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015) relationships were observed between milk or blood BHB and milk yield. Negative genetic associations with milk protein, lactose and urea content were generally consistent in literature (Buitenhuis et al., Reference Buitenhuis, Sundekilde, Poulsen, Bertram, Larsen and Sørensen2013; Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b), and a positive genetic correlation with milk fat percentage has been reported by Belay et al. (Reference Belay, Svendsen, Kowalski and Ådnøy2017b), probably due to the larger fat mobilisation required in early lactation by selecting for high milk production. A general consensus in the literature described milk BHB and F : P as positively genetically correlated (Koeck et al., Reference Koeck, Jamrozik, Schenkel, Moore, Lefebvre, Kelton and Miglior2014; Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016) and blood or milk BHB to be strongly positively correlated with acetone (van der Drift et al., Reference van der Drift, van Hulzen, Teweldemedhn, Jorritsma, Nielen and Heuven2012b).
On average, genetic correlations of BHB with diseases and milk production or composition traits were stronger in early lactation (Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015; Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b) and for primiparous cows (Penasa et al., Reference Penasa, Pretto, Varotto and De Marchi2015; Jamrozik et al., Reference Jamrozik, Koeck, Kistemaker and Miglior2016) compared with later stages of lactation and parity orders. Nevertheless, a clear explanation for the stronger correlations in early lactation and primiparous cows has not been provided yet.
Genetic perspectives for analysis of HYK and ketosis have been emerging in recent years. For instance, Weigel et al. (Reference Weigel, Pralle, Adams, Cho, Do and White2017) reported that the incorporation of genomic data to pedigree-based analyses enhanced estimates of HYK heritability, breeding values and predicted phenotypes. Parker Gaddis et al. (Reference Parker Gaddis, Megonigal, Clay and Wolfe2018) observed that susceptibility to ketosis in Jerseys was affected by numerous regions along the genome, involving genes related with several pathways as immune system, insulin regulation and lipid metabolism. However, in this study ketosis events were retrieved from a voluntary producer-recorded database which increases the error of diagnosis because of the several producers involved and the declarations collected on a voluntary basis.
Economic aspects
Recent studies have been conducted to evaluate the economic impact of HYK in the dairy herd. The HYK cost in European countries has been evaluated using a stochastic model with distribution laws as input parameters (Raboisson et al., Reference Raboisson, Mounié, Khenifar and Maigné2015) and a dynamic stochastic simulation model (Mostert et al., Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018). The economic impact of HYK in United States has been assessed using a deterministic model (McArt et al., Reference McArt, Nydam and Overton2015; Liang et al., Reference Liang, Arnold, Stowe, Harmon and Bewley2017), even if in the deterministic model of Liang et al. (Reference Liang, Arnold, Stowe, Harmon and Bewley2017) several variables were modelled stochastically. While McArt et al. (Reference McArt, Nydam and Overton2015) calculated the cost for HYK with blood BHB concentration ⩾1.2 mmol/l, Liang et al. (Reference Liang, Arnold, Stowe, Harmon and Bewley2017) did not clearly define the diagnostic method.
All the studies observed that the cost of HYK, which can be increased twice considering diseases related to HYK (mastitis, metritis, displaced abomasum, lameness and CK), is mainly due to impaired reproductive performance and milk loss. Despite the variation of prices (e.g. milk, feed, replacement and slaughter prices) on the market of each region and year, observed results followed the same trend. The total average cost of HYK has been estimated between $77 (Liang et al., Reference Liang, Arnold, Stowe, Harmon and Bewley2017) and $289 (McArt et al., Reference McArt, Nydam and Overton2015) per case and year in United States, and between €130 (Mostert et al., Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018) and €257 (Raboisson et al., Reference Raboisson, Mounié, Khenifar and Maigné2015) per case and year in Europe. In general, the cost is at least twice greater in multiparous than primiparous cows (Liang et al., Reference Liang, Arnold, Stowe, Harmon and Bewley2017; Mostert et al., Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018). However, a higher cost for primiparous ($374) than multiparous animals ($256) has been reported by McArt et al. (Reference McArt, Nydam and Overton2015). Although cost distribution was difficult to compare among studies due to the different variables considered in each formula, McArt et al. (Reference McArt, Nydam and Overton2015) and Mostert et al. (Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018) clearly reported that the most important cost was related to impaired reproductive performance (34% to 36%) and milk production loss (24% to 26%). Interestingly, the cost of the milk that was discarded following the treatment of HYK-related diseases (14%) has been additionally considered by Mostert et al. (Reference Mostert, Bokkers, van Middelaar, Hogeveen and de Boer2018). Most of HYK total costs (80%) were attributable to several HYK-related diseases (e.g. displaced abomasum, lameness, clinical mastitis and metritis) and consequently early culling, allocating less relative importance to milk production loss (11%) and prolonged days open (9%) in Raboisson et al. (Reference Raboisson, Mounié, Khenifar and Maigné2015). On the other hand, Liang et al. (Reference Liang, Arnold, Stowe, Harmon and Bewley2017) assigned most of the total costs to veterinary interventions and treatments (68%) or to extended days open (47%) for primiparous or multiparous cows, respectively.
Conclusions and perspectives
The present review summarised the major impacts of elevated blood or milk BHB concentrations on productive, fertility and economic aspects in early lactation dairy cows. A general consensus defined HYK as cause of increased risk of health problems during early lactation, with consequent negative effects on herd profitability. Nevertheless, controversial results have been observed for milk production and somatic cell count. The associations between BHB concentrations and milk yield are still not well defined both from a phenotypic and genetic point of view, and further studies are necessary to better understand the mechanisms underlying these relationships. Although the reviewed literature is consistent in reporting that elevated blood BHB concentration is detrimental to reproductive performance, Ospina et al. (Reference Ospina, Nydam, Stokol and Overton2010a and Reference Ospina, Nydam, Stokol and Overton2010b) highlighted that NEFA concentration is a stronger predictor of fertility depletion than BHB. A debate about the most convenient indicator concerns also milk composition traits, for which routinely predicted traits as acetone, F : P and FA profile have been assuming increasing importance. Moreover, the interest of using multiple measurable indicators to determine HYK has been emerging because until now most papers established HYK on the basis of a single predictor.
Therefore, the following relevant points deserve further investigations:
(i) Identification and validation of trustworthy methods to discriminate between cows affected by HYK and healthy animals in field conditions. Although the determination of blood BHB concentration is the most common method to identify HYK, it is not a useful tool in field conditions both from the economic and animal welfare point of view. The possibility to predict BHB in milk using MIRS is currently the most concrete and feasible way to collect phenotypes at population level and some studies have demonstrated that this approach is useful for monitoring HYK in dairy cows. Nevertheless, MIRS does not allow to collect detailed individual milk BHB concentrations with enough accuracy; this issue has been widely discussed in several papers and it is one of the main topics that lead researches on MIRS and metabolic-related indicators. The combination of several milk test-day predicted traits (e.g. BHB, acetone, F : P, FA) and performance variables has been proposed as an interesting strategy to predict HYK (Chandler et al., Reference Chandler, Pralle, Dόrea, Poock, Oetzel, Fourdraine and White2018). Another approach that has been recently suggested is the use of BHB concentration in blood rather than in milk as reference method to calibrate MIRS devices (Belay et al., Reference Belay, Svendsen, Kowalski and Ådnøy2017b). The possibility to predict the metabolic profile of cow by MIRS is a great challenge for the dairy sector.
(ii) Some of the controversial results highlighted in this review between the effect of elevated BHB concentration and health, milk production and composition, and reproductive performance, as well as HYK economic cost and genetic aspects could be related to data quality. Considering that ketosis is a complex metabolic disease, the diagnosis by using a single cut-off could be not enough to correctly discriminate between healthy and hyperketonemic animals, or to properly separate between SCK and CK. Further research should focus on BHB variability within and between cows, in order to provide essential information for the development of a more accurate diagnosis method. Moreover, when dealing with health events, results could vary if they come from a voluntary declaration, if the declaration comes from the farmers or the veterinarians, or if the health event is registered by only one person or different persons. In addition, further investigation using ordinal or multinomial logistic regression to assess the relation between HYK (or CK) and various predictors is needed. A more detailed review focussed on data quality could help to better understand how the quality of recorded data may affect the impact of HYK on cow’s health and performance.
(iii) Quantification of phenotypic and genetic variation of HYK in different breeds and environmental conditions. This issue is very relevant for scientists and technicians, and the possibility of recording BHB concentration or other predicted traits at population level, as mentioned above, would help investigate this topic. Several impacts of HYK on cow performance are controversial or not quantified yet, and the difficulties to have large and accurate data in the 1st days after calving is one of the main concerns for future research. Moreover, the possibility of combining metabolic information of dairy cows in early lactation with milk production and composition will support farmers to achieve an early detection of metabolic problems minimising the economic losses.
Acknowledgements
This review is based on an invited presentation at the 68th Annual Meeting of the European Federation of Animal Science held in Tallinn (Estonia) from 28 August to 1 September 2017. The authors gratefully acknowledge the financial support from the University of Padova, Italy (project BIRD163298/16 – ‘Studio degli aspetti fenotipici e genetici del contenuto di β-idrossibutirrato (BHB) nel latte di bovine di razza Frisona Italiana’).
Declaration of interest
The authors declare that they have no conflicts of interest.
Ethics statement
No ethical concerns.
Software and data repository resources
None of the data were deposited in an official repository.