Implications
Sheep are seasonal breeders. Although this characteristic is admittedly inherited from their ancestor, the Oriental Mouflon (Ovis orientalis Gmelin 1774) for the Western Eurasiatic sheep, the history of its evolution over 10 millennia of domestication still needs to be documented; our method was developed to attain this goal. The biogeochemical composition of enamel retains a record of the seasonal cycle during tooth formation, which can be retrieved from archaeological sheep teeth and used to infer their seasonality and season of birth. The investigation of European sheep teeth of between 8000 and 5000 years old lays the foundation for research that will link present day sheep to their earliest domestic ancestors.
Introduction
In temperate latitudes, sheep have a seasonal reproductive behaviour. On an annual scale, they alternate between a period of sexual activity and a period of sexual rest, with no behavioural or ovarian activity in the ewe (Hafez, Reference Hafez1952) and dramatically reduced sperm production in the male (Dacheux et al., Reference Dacheux, Pisselet, Blanc, Hochereau-de-Revier and Courot1981). This cycle, modulated by melatonin secretion, is under strong photoperiodic control (Karsch et al., Reference Karsch, Bittman, Foster, Goodman, Legan and Robinson1984; Malpaux et al., Reference Malpaux, Viguié, Skinner, Thiéry and Chemineau1997; Thiéry et al., Reference Thiéry, Chemineau, Hernandez, Migaud and Malpaux2002). In Europe, most domestic sheep breeds have a breeding season from late summer to early winter, leading to births occurring 5 months later from winter to early summer. This characteristic imposes strong constraints on husbandry, in terms of work organization and seasonal availability of animal products (Chemineau et al., Reference Chemineau, Guillaume, Migaud, Thiéry, Pellicer-Rubio and Malpaux2008). Researches in animal agriculture over the last 50 years have focused on understanding the physiological and environmental mechanisms driving this cycle, and trying to find ways to control it using hormonal treatments, exposure to artificial light regimens or through the manipulation of socio-sexual signals – the male effect (Thimonier, Reference Thimonier, Cognié, Lassoued and Khaldi1981; Martin et al., Reference Martin, Oldham, Cognié and Pearce1986).
These recent advances have revolutionized the tempo of multimillenary small ruminant husbandry system. Over 10 500 years of manipulation separates today’s European domestic sheep breeds from their wild ancestors, during which time population redistributions, management of demography and diet, and adaptation to economic rules have modified their ancestral heritage (Hafez, Reference Hafez1952). In spite of this, small ruminants have retained seasonal breeding behaviour, although differences have been observed between present day sheep breeds with a regionally focused history (Hafez, Reference Hafez1952; Thimonier and Mauléon, Reference Thimonier1969). Reconstructing the history of sheep breeding seasonality over a long time scale, starting with the unimproved domestic herds from prehistory, could assist with a better understanding of the reasons for the retention of this characteristic and its variability over millennia of domestication.
This perspective echoes archaeologists’ current considerations. European sheep (Ovis aries) lineages originated in the Near East (Poplin, Reference Poplin1979). Zooarchaeology and population genetics (Handley et al., Reference Handley, Byrne, Santucci, Townsend, Taylor, Bruford and Hewitt2007) have demonstrated that the sheeps’ wild ancestor (O. orientalis – absent from Europe) was initially domesticated in southeastern Anatolia, The earliest evidence of morphologically modified sheep dates to 8500 BC, as a result of man’s increasing control over wild animals that probably started several centuries earlier (Peters et al., Reference Peters, von den Driesch and Helmer2005; Meadows et al., Reference Meadows, Cemal, Karaca, Gootwine and Kijas2007; Vigne et al., Reference Vigne, Balasse, Gourichon, Helmer, Lesur, Mashkour, Tresset and Vila2012). Domestic sheep lineages were introduced to Europe through Greece and the Balkans in the early 7th millennium BC. From there, they spread along the northern coastline of the Mediterranean and inland Europe through the Danubian corridor and adjacent rivers, to reach the coasts of northwestern Europe by 5000 BC. They eventually colonized the British Isles at the start of the 4th millennium BC (Tresset and Vigne, Reference Tresset and Vigne2011). Along this westward and northward diffusion, sheep adapted to a diversity of climates and landscapes across a wide latitudinal gradient. Strong environmental constraints may have had definitive bearings on early European husbandry, some of which influenced the reproductive cycle of domestic animals. As now, the timing and the length of the lambing season constituted key parameters of ancient husbandry. Although economic issues were different in terms of the market, constraints at a husbandry system scale were probably just as strong with regards to the organization of agro-pastoral tasks, the availability of animal production on a seasonal scale and residential strategy (Digard, Reference Digard1981; Balasse and Tresset, 2007).
Across prehistoric Europe, it is reasonable to consider that in early domestic animals, births were restricted to a defined period of the year. The objective of this research is not to confirm the seasonality of births in early European domestic sheep, but to define the duration of this period and determine the time of year they occurred. This could, hopefully, lead to a clearer picture of seasonal rhythms and associated socio-economical constraints affecting early husbandry systems, including variability at a regional and interregional scale. In this study, we recall the methodological steps that led to the elaboration of a procedure to determine birth seasonality in past sheep herds, from the analysis of the stable oxygen isotoe composition of tooth enamel. We then provide a review of the data from European archaeological contexts dated to the 6th to the 3rd millennia BC (calibrated dates). This set of sites located under contrasted latitudes (43°N to 46°N and 59°N) also provides an interesting case for observation, due to the photoperiodic control on sheep reproductive activity and the latitudinal variation of this parameter (Hafez, Reference Hafez1952).
Material and methods
Principles of the sequential stable oxygen isotope analysis in tooth enamel
Stable oxygen isotope ratios (δ 18O) from the mineral fraction of vertebrate skeleton are linked to that of body water, derived from ingested water, whose primary source is precipitation (Land et al., Reference Land, Lundelius and Valastro1980; D’Angela and Longinelli, Reference D’Angela and Longinelli1990). The precipitation δ 18O values are affected by ambient temperature and its seasonal variations. At high and middle latitudes in Europe, the highest δ 18O values of precipitation occur during the warmest season and the lowest during the coldest season (Rozanski et al., Reference Rozanski, Araguas-Araguas and Gonfiantini1993). During formation, teeth build and retain a chronological record of the individual’s stable isotope history. This history may be reconstructed through sequential sampling of dental tissues following growth directions (Figure 1a). In particular, δ 18O sequences have been retrieved from enamel, showing cyclical variations reflecting seasonal trends (Figure 1b).
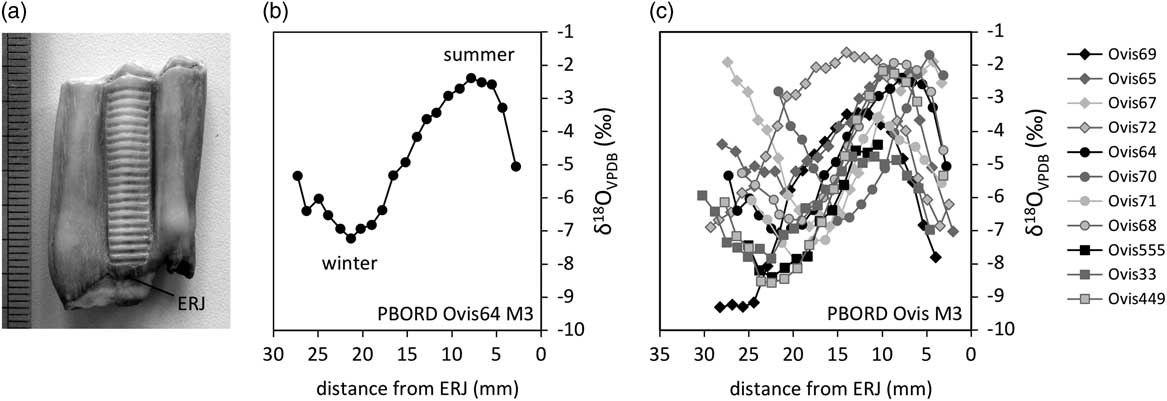
Figure 1 Description of the methodological approach: enamel sequential sampling of a sheep’s lower third molar (a), retrieval of the seasonal cycle in the sequence of δ 18O values; measured against Vienna Pee Dee Belemnite (VPDB) (b) and comparison of inter-individual variability (c). Example from the Chalcolithic site of Borduşani-Popină. Samples are located in the tooth crown using their distance from the enamel root junction (ERJ).
Because the timing of tooth growth is fixed within a species, inter-individual variability in the δ 18O sequences retrieved from enamel (Figure 1c) is related to variability in the season of birth (Bryant et al., Reference Bryant, Froelich, Showers and Genna1996; Fricke and O’Neil, Reference Fricke and O’Neil1996). This variability may be described through the observation of the position of the optimum value of the δ 18O cycle in the tooth crown (Balasse et al., Reference Balasse, Smith, Ambrose and Leigh2003). A quantitative estimation of the variability involves a normalization procedure, where the δ 18O sequences are modelled using an equation derived from a cosine function (Balasse et al., Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b). This allows to objectively define the position of the optimum value and to determine the period of the cycle (the distance over which the isotopic record covers one annual cycle), which may be used to link distance to time (Supplementary Figure S1a). The period of the cycle is then used to normalize the position of the optimum in the tooth crown, in order to eliminate the tooth size factor (Supplementary Figure S1b).
The birth season is determined by comparison with modern reference sets, assuming a similar timing of tooth development for modern and ancient sheep breeds. In sheep, these include the Carmejane (CAR) reference set for late winter and late summer births (Blaise and Balasse, Reference Blaise and Balasse2011) and the Rousay (ROU) reference set for mid-spring births (Balasse et al., Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b). Analyses are conducted on the second (M2) and the third molars (M3).
The archaeological data set
The archaeological assemblages (Figure 2 and Table 1) belong to central and southeastern Europe (early 6th to second half of 5th millennium BC), France (Middle and Late Neolithic, 4th and 3rd millennia BC) and the Northern Isles of Scotland (Orkney, first half of 4th to mid 3rd millennium BC). A brief description of all animal husbandry contexts and orientations are provided in Supplementary Material S1. Several millennia separate these husbandry systems from the earliest times of domestication. Similar studies were recently initiated on earlier Near Eastern sites but are still at a preliminary state (Tornero et al., Reference Tornero, Balasse, Molist and Sana2016).
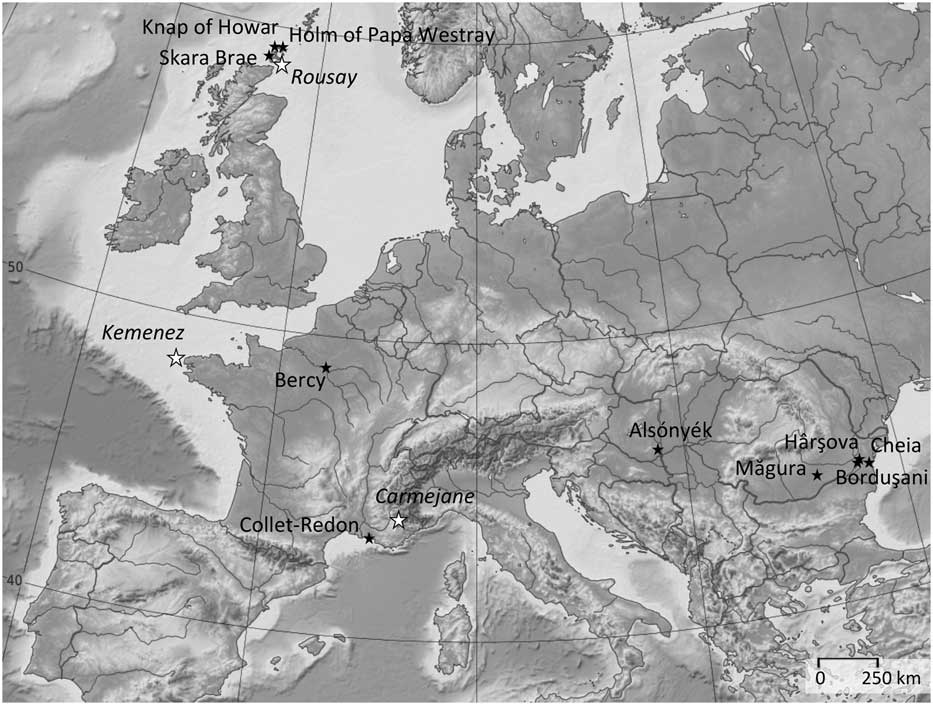
Figure 2 Location of the archaeological sites where sheep birth distribution was investigated (![]() ) and modern farms which provided reference sheep with known birth season as comparative material (
) and modern farms which provided reference sheep with known birth season as comparative material (![]() ).
).
Table 1 List of archaeological sites providing sheep teeth included in the study
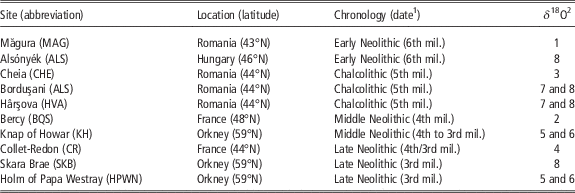
mil.=millennium.
1 Calibrated BC.
2 Original δ 18O data set published in 1: Balasse et al. (Reference Balasse, Bălăşescu, Janzen, Ughetto-Monfrin, Mirea and Andreescu2013); 2: Balasse et al. (Reference Balasse, Boury, Ughetto-Monfrin and Tresset2012a); 3: Tornero et al. (Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013); 4: Blaise and Balasse (Reference Blaise and Balasse2011); 5: Balasse et al. (Reference Balasse, Tresset and Ambrose2006); 6: Balasse and Tresset (Reference Balasse and Tresset2009); 7: Balasse et al. (Reference Balasse, Bălăşescu, Tornero, Frémondeau, Hovsepyan, Gillis and Popovici2017); 8: this study.
The modern reference sets
The ROU is composed of the lower M2 from sheep from a Shetland cross, raised on a working farm on the island of Rousay in Orkney (Figure 2). They provide a reference for April/May births (Balasse et al., Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b). The CAR is composed of five M2s from Préalpes du Sud ewes raised on an experimental farm in southern France (Digne, Alpes de Haute Provence, Figure 2). They provide references for late January/early February births and September births (Blaise and Balasse, Reference Blaise and Balasse2011).
An additional reference set was introduced to this study, composed of three sheep from a Ouessant×Landes de Bretagne cross, raised on the island of Kemenez (KMZ; Molène, Finistère) (Figure 2). Uncontrolled reproduction leads to lambing which occur almost exclusively in February. The specimens composing the data set were retrieved from the semi-articulated skeletons of individuals who had died of natural causes, collected on the shoreline of the island in 2013 and 2014. The stable isotope data set is currently under constitution; in this study we include the first results obtained from three M2s and one M3 (Table 2).
Table 2 The modern sheep reference sets
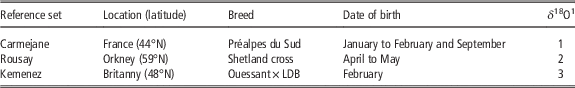
LDB=Landes de Bretagne.
1 Original δ 18O data set published in 1: Blaise and Balasse (Reference Blaise and Balasse2011); 2: Balasse et al. (Reference Balasse, Boury, Ughetto-Monfrin and Tresset2012a); 3: Tornero et al. (Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013).
In all locations, the δ 18O values of modern precipitation are driven by ambient temperature as the main factor, and show similar patterns of seasonal variation, with the highest values measured in July/August (IAEA/WMO, 2016).
Analytical protocol
Enamel samples were drilled (Balasse et al., Reference Balasse, Smith, Ambrose and Leigh2003), on the posterior (M2), middle (lower M3) or anterior lobe (upper M3). The archaeological enamel powders were pre-treated for elimination of diagenetic carbonates following Tornero et al. (Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013). Isotope ratios were measured on a DeltaVAdvantage MS interfaced with a Kiel IV device (Thermo Scientific, Bremen, Germany). The analytical precision within each run, estimated from repeated analyses of our laboratory carbonate standard (Marbre LM, normalized to NBS19) was lower than 0.06‰ (n=8). The modelling procedure was applied to the data sets from KMZ, CTD, CR, HPWN, KH, SKB, PBORD, HVA and ALS (Table 1) following Balasse et al. (Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b). The data set from Cheia (CHE) (Tornero et al., Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013) was revised in order to correct the estimation of the location of the enamel root junction in teeth where mineralization was incomplete.
Results
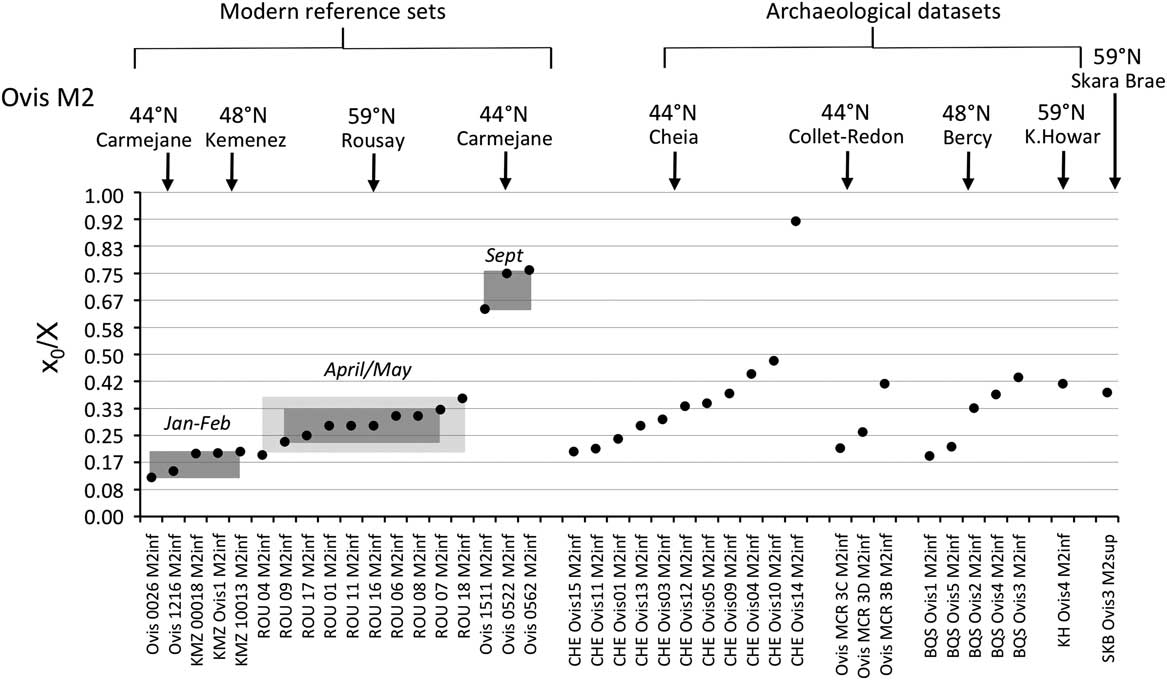
The distribution of births within the archaeological sheep, as investigated from the modelled δ 18O sequences, is shown in Figures 3 (M2) and 4 (M3). Results from the stable isotope analysis and modelling are presented extensively in the Supplementary Tables S1 and S2 and Supplementary Figures S1 to S6. In all instances, seasonal breeding is confirmed. Globally, in all assemblages from central/southeastern Europe (latitudes 43°N to 46°N: Măgura, Alsónyék, Cheia, Borduşani and Hârşova) the period of sheep births occurred at the same time of the year and over a comparable duration (x 0/X ratios ranging from 0.09 to 0.61 in the M3; Figure 4). The sheep from Collet-Redon (44°N) and Bercy (48°N) in France also belong to this group: the births observed at these sites occurred within the same time period as in Cheia (Figure 3). Two outliers to this middle latitudes group are observed, at Cheia (x 0/X ratio=0.88 on the M3) and Hârşova (0.99) (Figure 4).
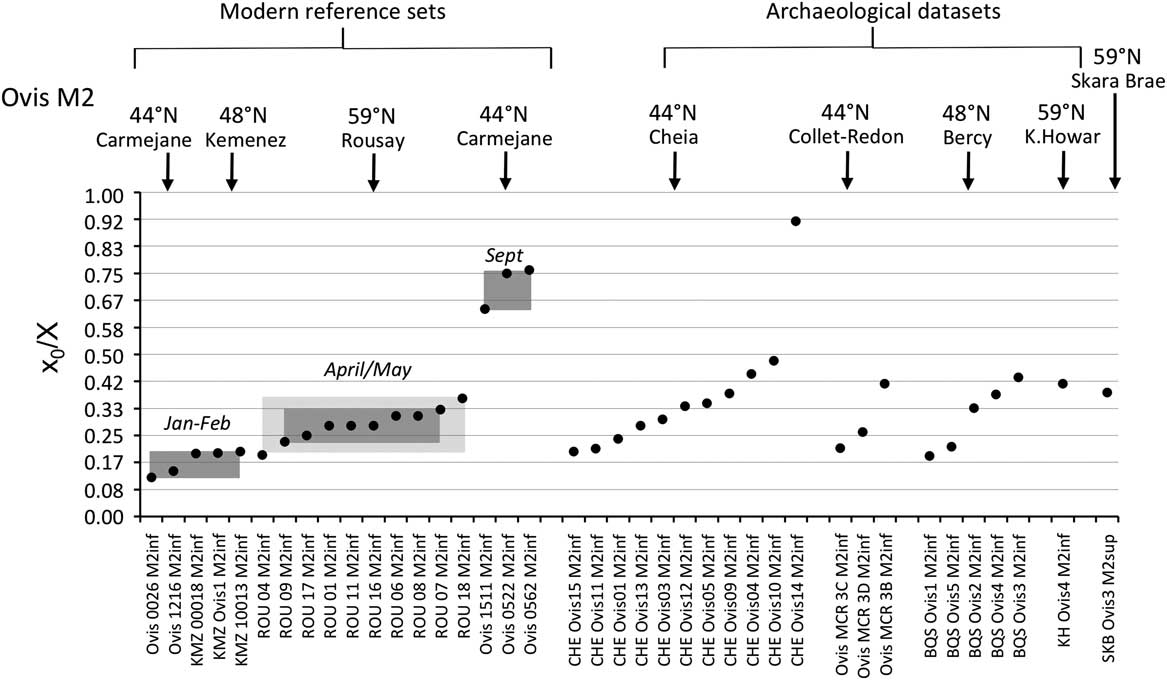
Figure 3 Results from normalization of the position in the M2 crown of the highest δ 18O value (x 0), using the period (X) of the cycle. Comparison of the archaeological data sets with the modern reference sets. For the Rousay reference, the rectangles indicate the whole variability (light grey) and 1SD around the mean (1σ; dark grey). Data: see Tables 1 and 2. ROU=Rousay; CHE=Cheia; BQS=Bercy; KH=Knap of Howar; SKB=Skara Brae.
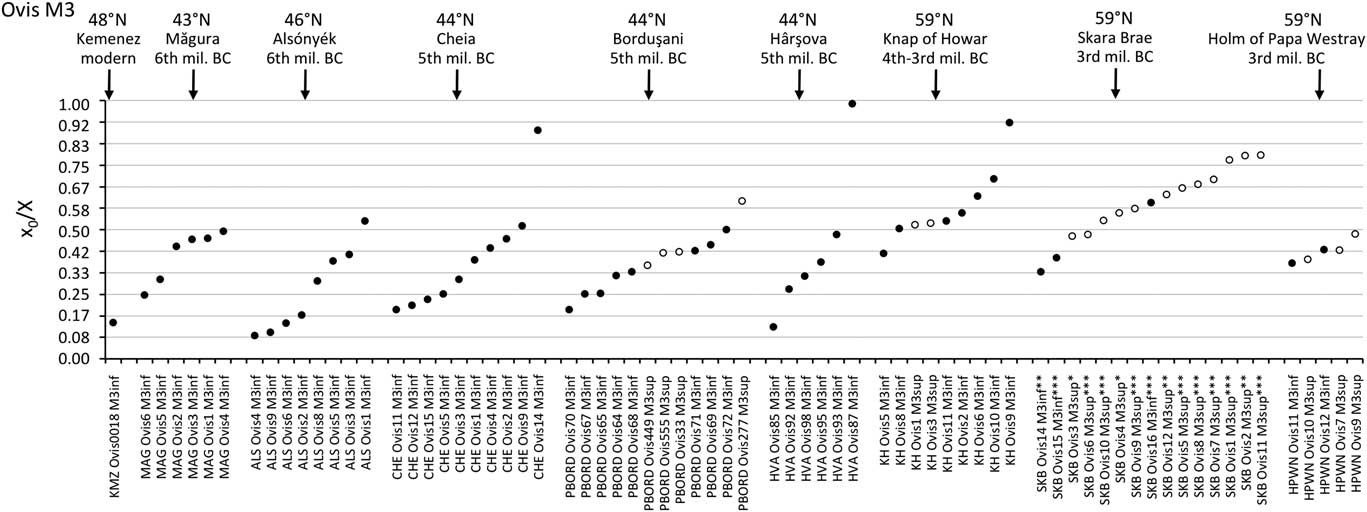
Figure 4 Results from normalization of the position in M3 crown of the highest δ 18O value (x0), using the period (X) of the cycle. The dark symbol refers to lower M3, the open symbols refer to upper M3. Skara Brae: first occupation phase (*); start of second occupation phase (**), end of second occupation phase (***). Data: see Tables 1 and 2. mil=millennium; MAG=Măgura; ALS=Alsónyék; CHE=Cheia; HVA=Hârşova; KH=Knap of Howar; SKB=Skara Brae; HPWN=Holm of Papa Westray.
By contrast to the group of middle latitude sites, the Orkney sites (latitude 59°N) showed a delayed onset of the breeding season, as reflected in significantly higher x 0/X ratios (Wilcoxon rank test, W=197.5, P=2.10−6), globally ranging between 0.34 and 0.79 on the M3 (Figure 4). The difference remains highly significant (Wilcoxon rank test, W=94.5, P=0.0025) when considering lower teeth in isolation. A delayed onset of the breeding season in Orkney is also supported by the results obtained on the M2, where the births at Skara Brae and Knap of Howar occurred within the latest observed at the middle latitude sites of Cheia, Bercy and Collet-Redon (Figure 3).
Results from the modern sheep molars confirm and enlarge the previous reference data sets (Figure 3). A difference of 6.2 months (0.52 in x 0/X ratios, i.e. 52% of a year) is observed between the KMZ and the CAR sheep born 7 months apart; the KMZ sheep (February births) yielded slightly higher x 0/X ratios than those measured in the CAR sheep (late January/early February). A slight disagreement appears between the KMZ (February) and ROU (late April/early May) data sets, separated on average by only 1 month when a larger difference would be expected. The x 0/X ratio measured on the KMZ M3 at 0.14 provides the first reference point for the record of a winter birth in the third molar (Figure 4).
Discussion
Sheep births distribution in the archaeological contexts
All results indicate seasonal breeding in archaeological sheep. The duration of the period of births is estimated to ~3 to 4 months (Figures 3 and 4). This is significantly higher than what is observed today in the European mouflon, where births occur during a period of only 1 to 2 months (Hafez, Reference Hafez1952; Pfeffer, Reference Pfeffer1967; Garel et al., 2005; Tornero et al., Reference Tornero, Balasse, Molist and Sana2016). Although the affiliation between the European mouflon and the domestic sheep’s wild ancestor is not straightforward due to a complex history of fertilization and hybridization between wild and domestic populations (Poplin, Reference Poplin1979; Schröder et al., Reference Schröder, Lieckfeldt, Lutz, Rudloff, Frölich and Ludwig2016), the European mouflon remains the closest living representative of early domestic breeds. A 3- to 4-month period is also longer than what was observed in the 8th millennium BC domestic sheep in the Near East (Tornero et al., Reference Tornero, Balasse, Molist and Sana2016), while it is shorter than what is observed in most improved breeds (Hafez, Reference Hafez1952). This corroborates the idea that domestication led to an extension of the period of sexual activity in sheep (Hafez, Reference Hafez1952), but also that this trend was accentuated over the long term.
Some individuals escaped the rule. The outliers at Cheia and Hârşova reflect out-of-season births. In both instances they occur ~4 to 6 months later than the latest births from the main group. Out-of-season breeding is observed today in some herds where a few females happen to present sexual receptivity at a time when the remaining ewes are in sexual rest. The capability of breeding out of season differs according to the sheep breed (Thimonier and Mauléon, Reference Thimonier1969; Thimonier et al., Reference Thimonier and Mauléon2000). Our results suggest that this trait already existed in the 5th millennium BC sheep herds, and that these lambs born out of season survived in a context of prehistoric husbandry.
Variations with latitude
A later onset of the breeding season in the higher latitude sites (Orkney) compared with the middle latitudes sites (central/southeastern Europe and France) also meets current expectations. Currently, later lambing is observed at higher latitudes, where the photoperiodic cycle induces shorter periods and a later onset of sexual activity in the ewe (Hafez, Reference Hafez1952). In middle latitude locations in Europe, the onset of the sheep’s breeding season is in September with lambing occurring from February onwards. In contrast to this, present day ewes established in Orkney do not give birth until April/May. Similarly, the Soay sheep from the neighbouring archipelago of St Kilda, Outer Hebrides, does not start breeding until November and gives birth from April onwards (Blackbourn, Reference Blackbourn1995). Considering that the Knap of Howar farm constitutes the earliest occurrence to date of sheep in Orkney, a later onset of the breeding season would suggest rapid adaptation to the photoperiodic conditions of North Atlantic Europe.
The harsh winter conditions and consequences on neonatal survival (McCormick, Reference McCormick1998) might also explain some of the data set. At the Holm of Papa Westray, foetuses and neonatal individuals are extremely abundant (Tresset, Reference Tresset2003, Balasse and Tresset, Reference Balasse and Tresset2009; and Supplementary Material S1). A shorter birth distribution, with a later onset compared with middle latitude sites, could reflect the non-representation of the earliest births: the second and third molars’ formation is completed later in life (Weinreb and Sharav, Reference Weinreb and Sharav1964; Milhaut and Nézit, Reference Milhaud and Nézit1991), consequently the sample does not include neonatal or infantile mortality.
The season of birth
The season of birth is determined by comparison with the modern M2 reference set (Figure 3). The slight disagreement between the KMZ and ROU data sets might suggest interbreed variability in the timing of tooth development, which must be confirmed using larger data sets. Caution is required when interpreting these ratios on a monthly scale. At Cheia, Bercy and Collet-Redon, sheep births started in late winter and occurred throughout the spring to early summer. The out-of-season birth at Cheia occurred in late autumn. The assemblage from Cheia, on which analysis was conducted both on the M2 and the M3 (Tornero et al., Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013), may be used to fix the period of birth in the M3 data set, until strong reference sets are obtained for the M3. On all sites from the central/southeastern Europe group, sheep births occurred from late winter to early summer, possibly with a slightly earlier onset at Alsónyék (Figure 4). A late winter onset of the lambing season is also in agreement with the very first reference point obtained from the M3 of a Kemenez sheep born in February (Figure 4).
Temporal changes at the regional scale
When comparing results on a supra-regional scale, no diachronic trend is observed: the Chalcolithic sheep at Cheia (turn of 5th millennium BC), Borduşani and Hârşova (second half of 5th millennium BC) all had very similar birth periods, comparable with those of the Early Neolithic sheep at Măgura (early 6th millennium BC).
Inter-site variability within the Orkney group is interesting in several respects: first, because the Knap of Howar represents the earliest adaptation of sheep husbandry on the islands, whereas the settlements at Skara Brae and Holm of Papa Westray postdate it by several centuries; second, because a major adaptation of sheep husbandry systems occurred within the first millennia of their presence on these islands. This adaptation consisted of the introduction of seaweed to the sheep’s diet in winter, in order to cope with the scarcity of vegetation. Today, the sheep from the North Ronaldsay breed, endemic to Orkney, survive on a diet composed almost exclusively of seaweed, which they became adapted to after being confined to the shoreline since the building of a dyke encircling the island in the 1830s. These sheep attain optimal weight in winter, a time of the year when large amounts of kelp are driven ashore by storms (Fenton, Reference Fenton1978). In spite of this, lambing time is scheduled for late spring (April/May). The investigation of seaweed consumption by sheep in the archaeological record, involving stable carbon isotope analysis, has demonstrated no contribution of seaweed to the sheep’s diet in the early stages of husbandry at the Knap of Howar, but a significant and systematic contribution of seaweed to the sheep’s winter diet at the Holm of Papa Westray (Balasse et al., Reference Balasse, Tresset and Ambrose2006; Balasse and Tresset, Reference Balasse and Tresset2009). At Skara Brae, seaweed consumption is not attested to the earliest phase of occupation; however, it is detected in the second phase of occupation, where it occurred in the winter, though not on a systematic basis (Balasse et al., unpublished). Considering this nutritional parameter, it does not seem that the breeding period was different at the Knap of Howar before the introduction of a seaweed complementation in winter, whereas no change was observed within the Skara Brae assemblage before or after the first appearance of this practice (Figure 4). This would suggest that a major change in food availability throughout the annual cycle did not significantly impact the cycle of sheep fertility.
Conclusion
Of the sample set considered, zooarchaeology confirms the expected scheme of lambing over a restricted time of the year in European sheep from the 6th to 3rd millennia BC. A 3- to 4-month breeding season, longer than in the present day European mouflon and shorter than in current improved breeds, corroborates the idea that over the long term, domestication led to an extension of the period of sexual activity in sheep.
All conclusions are in agreement with what is currently known of animal physiology in present day sheep. Beyond the historical significance, this consistency contributes in reinforcing the methodological basis of the approach. Further studies should enable the definition of variability attributable to technical choices, within global schemes constrained by environmental and climatic factors. These choices should be understood with regards to the orientation of animal productions. Those include in particular milk, which was exploited from the earliest times of sheep husbandry (Helmer et al., Reference Helmer, Gourichon and Vila2007).
The capacity for out-of-season breeding, observed in today’s Mediterranean breeds, also deserves attention. From the archaeological data set, the occurrence of outliers, reflecting the survival to adulthood of out-of-season lambs, suggests that this trait already existed in the 5th millennium BC sheep herds. Could this have been a starting point for the emergence, through selection, of sheep breeding out of season? Future research may help clarifying this point.
Acknowledgements
The authors thank Allison Sheridan and the National Museums Scotland for permission to sample the Skara Brae sheep, and Anett Osztás and István Zalai-Gaál who led the excavations at Alsónyék-Bátaszék. The authors also thank Soizic and David Cuisnier from the Ferme insulaire de Kemenez for providing access to their property and sheep. Over the last 12 years, this work has been supported by an ECLIPSE II project 2004–2005 (dir. A.T. and M.B.) for the study of Knap of Howar, Holm of Papa Westray, Bercy and the Carmejane sheep; by funds from the SRA Provence-Alpes-Côte d’Azur to E.B. for the study of Collet-Redon; by a NERC project (NER/ B/S/2003/00223, dir. I. Mainland) for the study of the Rousay sheep; the ‘SIANHE’ ERC Starting Grant project (GA 202881, dir. M.B.) for the study of the remains from Măgura, Cheia, Borduşani-Popină, Hârşova-tell, Skara Brae, Rousay and Kemenez; by the CNCS – UEFISCDI project PN-II-ID-PCE-2011-3-1015 (dir. A.B.) for the study of the Romanian assemblages; and by the DFG Food Cultures project (IV 101/5-1, dir. M.I.) for the study of the material from Alsónyék-Bátaszék. The authors also thank Philippe Chemineau for his interest in our research and enlightening discussions. English language editing by Jill Cucchi.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731117001045