The emergence of the modern hospital
Sometimes it seems that the hospital is the health system. Whether in popular culture, such as the American television series ER, in political and popular discourse, with its focus on opening and closing of hospitals, in statistical databases that give prominence to numbers of hospital beds, or in budgetary breakdowns, showing that the bulk of health service spending is concentrated in hospitals, it is clear that the hospital is seen as being at the heart of the health system (Reference McKee and HealyMcKee & Healy, 2002). Even when the many other components of the health system are recognized, the hospital typically sits at the top of the pyramid. This is perhaps inevitable. Hospitals are highly visible. They are large buildings, well signposted, and adorned with the symbols of health care, such as red crosses. When politicians wish to make a statement on health services, they typically find a convenient hospital as a backdrop. Hospitals are also important for the public, not just when they are ill, but by providing reassurance that they will be cared for nearby if they become ill in the future. They play other roles too, as settings for the education of the next generation of health workers and through their contribution to the local economy. So even though they are only one part of the overall health system, they are an important part, and are recognized as such by almost everyone.
Yet the concept of the hospital is a relatively recent one. Before the 18th century most people were cared for in their own homes, usually by family members or traditional healers. Institutionalized care, to the extent that it existed at all, was often in the hands of religious orders, providing somewhere that those with incurable illnesses could spend their last days in peace and tranquillity (Reference PorterPorter, 1999). What changed was the scientific revolution. Advances in a number of different areas brought new opportunities. In physics, the discovery of X-rays made it possible to look inside the human body as never before (Reference ReedReed, 2011). Advances in optics paved the way for microscopes, and thus the development of histopathology (Reference WollmanWollman et al., 2015). In chemistry and biology, technical advances made it possible to gain new insights into a patient’s condition from samples of their bodily fluids (Reference MoodleyMoodley et al., 2015). Acceptance of the germ theory led to the emergence of bacteriology (Reference Roll-HansenRoll-Hansen, 1979). Meanwhile, the development of safe anaesthetics and an understanding of the importance of asepsis made possible surgical procedures inside bodily cavities (Reference JessneyJessney, 2012).
The technology required to exploit these developments was rudimentary and there were few with the necessary skills to take advantage of it. There was a need to concentrate resources. The hospital was an obvious setting to bring together laboratories, operating theatres, and X-ray departments. It was also the obvious place to train people in their use.
Throughout the 20th century the opportunities to intervene to save lives and reduce suffering advanced rapidly. Paradoxically, it was from the death and destruction of war that many of the most important developments arose, such as the mass production of penicillin (Reference NeushulNeushul, 1993) and advances in plastic surgery (Reference GeomelasGeomelas et al., 2011), the management of burns, and orthopaedic surgery (Reference DoughertyDougherty et al., 2004) during the Second World War, as well as new approaches to major trauma in the Korean and Vietnam Wars (Reference EisemanEiseman, 1967; Reference MolnarMolnar et al., 2004). The earliest treatments for cancer were derived from chemical weapons, such as mustard gas (Reference MukherjeeMukherjee, 2010).
All of these expanded the scope of work of the acute hospital. Yet there were also changes that were reducing the work of some hospitals. From the 19th century onwards public bodies in many countries had invested in large hospital facilities, typically away from urban centres, in which they could place those with infectious diseases, especially tuberculosis, as well as mental illness. By the early 1950s the introduction of streptomycin had transformed the management of tuberculosis. Death rates in many countries were falling year on year and it was no longer necessary to incarcerate patients for long periods of time in the hope of spontaneous recovery (Reference DanielDaniel, 2006). By the early 1960s new antipsychotics had transformed the management of schizophrenia. Coupled with new models of care in the community, the days of the large psychiatric hospital were numbered (Reference CliffordClifford et al., 1991). Similar changes were happening within the acute hospital. Improvements in hygiene, linked to better living conditions, brought about a dramatic reduction in the number of children requiring admission for infectious jaundice, gastroenteritis, and respiratory infections (Reference Wolfe and McKeeWolfe & McKee, 2014).
But much more was happening in the hospital. Populations were ageing, benefiting from a remarkable increase in our ability to control many common chronic diseases. The consequence was that patients who would have died in previous years, were now surviving but with growing numbers of clinical conditions, a phenomenon termed multimorbidity (Reference BarnettBarnett et al., 2012). Ultimately, many experienced what has been termed frailty, involving decline in a wide range of bodily functions (Reference Nicholson, Gordon and TinkerNicholson, Gordon & Tinker, 2016). When they became seriously ill, they could require inputs from a wide range of health professionals, working together. But it was not just changes in the characteristics of patients. New opportunities to intervene also required new models of working based on teamwork, whether the problem was cancer (Reference PradesPrades et al., 2015), gastrointestinal haemorrhage (Reference LuLu et al., 2014), or major trauma (Reference McCulloughMcCullough et al., 2014). The evidence was accumulating that a multidisciplinary team (MDT), using shared protocols, achieves the best results.
Sometimes, changes in patterns of disease have even more profound consequences. The epidemic of HIV infection that began in the 1980s led to widespread changes in some of the fundamental elements of health care. These ranged from new approaches to infection control, in particular the risk of transmission of infection through surgical and medical procedures, to a new way of thinking about patient confidentiality and informed consent (Reference HayterHayter, 1997). Similarly, the growth of antimicrobial resistance has major consequences for many aspects of care delivered in hospitals and, in the future, is likely to have even greater impact, potentially threatening the fundamental principles on which hospitals are organized (Reference GoffGoff et al., 2017).
At the same time it became increasingly apparent that what was important in achieving the best outcomes was not where treatment was provided but how. In particular, waiting for the patient to arrive at hospital often meant missing important opportunities. Innovative treatments, such as thrombolysis for patients with myocardial infarction, could be initiated in an ambulance on the way to hospital, thereby reducing delays in this time-critical treatment (Reference McCaul, Lourens and KredoMcCaul, Lourens & Kredo, 2014). The use of advanced techniques to stabilize patients at the scene of major trauma meant that they arrived at the hospital in much better condition (Reference WilsonWilson et al., 2015).
It is not, however, only those things that happen before the patient gets to hospital that are important. Changes in family structure and in labour mobility mean that growing numbers of older people, including those with multiple disabilities, are living alone. Once they have completed active treatment in hospital they may have inadequate support at home, reflecting both the breakdown of traditional extended family structures and reductions in services, exacerbated since 2008 in countries that have imposed austerity policies leading to cuts in social care (Reference LoopstraLoopstra et al., 2016). The result in some countries is that much-needed hospital beds are occupied by patients who would be much more appropriately cared for elsewhere, if only appropriate accommodation and support structures existed (Reference Turner, Nikolova and SuttonTurner, Nikolova & Sutton, 2016).
Other technological changes have challenged some aspects of the rationale for the hospital. The original justification for concentrating resources in hospitals stemmed from the need to avoid duplication of three sets of resources: imaging equipment, laboratories, and operating theatres. However, the advent of portable ultrasound machines, coupled with mobile magnetic resonance imaging (MRI), offered new means of seeing inside the human body. Advances in near-patient testing, from the first simple test strips to complex micro-arrays (Reference VoswinckelVoswinckel, 1994), have challenged the role of the laboratory. Injectable anaesthetics, endoscopic procedures, and minimally invasive surgery have enabled what were once major procedures to be undertaken outside hospital. Many treatments that still need to take place in hospital can be completed in hours rather than days, and the pace and intensity of hospital work has changed beyond recognition; however, many processes, ways of working, and individual professional roles have struggled to keep pace.
In summary, the challenges facing hospitals have changed enormously in recent decades. The factors involved are extremely complex and interlinked. However, in broad terms, they can be divided into: changes in technology, including diagnostics and treatments; changes in patients, who have become older, frailer, and often more socially isolated; changes in models of care, involving networks and integrated pathways; and changes in staffing, affecting the need for both specialists and generalists.
The changing policy context within which hospitals operate
The preceding paragraphs have outlined the clinical changes that have driven developments in hospitals. However, there have also been many changes in the broader policy context within which they operate.
The first of these changes is in relation to accountability. For most of the 20th century what a hospital did, and how it did it, was determined largely by the medical profession (Reference FreidsonFreidson, 1974). Typically, each department was headed by a specialist physician or surgeon whose rule was absolute. Each department was largely autonomous, maintaining strict control over staff and resources. There was a tacit assumption that the senior physicians knew best, drawing on their long experience and status. It was inconceivable that their decisions would be questioned, no matter how idiosyncratic they seemed. Their relations with other health professionals, their junior staff, and patients were characterized by deference and, in some elite hospitals, their ward rounds could assume the trappings of a royal visit (Reference OsterbergOsterberg, 1990).
This situation reflected the prevailing approach to the professions. Professions were granted certain rights, in particular that of self-regulation, and high status. Members of professions had accumulated knowledge through a long process of apprenticeship. They were expected to exercise complex judgement, often in the face of uncertainty. It was not clear how anyone from outside the profession could second-guess them. In return, they were expected to maintain high ethical standards and obligations to the public (Reference FreidsonFreidson, 1988).
In all but a few places such situations are no more. There are many reasons. One is a wider societal rejection of deference to authority of all sorts. Another is a recognition that sometimes the professions fail to live up to the high standards they are expected to adhere to, whether in terms of competence or probity (Reference KaplanKaplan, 2007). A third relates to the growing commercialization of health care in some countries, whereby professional knowledge and status are seen as a barrier to the operation of the free market. Although health professionals remain among the most trusted groups in society (Reference Appleby and RobertsonAppleby & Robertson, 2016), politicians and the media are unwilling to countenance the high level of professional autonomy that once existed (Reference RaoRao et al., 2017). The extent to which this has happened varies enormously among countries and in some the concept of the liberal profession still holds sway. In others, however, health professionals are finding their work increasingly subject to high levels of regulation and monitoring, impacting adversely on morale and levels of burnout (Reference ChamberlainChamberlain, 2016; Reference RaoRao et al., 2017).
A second development relates to the explosion in data for monitoring. Health professionals have been monitoring outcomes of patients at least since the days of Florence Nightingale, albeit in very basic ways (Reference CaelleighCaelleigh, 1997). Advances in information technology, psychometrics, and health services research more generally have led to new ways of monitoring health outcomes, often using linked data, for example, to the deaths occurring after discharge from hospital, as well as a wide range of patient-related outcome measures (Reference BlackBlack, 2013).
These developments have facilitated a revolution in methods for assessing quality of care since the 1980s. However, this brings both opportunities and risks. In particular, publication of outcomes by individual health professionals has proven highly controversial, for several reasons. One is the challenge of adjusting adequately for case-mix or attributing an outcome to the action of an individual when the care is provided by a team (Reference Jacobson, Mindell and McKeeJacobson, Mindell & McKee, 2003). A second is the potential for opportunistic behaviour, which can range from changes in recording of patient characteristics to avoidance of those patients at greatest risk of an adverse outcome (Reference BurnsBurns et al., 2016). Finally, there are questions about whether publication accelerates or slows improvements in outcomes (Reference JoyntJoynt et al., 2016). Notwithstanding these concerns, it is clear that hospitals now and in the future will increasingly be evaluated in terms of the health gain that they bring about and not just the money they spend and the patients that flow through their wards.
A third issue, also related to the first two, has been the emergence of what has been termed “patient safety” on the policy agenda (Reference LongoLongo et al., 2005). While overlapping to some extent with the concept of quality of care, this explicitly reflects a recognition that hospitals may, on occasions, damage health. This can happen in many ways (Institute of Medicine, 2001). Failures to put in place appropriate procedures can lead to patients receiving the wrong treatment, for example, an incompatible blood transfusion, a drug to which they are allergic, or even a surgical procedure on the wrong patient or on the wrong side of the right patient. Recognition that this is a problem has led to new organizational structures, to ensure that problems are identified early and dealt with effectively. Lessons have been learnt from other sectors, such as the system used by airline pilots experiencing near-misses (Reference Nicholson and TaitNicholson & Tait, 2002).
A fourth issue is a change to the way in which hospitals are funded. Traditionally, hospitals receive their funding in a number of ways, including historical budgets and payments per patient or per bed day (Reference McKee and HealyMcKee & Healy, 2002). However, the recognition that patients with different conditions incurred very different levels of expenditure created pressure
for a much more differentiated system. The result in many countries has been the implementation of some form of activity-based system, typically based on the diagnosis of the patient and the procedures they undergo, with the best-known being versions of the American Diagnosis Related Groups (Reference Busse, Geissler and QuentinBusse, Geissler & Quentin, 2011). These systems are designed to incentivize hospitals to increase their efficiency, treating each patient with the minimum necessary resources. One consequence has been to bring about reductions, often substantial, in length of stay. Often this is a good thing, given the risks associated with being in hospital for prolonged periods (Reference AsherAsher, 1947). However, it presupposes that patients have somewhere safe and supportive to go to.
A final set of issues facing hospitals relates to the broader political context and, specifically, whether health care is seen as a tradable or a public service (Reference StarrStarr, 2008). In some countries, where the latter view has so far prevailed, hospitals are increasingly being seen as corporate entities and profit centres. This creates a powerful incentive to work in isolation, notwithstanding the importance of collaboration across the entire patient journey. Elsewhere, there is an increasing emphasis on networks, allowing patients to move freely within a system, obtaining routine care close to home when needed, but also access to advanced specialized services and specialized facilities if required. In a number of countries there has also been a significant growth in the number of hospitals that are part of groups, partly as a way of responding to some of the challenges detailed here but also as a method of reducing costs and improving quality through standardization and a greater role for professional management.
As with the changing clinical context, these issues are well recognized by those working in hospitals, but less often by those elsewhere who may be responsible for decisions that have profound consequences for hospitals and those who work in them. We believe that there is a need to bring all of these issues together: something that we have attempted to do in this book.
Rather than seeing hospitals as discrete entities within the health system that are often viewed in a mechanistic way through metrics such as numbers of beds or physicians, we view hospitals as complex adaptive systems, each containing a multiplicity of subsystems, some dealing with patients with particular conditions, such as a surgical department for example, while others provide resources that are shared among many of the other systems, such as operating theatres and pharmacies.
All of these systems interact with each other and are shaped by these interactions (Reference ChecklandCheckland, 1981).
We can only understand how they operate by looking at all levels, from the individual interaction between the patient and health professional through to the design and operation of the facility. However, this approach also recognizes that hospitals are situated within a broader health system, the optimal functioning of which depends on the linkage of many parts. This includes prehospital and post-discharge care. It also includes linkages to the training of health professionals, and the research and development that generates the knowledge on which effective care should be based. All of these systems and subsystems are operating in a rapidly changing environment, involving: the patients and their conditions; the opportunities to intervene, including technological advances and evidence on innovative models of care; and the broader policy and political context in which health care is delivered.
Consistent with the wider discourse in health policy, we have chosen to take a patient-centred approach. Pragmatically, this creates a problem. On the one hand, as we have noted, growing numbers of patients have multiple, complex needs and cannot easily be placed into individual categories. On the other hand, it is necessary to simplify our approach to make sense of the complexity. Consequently, in this book we have focused primarily on the acute general hospital rather than single speciality or specialized hospitals, long-stay facilities, and those providing restricted services or mainly convalescence (although in some chapters we do consider specialist hospitals too). We have looked at a number of the most important activities in which hospitals engage, defined by the conditions of their patients.
Meeting the needs of patients
We now look at the areas of hospital activity that are discussed in this volume. It is impossible to cover everything that is done within the hospital. Nor is it easy to create a simple taxonomy of the areas we could have covered. Consequently, we have selected a series of examples, looking at different patient groups, defined variously by age, disease process, and type of treatment, as well as some other areas where scientific advances have led to changes in patient management, such as imaging and laboratory science. While each contains a number of issues specific to the topic of the chapter, collectively they highlight many issues that have applicability more widely.
We start with children, the subject of Chapter 2 in this book. As noted above, the population of children in hospital has changed beyond all recognition in the last four decades. The wards that were once filled with children with common infectious diseases have gone. So has the generic paediatrician who once would have cared for children from birth to adolescence. Instead, there has been a remarkable diversification, of necessity given the high level of specialist skills required in many of the new areas that have emerged. This is perhaps most apparent with neonatal care. In 1975 one in every two premature newborns with a birthweight of less than 1500 g died in the perinatal period. By 2009 this had fallen to one in eight. Moreover, an increasing proportion of births in some countries are at low birthweight, as a consequence of multiple pregnancies related to in vitro fertilization. This has had enormous implications for both obstetrics and neonatal paediatrics, although not without controversy, as it has brought into sharp relief the tension between centralization, specialization, and medicalization on the one hand and a vision of birth as a natural event, involving a partnership between the mother and her midwife that is usually free from complications. Clearly there is a challenge in getting the balance right. However, this can only be done by close coordination between the different facilities providing obstetric and neonatal care. It illustrates perfectly the need for clinical networks of hospitals and other settings for childbirth working together collaboratively.
The chapter also looks at developments in care for older children. This is also an area that has been transformed by the creation of new knowledge (Reference WolfeWolfe et al., 2013), although there is enormous diversity among European countries (Reference EhrichEhrich et al., 2015). One result is increasing specialization. As with adults, it is not possible to expect a single physician to be an expert in the many body systems in which problems may arise. Moreover, as is frequently pointed out, children are not simply small adults. Consequently, there is a need for the specialist knowledge that paediatricians bring to these areas. The difficulty is that many of these diseases are relatively uncommon. Services must be concentrated to be viable, leading to the growth of highly specialized paediatric centres. This can be a major challenge for many small countries, in this case calling for networks that extend beyond national frontiers (Reference SalibaSaliba et al., 2014). Finally, it should never be forgotten that children should be kept out of hospitals as much as possible. Their physical, mental, and social development is best achieved at home with their families. As the chapter shows, there is much that can be done to make the hospital as friendly as possible for children (Reference EhrichLenton & Ehrich, 2015). However, although it may sometimes be needed, admission of children to hospital should always be a last resort.
The third chapter moves to the opposite end of the age spectrum, looking at one of the most common afflictions of middle and old age: stroke. Fortunately, the incidence of stroke has been falling dramatically in many high and middle income countries, largely as a result of improvements in the detection and management of hypertension (Reference LacklandLackland et al., 2014). However, as populations age, the absolute number of people affected by stroke is rising. The management of stroke has been transformed in recent years. Even as recently as the 1990s, many patients with stroke would simply be admitted to hospital to await a hopefully spontaneous recovery. Now, the focus is on early recognition of symptoms and signs, rapid transfer to hospital, early diagnosis using brain imaging, and definitive treatment. All of this must be achieved within a few hours and, if it can be, levels of disability can be reduced greatly. In a number of places, stroke services are organized on a population basis, reaching outside the hospital to begin the process of restoring blood supply to the affected part of the brain as soon as possible (Reference Alonso deAlonso de Lecinana et al., 2016; Reference Turner, Nikolova and SuttonTurner et al., 2016), in some cases using ambulances with computerized tomography (CT) scanners linked by telemedicine to specialist centres (Reference EbingerEbinger et al., 2014). However, this is only the beginning of the process, with subsequent management seeking to tackle the reasons why the stroke occurred, to prevent it recurring, and to provide the rehabilitation necessary to make as full a recovery as possible.
Once again, this chapter makes a very strong argument for a comprehensive approach to, in this case, a particular condition. This involves measures that address all of the building blocks of the health system, including a trained workforce, appropriate technology, and high levels of training. Yet, as it also shows, there are many barriers to achieving this and – still – great variation in the outcomes of treatment. This is an area where there are many opportunities for shared learning and comparisons of policies and practices.
The fourth chapter looks at a group of people whose numbers are growing rapidly but who often fall through the gaps in the hospital system (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). These are frail elderly people. Successes of modern medicine have allowed many more people to live into old age, albeit while experiencing the consequences of multiple disorders and declining bodily functions. Yet, with appropriate support, they can still live a fulfilling and satisfying life. The loss of functional reserve does, however, mean that they will require specialist advice in hospital outpatient clinics from time to time, and are prone to episodes of illness when they will require treatment in hospital. The challenge, in an increasingly specialized hospital system, is how best to design hospitals that are appropriate to their often complex needs (Reference Crews and ZavotkaCrews & Zavotka, 2006) and how to manage individuals who may have disorders of four or five different body systems, drawing on evidence such as that showing how procedures like comprehensive geriatric assessment can improve management and outcomes (Reference EllisEllis et al., 2011).
This chapter looks at some of the more innovative approaches to responding to the needs of this vulnerable group of people. It includes the creation of care coordination mechanisms, whereby they are helped to navigate through the complexities of the health care system, and in particular, avoiding the risk of falling through the gaps. It also includes the availability of rapid access and response teams, located either in hospitals or in the community, but able to provide assessment and treatment wherever it is needed (Reference WrightWright et al., 2014). In some ways, the process of ageing is the mirror image of development in childhood. Just as with children, frail elderly and, especially, confused people can find hospitals unfamiliar and disorientating. Yet, as with children, there is much that can be done to ensure that hospitals are friendly to older people when they do need to be admitted. One solution is the creation of dedicated frailty units, where patients can be cared for by specialized nurses with experience in issues such as falls, dementia, and incontinence (Reference ConroyConroy et al., 2014). And finally, it involves attention to hospital design, to ensure that the accommodation in which frail elderly people find themselves can meet their needs and expectations as effectively as possible.
The fifth chapter looks at another complex problem: cancer. This is an area that has been in the forefront of developing networks and multidisciplinary teams, recognizing the need for patients to be able to move seamlessly through a complex system from diagnosis to treatment and, if this is unsuccessful, to palliation. Often the management of cancer is straightforward, with surgery or radiotherapy achieving high levels of cure. But in many cases it is extremely complex. There have been remarkable advances in our understanding of the biology of cancer cells, leading to innovative new treatments that target them precisely. However, this can only be achieved with close working between a wide range of specialists. As with many of the other areas considered in this book, this increased knowledge has brought about a high level of specialization, with oncologists, or in some cases teams of surgeons, interventional radiologists, oncologists and others working together, now increasingly specializing in cancer of a single organ.
Cancer care has also been at the forefront of monitoring and evaluation, with most countries having well-functioning cancer registries. This has made it possible to identify, and in many cases explain, variations in outcomes. In some countries this knowledge has contributed to major reorganizations of cancer services, and in particular the creation of integrated networks. Yet again, cancer reveals the importance of organization, with collaboration rather than competition among hospitals.
The burden of disease in high income countries is dominated by chronic disorders. Increasingly, these are managed out of hospital. This was not always the case, and even now in many countries people with diabetes spend long periods in hospital, especially if they have complications. To illustrate the challenges involved in the hospital management of chronic diseases, we have selected, for the sixth chapter, one condition: chronic obstructive pulmonary disease (COPD). In most cases, those affected will be managed outside hospital, but they will, from time to time, often experience exacerbations that require admission. As with stroke, in the past such patients were often admitted, treated, and discharged. Many of them would return frequently, especially in winter, so they became well known to the hospital staff. As this chapter shows, treatment has been revolutionized by new approaches to the active management of this condition, and in particular a major focus on prevention, involving measures to improve lung function. Yet, as with stroke and cancer, there are still large variations across and within industrialized countries in the extent to which services for these people have moved from a reactive model to one that actively seeks to restore them to as good health as possible.
The seventh chapter deals with that part of the hospital that has come, in the popular imagination, to represent acute health care. This is the case of emergency medicine. As with all of the other areas, this has changed remarkably. Traditionally, the emergency department functioned as the front door of the hospital, through which an undifferentiated mass of people, with problems ranging from the trivial to the life-threatening, would pass. Those in the emergency department were confronted with the challenge of sorting them out, deciding which required immediate treatment and which could wait. Mixed among them were children, often exposed to sights that they would be forbidden from watching in a movie theatre. Now, however, the management of the acutely ill patient often begins before they ever reach the hospital, with trained paramedics commencing treatment in the patient’s home, at the roadside, or in the ambulance. Once they reach the hospital, they are triaged rapidly, their needs prioritized, and appropriate treatment begun as rapidly as possible. As with the conditions discussed in the other chapters, technological advances have transformed many aspects of emergency medicine. There has been a growing recognition of the importance of early stabilization and resuscitation and in many cases definitive treatment. Yet again, this demands a high level of organization. Teams need to be brought together, they need shared protocols, and they need to be present, with the appropriate equipment and facilities, at all times.
The eighth chapter looks at another aspect of the work of the hospital that, for many people, characterizes it. This is what happens in operating theatres, but now increasingly also what happens before patients get to theatre and how they recover afterwards. Technological advances, for example in intravenous anaesthesia, allowing people to recover rapidly, as well as in minimally invasive surgery and interventional radiology, have transformed surgery. For many people, especially if they are young and healthy, this means that a procedure that would once have required an admission over several days can now be completed within hours, allowing them to return home that evening. However, these advances have also lowered the threshold for intervention, especially with regard to those whose conditions might once have precluded surgery (Reference MougMoug et al., 2016). This, coupled with new opportunities for the more complex types of surgery, means that there is an increasing need for post-operative care, which has developed into a specialty in its own right. Again, this is something that requires careful planning, not just to put the systems in place, but to ensure the flow of patients through the hospital.
The final two chapters look at two of the reasons why the modern hospital developed in the first place: laboratories and imaging. As noted earlier, these are areas that have changed remarkably, in many different ways. Once, an imaging department depended on X-rays to look inside the body. Now, it can call upon ultrasound and MRI, with the bodily
organs highlighted using a multiplicity of contrast agents and, in some cases, radioactive tracers. It can do so with a precision undreamt of in the past, allowing the radiologist to view the patient in three dimensions and creating a form of virtual reality. In parallel, a new specialization has emerged. This is interventional radiology, where endoscopic instruments are manipulated under radiographic guidance, making it possible to undertake major proceedings without actually opening the body cavities. However, this has created tensions in some countries, with demarcation disputes between this new group of interventional radiologists and surgeons (Reference Baerlocher and DetskyBaerlocher & Detsky, 2009).
Laboratory medicine has also changed, again driven by advances in technology. There has been a remarkable growth in opportunities for near-patient and self-testing (Reference Larsson, Greig-Pylypczuk and HuismanLarsson, Greig-Pylypczuk & Huisman, 2015) but this has also created challenges as the results must frequently be interpreted by those with the expertise and ability to make an assessment of the whole patient. Increasingly, this means that pathologists are moving out of the laboratory, becoming part of the MDT caring for the patient, advising on the most appropriate tests that should be done and how their results should be interpreted, especially in patients that have multiple disease processes simultaneously.
Conclusion
As this introduction shows, the work of the hospital has changed beyond all recognition in a few decades. Yet its design has often failed to keep pace with these developments. In the final chapter, we will look at some of the challenges that face the hospital in the future. These include the growth of antimicrobial resistance, a problem that has largely been created by hospitals in the way that they operate. Yet there is now extensive evidence that the design and function of hospitals can do much to prevent its emergence. They also include the need to design hospitals in ways that take account of the needs of different groups of patients (Reference Rechel, Wright and EdwardsRechel, Wright & Edwards, 2009). As discussed already, these include children and frail elderly people. There are already many examples of good practice, with designs that address their needs, but too often there is a sense that the hospital has been assembled with no thought about those who will use it, whether this involves the use of materials that amplify noise at night, thereby preventing people from sleeping (Reference DuBose and HadiDuBose & Hadi, 2016), or the lack of signposting that allows people to get lost (Reference Wright, Hull and LickorishWright, Hull & Lickorish, 1993). Finally, it is often forgotten that those who spend the most time in hospitals are not the patients but the staff. At a time when many countries are facing acute shortages of health workers, it is essential that the hospital is configured in a way that is welcoming to them and allows them to do their work as effectively and efficiently as possible.
Above all, the pace of change is so rapid that it is essential that those facilities being designed today are built in a manner that is flexible, and allows them to adapt to these changing circumstances. We hope that this book will assist those who, in whatever role, are interested in hospitals and, in particular, how they can best meet the needs of patients and staff in the future.
Child health care in Europe
The role of the hospital in caring for children has changed beyond recognition in the past five decades. On the one hand, the conditions that were once responsible for most bed occupancy, such as respiratory tract infections, gastroenteritis, and hepatitis A, are now far less common and, when they occur, are managed at home in all but the most severe cases. On the other hand, advances in medicine and technology, coupled with better understanding of genetics, metabolic and neonatal medicine, new treatments for cancer and acute/chronic organ failure, advances in surgical techniques, and new ways of managing severe mental disorders, have created a need for services that did not previously exist (Reference WolfeWolfe et al., 2013). Consequently, the hospital continues to play a key role in the health care of children, albeit one that is rapidly adapting to the changing needs of sick foetuses, newborns, infants, children, and adolescents. Hospital services for children must also be able to work closely with other parts of the health system and beyond, reaching out to wider services for children including education, prevention, long-term outpatient care for children with rare diseases, and primary care out of normal hours. Yet a survey conducted in 2015 revealed great diversity in hospital services for children in the 53 countries of the World Health Organization’s European region (Reference Ehrich, Namazova-Baranova and Pettoello-MantovaniEhrich, Namazova-Baranova & Pettoello-Mantovani, 2016). Differences are apparent even at the most basic level: the definition of a child. The age at which young people are no longer managed in children’s hospital services varies among countries. In 53% of countries childhood is defined as up to 18 years of age, but in one country it is up to 11, in three up to 14, in four up to 15, in six up to 16, and in one up to 17 years of age. Two countries reported the upper age limit for children in paediatric services to be 19 and in one country it is 26 years (Reference EhrichEhrich et al., 2015a).
There are also considerable variations in the settings in which children receive hospital care. A 2009 survey conducted by the European Paediatric Association identified four different types of children’s hospital in Europe: 1) general children’s hospitals and paediatric units (or paediatric wards) within larger hospitals for adults; 2) stand-alone children’s hospitals; 3) university children’s hospitals; and 4) mother and child centres. Day clinics and neonatal intensive care units (NICUs) were found in all four types of hospital. There are also major differences in infrastructure, such as diagnostics and therapeutics, especially high technology equipment, as well as organizational arrangements and markers of quality.
As with every other aspect of medicine, health services for children must adapt to a rapidly changing landscape. One way in which this landscape is changing is the demography of Europe. With a falling birth rate, Europe is facing a declining child population. The mean shares of the total population aged 0–14 years and 0–4 years in Europe were 21.2% and 6.4% respectively in 1982 but these figures had fallen to 15.2% and 5.0% in 2014. The scale of the change can be seen from looking at three countries, Belgium, Ireland, and Portugal, where the fertility rate decreased from 2.54, 3.78 and 3.16 respectively in 1960 to 1.75, 1.96 and 1.21 in 2013 respectively (Eurostat, 2016).
This demographic change, coupled with changes in disease patterns and treatment settings, has contributed to a large reduction of hospital beds and to the closure or merging of children’s hospital facilities and thus is challenging conventional ways of thinking about hospital facilities for children. Traditionally based on a division between primary, secondary, and tertiary care, new models of care seek ways to innovate and improve the whole system. The changes are complex and do not simply involve crude reductions in hospital capacity. For example, on the one hand, the decline in the incidence of communicable diseases through immunization programmes, as well as injuries through injury prevention programmes, has caused a decrease in the need for care and consequently hospital admissions. On the other hand, medical and surgical advances, in areas such as neonatal surgery and intensive care, oncology, and interventions for inherited diseases, are increasing the need for highly specialized care that can only be provided in tertiary hospitals. At the same time, there is a continuing burden of chronic diseases – some attributable to increasing risk factors, such as childhood obesity, and some to improved survival of previously fatal conditions, such as malignancies and certain inherited disabilities. This has created a greater need for specialist care that transcends the hospital and the community (Reference Wolfe and McKeeWolfe & McKee, 2014), a need that is also increasing because of the improved survival of very low birthweight babies, some of whom are living with long-term disabilities. These children require sophisticated diagnostic and therapeutic interventions delivered by well-trained personnel using technologically advanced infrastructure.
In addition to the complications created by the increasing specialization of care provided for children in hospitals, there is a growing recognition of the importance of designing systems from the perspective of the user rather than the provider of services. This is exemplified by the call to design “a hospital that does not feel like a hospital”. This thinking has been captured in the Council of Europe’s “Child-Friendly Health Care” approach, which was endorsed by 47 ministers representing the nations of Europe (Reference Lenton and EhrichLenton & Ehrich, 2015) (Box 2.1). This approach brought systems thinking and values based on the United Nations Convention on the Rights of the Child together into a practical framework to plan, deliver and improve services for children and families. The child-friendly health care approach builds on patient-centred care and patient pathways. The responsibility of the health system is to ensure that all the component parts are in place and working well together to achieve the best possible outcomes. This can only happen if appropriate child health care networks, based on collaboration (Future Ho . This spital Commission, 2013), can work together to improve quality continuously. Crucially, the hospital is a key element of these networks. Despite the shift from inpatient to outpatient care and the fall in the mean duration of stay in hospital the hospital is still very important. Although their work often extends beyond the walls of the hospital into the community, it is still the case that in many European countries about half of all paediatricians are still hospital-based (Reference EhrichEhrich et al., 2015a).
Box 2.1 Extract from the terms of reference of the Council of Europe on child-friendly health care
Five principles are particularly relevant to the child-friendly health care approach:
1. Participation Participation means that children have the right to be informed, consulted, and heard, to give their opinions independently from their parents, and to have their opinions considered. It implies the recognition of children as active stakeholders and describes the process by which they take part in decision-making. The level of child participation depends both on his or her age, evolving capacities, maturity, and on the importance of the decision to be taken.
Parents and families should encourage children to participate in family, community and society decision-making – encouraging increasing independence and reducing their support as the child’s capacity for autonomy and independence develops.
2. Promotion Health promotion is “the process of enabling people to increase control over their health and its determinants and thereby improve their health”. Promotion therefore includes all actions that allow
children to become more involved in their own health and increase their exposure to positive determinants of health (defined as factors which will improve health or well-being). Health promotion covers not only activities in families and communities, directed at health determinants or lifestyles, but also factors in health care services and settings which will improve outcomes.
3. Protection Health protection includes all actions that either limit or avoid children’s exposure to any hazard which can be defined as a factor that has the potential to cause harm. Hazards can occur in families, communities and health services. Medical interventions can cause harm and patient safety perspectives highlight the fact that children are particularly vulnerable to medication errors and hospital-acquired infections.
4. Prevention Prevention is an active process the aim of which is to avoid future health, social or emotional problems to enable the fullest realisation of human potential. This includes action to reduce adverse health determinants, to prevent the development of a disease or condition, to avoid complications of a disease or condition, to prevent the impact of a disease or condition on the lifestyle or aspirations of an individual, and to prevent harm caused by a service or intervention.
5. Provision Provision refers to any service which contributes to the health and well-being of children and families, and therefore includes more than just traditional health services. “Pathway-based provision” is a concept that describes all the component parts that need to be in place and working well together to achieve an excellent patient experience which brings about optimal outcomes for children and families in their journey through services.
Clearly, in the light of the preceding discussion, there is no simple way to structure a chapter on the care of children in hospital. Consequently, we have taken a pragmatic approach, looking first at the highly-specialized care of newborn infants, followed by the care of older children with common conditions requiring hospitalization, and then the provision of highly specialized care in tertiary hospitals. We then review some common patient pathways, illustrating the inter-linkages between the different elements of the system before looking to emerging developments that may impact on the health system response to children in the future.
Maternal, neonatal and follow-up care in specialized facilities
Trends in obstetric and neonatal care
The management of pregnancy and childbirth has been transformed in recent decades, both organizationally and in terms of the technology and knowledge base required to achieve improved outcomes. These changes have been accompanied by a marked improvement in outcomes. Neonatal mortality has improved substantially during the last four decades. In 1975 the 28 current European Union Members (EU28) experienced 12.84 neonatal deaths per 1000 live births, yet by 2014 this had fallen to 2.52 per 1000 live births. This reflects several factors, including a reduction in low birthweight babies, and especially, survival among those born prematurely. Consequently, in 1975 half of all premature newborns with a birthweight less than 1500 g died during the postnatal period but this fell to 14.3% and 12.4%, in 2000 and 2009 respectively (European Society for Neonatology, 2015). Maternal morbidity and mortality have also improved significantly.
These changes have been accompanied by several, often conflicting, trends taking place in the organization of services during childbirth in European countries that have implications for the future role of the hospital. In the past, many deliveries took place in small local hospitals, close to where people lived. These hospitals often had limited facilities but had the advantage of convenience for the mother and her family. However, the falling birth rate in many countries and the subsequent reduction in demand for delivery facilities threatens the viability of these hospitals, many of which have closed for other reasons, such as the inability to provide comprehensive, 24-hour, advanced medical and surgical services. Moreover, the smaller hospitals were unable to provide the facilities required when complications arose during childbirth. Yet, while many of the other services that these hospitals once provided have been transferred to larger hospitals with more sophisticated equipment, there is also pressure to de-medicalize childbirth, leading either to increased home births or to the development of stand-alone family friendly facilities, separate from acute hospitals.
Such facilities clearly meet the needs of the majority of expectant mothers. However, there are some who have other conditions that place them at high risk, such as advanced cystic fibrosis or cardiac insufficiency, or who are post-transplant, who require careful monitoring by a MDT that brings together adult medical, obstetric, and neonatal care. In the past, many of these mothers would not have survived into adulthood, and those who did would have been advised against becoming pregnant. With individualized care planning for delivery, coupled with advances in intra-partum care, they can now expect to have a healthy baby. However, this intensity of management, and especially the involvement of multidisciplinary teams, can only be undertaken from a well staffed and equipped hospital facility, even though those expectant mothers that do require specialized medical or surgical intervention can often receive it on an ambulatory basis. An added complication is that in some countries there have been increases in the number of infants who require intensive care and specialist intervention, in part reflecting later pregnancies and multiple births following in vitro fertilization. Finally, as often noted, a normal delivery can only be assessed as such in retrospect.
For these reasons, there is a need to ensure close coordination between facilities undertaking deliveries, whether stand-alone or within acute general hospitals, and those facilities providing specialized neonatal care. Ideally, any pregnancy identified as high-risk should be delivered in a setting where the delivery suite and the NICU are adjacent, or at least on the same site, but it is also important to recognize that, while unanticipated complications of delivery are fortunately rare, they do happen, so there should also be mechanisms in place to enable early referral and rapid intervention to save the life of the mother and baby in stand-alone facilities.
There are other reasons for close collaboration between obstetric and neonatal services, including shared training and participation in research, especially that responding to the needs of mothers and babies with complications. Obstetrician involvement in postnatal NICU ward rounds and discussions with parents can improve the knowledge base for antenatal counselling, which should also ideally involve the MDT, including the obstetrician, neonatologist, and, where appropriate, teams providing surgical and highly specialized paediatric expertise.
This is, however, an area where technology and knowledge continue to advance rapidly. New resuscitation guidelines have recently been published by ILCOR/ERC/AHA in 2015 (Reference WyllieWyllie et al., 2015), including changes in resuscitation practice, such as resuscitation closer to the mother to allow delayed cord clamping, but requiring greater involvement of specialist neonatal care in the delivery suite. At the same time, advances in remote monitoring are making it possible for senior staff to provide input remotely during resuscitation. Other advances include greater use of point-of-care testing (POCT), discussed further in Chapter 10, but this will require adaptation, including the use of nanotechnology, to take account of the very small volumes of blood that can be taken from extremely premature infants.
Provision of NICU
Existing guidance suggests that in a typical western European country, based on contemporary practice, there is a need for 0.75 cots per 1000 births for intensive care, 0.75 cots per 1000 births for high dependency care, and 4.4 cots per 1000 births for special care (Reference LaingLaing et al., 2004). However, this must also take account of changes in the frequency of preterm births, such as the increases in several countries including the USA, Canada, Australia, Sweden, Scotland, and Wales (Reference HallsworthHallsworth et al., 2008). In Europe, in countries with comparable levels of development and health care systems, preterm birth rates vary markedly, ranging from 5% to 10% among live births. A second question relates to the distribution of facilities. A German population-based study found that 28-day mortality was more closely associated with the numbers of neonates looked after in a NICU than with the number of births in the hospital, with the effect greatest for infants of less than 29 weeks’ gestation (Reference HellerHeller et al., 2007), although a study in the USA found that, while both the number of very low birthweight babies and the numbers treated in NICUs were important determinants of good outcomes, the former was more important. Other researchers found that mortality in small NICUs is significantly increased (Reference BartelsBartels et al., 2006). This evidence has led the American Academy of Pediatrics, in its Committee on Fetus and Newborn report on Levels of Neonatal Care, to support larger-volume NICUs.
NICU design and environment
As survival of preterm infants has improved dramatically during recent decades, there has been a marked increase in the number of children treated in NICUs. Initially, in the late 1970s and 1980s, NICUs were designed as multipatient wards with some private rooms to isolate infants with infections. In the 1990s, as survival became commonplace, new ideas began to emerge about possible effects of the physical environment on the fragile, growing brain of newborns. In 1992 Reference White and WhitmanWhite and Whitman (1992) recommended some private rooms for neonates. Many studies have now found that preterm infants are influenced by the physical conditions in NICUs, such as noise (Reference Long, Lucey and PhilipLong, Lucey & Philip, 1980) and lighting (Reference MannMann et al., 1986). Box 2.2 sets out suggested environmental and building standards.
Box 2.2 Environmental and building standards for NICUs
Noise: Sound levels should be kept at less than 40 dB. Private rooms provide a decrease in the number of adults in the room, and a study by Reference Robertson, Cooper-Peel and VosRobertson, Cooper-Peel & Vos (1999) showed clearly that decreasing conversation had the greatest effect on decreasing noise levels in a NICU.
Light: Adjustable lighting between 0.5 and 60 ft-candles (5–600 lux) is appropriate for general lighting levels in NICUs and an indirect room lightening should be preferred. A circadian lighting scheme should be used in the patient care area.
Air quality: NICUs should be air-conditioned to the highest standards, with air temperature at 22–26 degrees Celsius, 30–60% relative humidity, and a minimum of six air-changes per hour.
Design: Careful design is needed, with extensive additional space for family, overnight stays, privacy and staff.
Private rooms: Single-family rooms (private rooms) allow infants to be cared for in a room where they are shielded from medical or social activity at a neighbouring bed. The risk of cross-contamination may also be reduced.
The first all-private room NICU in Europe was built in Brest, France, for the express purpose of minimizing nosocomial infection (Reference WhiteWhite, 2011), although no study has yet found that private rooms in NICUs enhance infection control. A study from the Karolinska group of hospitals in Stockholm compared the results of two different types of NICU: those with private rooms versus those with four-bed open rooms (OReference Ortenstrandrtenstrand et al., 2010). Premature newborns cared for in private rooms showed marked reduction in ICU and total hospital days, as well as a reduction of bronchopulmonary dysplasia. However, recent research has highlighted the need for greater attention to the sound in the NICU as infants nursed in single rooms had significantly altered MRI findings compared to those in an open ward (Reference SmithSmith et al., 2011).
Guidelines developed within the WHO/UNICEF Baby-friendly Hospital Initiative (BFHI) (World Health Organization, 2016) propose that newborns who do not need NICU facilities should be cared for in their mother’s room, with the support of specialized nurses to encourage and support bonding and support breastfeeding. Family rooms that allow parents to “room-in” and care for their infants also offer a means for siblings to meet and bond with the new baby without creating infection-control issues for the NICU.
Levels of newborn care
Newborns need different levels of care in NICUs. The 2012 classification developed by the American Academy of Paediatrics is used widely (American Academy of Pediatrics, 2012). These facilities are divided among those providing basic care (level I), specialty care (level II), and subspecialty intensive care (level III, level IV). Level I facilities (well newborn nurseries) provide a basic level of care to neonates who are low risk. Neonatal resuscitation can be undertaken if required for every delivery in these units and healthy newborns can be evaluated and receive routine postnatal care. In addition, Level I units can care for preterm infants at 35 to 37 weeks’ gestation who are physiologically stable, and can stabilize newborn infants who are less than 35 weeks’ gestation or who are ill until they can be transferred to a facility at which specialty neonatal care is provided. Care is provided by paediatricians, family physicians, and nurse practitioners. The recommended ratio of nurses to babies is 1:4. Interestingly, a study of NICUs in California found no difference in quality across levels of NICU (Reference ProfitProfit et al., 2016).
The British Association of Perinatal Medicine (BAPM) has published guidelines suggesting that units with fewer than 50 infants with birthweight less than 1500 g should plan to amalgamate with other units to ensure clinical skills and expertise are retained (British Association of Perinatal Medicine, 2010). The BAPM guidelines also highlight the importance of hand-washing facilities for parents and staff, as well as adequate space to prevent cross-infection between babies and isolation facilities for infected infants.
Care in a specialty-level facility (level II) should be reserved for stable or moderately ill newborn infants who are born at ≥32 weeks’ gestation or who weigh ≥1500 g at birth but have problems that are expected to resolve rapidly and who would not be anticipated to need subspecialty-level services on an urgent basis. Level II nurseries may provide assisted ventilation until the infant’s condition either soon improves or the infant can be transferred to a higher-level facility. Care is provided by neonatologist and neonatal nurse practitioners (NNPs) in addition to level I staff. The recommended ratio of nurses to babies is 1:2.
Infants who are born at less than 32 weeks’ gestation, weigh less than 1500 g at birth, or have major medical or surgical conditions, regardless of gestational age, should be cared for at a level III facility. Level III facilities should be able to provide ongoing assisted ventilation for 24 hours or more, which may include conventional ventilation, high-frequency ventilation, and inhaled nitric oxide. A broad range of paediatric medical subspecialists and paediatric surgical specialists should be readily accessible on site or by prearranged agreements. Level III facilities should have the capability to perform advanced imaging with interpretation on an urgent basis, including CT, MRI, and echocardiography. Care is provided by paediatric medical subspecialists, paediatric anaesthesiologists, paediatric surgeons, and paediatric ophthalmologists in addition to level II staff. The recommended ratio of nurses to babies is 1:1.
Level IV units include everything that is available at level III with additional ability to care for the most complex and critically ill newborn infants. Such units should have specialist paediatric medical and surgical consultants continuously available 24 hours a day. Level IV facilities also include the capability for surgical repair of complex conditions (such as congenital cardiac malformations that require cardiopulmonary by pass with or without extracorporeal membrane oxygenation). Care is provided by level III staff plus paediatric surgical subspecialists.
Good outcomes for neonates in the NICU are dependent on the availability of sufficient numbers of skilled neonatal nurses. Developing NNPs can help maintain skills and continuity of care as medical staff change frequently. In many countries NNPs are expanding into roles such as neonatal transport and NNP-led clinics. Clinical nurse specialists provide vital services in the areas of discharge planning, support for lactation, and resuscitation. In some areas community neonatal nurses provide pre- and post-discharge care and visits. Clinical nurses and midwives specializing in bereavement play an important and expanding role in maternity hospitals, supporting parents faced with an antenatal diagnosis of a potentially lethal condition or who have newborns who die in the first few weeks of life. The United Kingdom’s Royal College of Paediatrics and Child Health (RCPCH) framework on withholding or withdrawing life-sustaining treatment in children and the neonatal palliative care guidelines offer guidance to health care providers in these situations. These health professionals can also address issues such as neonatal organ donation.
Speech and language therapists, dieticians, physiotherapists, psychologists, medical social workers and occupational therapists are all essential to the operation of a level III NICU. Neonatal dieticians are playing an increasing role in optimizing nutrition. The extended role pharmacist will become more important in contributing to staff and parental education on medication use and prevention of prescribing errors, as well as new pharmaceutical agent use in the NICU. The role of clinical engineers has expanded with newer devices with a broader range of uses, such as neonatal ventilators and monitoring equipment for transport and care of infants with neuro-critical conditions. Neonatal transport is an essential part of an integrated neonatal network. This service needs to be available round-the-clock, including specialized equipment such as that required for hypothermia therapy.
Beyond the classification of NICUs set out above, there is a need to consider separately the provision of extracorporeal membrane oxygenation (ECMO), a life support mechanism which allows blood to be taken from the body, oxygenated outside the body and returned, and carbon dioxide and oxygen exchanged. A randomized controlled trial conducted in the United Kingdom showed a clear benefit for newborn infants with severe respiratory failure.
Education for staff and families
The European Society of Neonatology (ESN) Curriculum for Training in Neonatology in Europe (European Society for Neonatology, 2015) was developed to support national training programmes. The ESN has created a database of national training programmes to encourage transparency and harmonization of subspecialist training in neonatology.
Technological advances have enabled simulation to become a core element of training, with dedicated simulation laboratories equipped with high-fidelity mannequins in many new level III units. This development has been encouraged by the implementation of the European Union (EU) working time directive, designed to reduce the known risks associated with long working hours. However, despite the clear benefits for patient safety, it has posed problems in enabling medical trainees to obtain adequate practical experience. Simulation offers a means to deliver carefully designed, well supervised training experiences that are a significant improvement over the ad hoc approaches used previously. Simulation also offers a means to provide coordinated training for the multidisciplinary teams whose work is now so essential in NICUs, allowing them to develop their skills in a team setting where they can realistically model clinical scenarios.
Reflecting the important role that parents play in the care of newborn infants, it is important that training should not be limited to staff. Although parents are supported as they come to terms with the health of their newborn infants, there is considerable scope to develop this more formally in association with NICUs, including preparation for those expectant parents where it is anticipated that their babies are likely to require a stay in a NICU, as well as preparation for the post-discharge period.
Secondary care for children
While much childhood illness can be managed in primary care, there will inevitably be children who require hospitalization in secondary care facilities. Table 2.1 sets out some examples of such conditions. However, the numbers involved will depend not only on the burden of disease in the population but also on the scope and quality of primary care, which varies greatly around Europe. Policies in many countries have sought to reduce unnecessary admissions to hospital as they are
Table 2.1 Selected examples of indications for admission of children to hospitals
| Paediatric subspecialty care | Standard indications | Optional* indications |
|---|---|---|
| Neurology | developmental disorders, di-/tetraplegia, gait disorders, headache, neuropathies, seizures, etc. | myopathies, motor, hearing, visual, mental and skeletal disabilities, specific sleep disorders, etc. |
| Ear, nose and throat | tonsillitis, otitis, sinusitis, lymphadenopathies, etc. | cholesteatoma |
| Cardiology | arterial hypertension, arrhythmias, myocarditis | cardiomyopathy |
| Pulmonology | laryngitis, bronchi(oli)tis, asthma, pneumonia | cystic fibrosis |
| Hepato-gastroenterology-Nutrition | gastroenteritis, appendicitis, hepatopathies, abdominal pain, etc. | chronic inflammatory bowel disease, intussusception, cholestasis, etc. |
| Hematology/Hemostaseology | anaemia(s), leukopaenia, immune thrombocytopaenia (ITP), preoperative screening | coagulopathies |
| Infectious diseases | meningitis, encephalitis, upper airway infections, hepatitis, borreliosis, etc. | tuberculosis |
| Urology/Nephrology | urinary tract infection, hydronephrosis, glomerulonephritis, etc. | common nephritic and nephrotic syndrome |
| Dermatology | all kinds of rashes, atopic dermatitis | haemangioma |
| Mental disorders | somatoform disorders | ADHD, depression |
distressing for children, cause problems for parents, and are usually a less cost-effective way of treating acute illness. On the other hand, delayed referral of severely sick children to hospital may lead to preventable complications and death. Closing the organizational gaps between primary and secondary care for children is therefore an important task.
It is important to see the hospital as only one element within the wider health system. However, the way in which the hospital interacts with the other elements of the health system will vary, influenced by the organizational characteristics of the system. Across Europe, the responsibilities of hospitals caring for children are not uniformly defined and vary between countries and even regions within countries. Furthermore, they may vary according to whether the hospital is publicly or privately owned, with the former typically responsible for providing a comprehensive range of services while the latter can select those areas that are most profitable and incur least risk to the provider. Figure 2.1 illustrates these relationships, with the hospital bringing together a range of specialist expertise.

Figure 2.1 The position of hospital care for children within the health system
Where in the system a child is treated will vary according to a range of factors. However, even within a single system, the boundaries are not necessarily clear. Decision-making processes relating to treatment and referral are subject to different rules and regulations of the health systems, but also policies about what services to offer in what facility, themselves influenced by the interests of the health care personnel involved. For instance, the management of many long-term conditions, such as asthma, diabetes, or coagulopathies, may be provided in different settings in different countries. Despite the existence of such variations, it is desirable that those responsible for managing and providing care in hospitals should find ways to achieve consensus with primary care physicians and tertiary care paediatricians on standards to be adopted for infrastructure, facilities, staff, and quality of medical treatment.
Recognizing that, where possible, children and adolescents should be managed in settings other than in hospitals, when they are admitted they are entitled to have certain expectations:
To be welcomed by friendly staff, whether doctors, nurses or others
To be adequately informed about what is to be done and when;
To have the option to be accompanied by mother, father, and other relatives;
To be treated according to modern standards of evidence-based medicine;
To experience as little pain as possible;
To receive age- and disease-appropriate treatment;
To be treated by staff trained to communicate with children and parents;
To experience a private and respectful atmosphere whenever possible;
To be properly informed about all procedures and results of investigations;
To stay in hospital for as short a time as possible;
To receive adequate information about further treatment;
To receive full treatment free of charge.
These expectations of patients and their care takers are consistent with the Charter of the European Association for Children in Hospital (EACH) (Box 2.3) (European Association for Children in Hospital, 2015).
Box 2.3 Charter of the European Association for Children in Hospital (EACH)
Article 1 Children shall be admitted to hospital only if the care they require cannot be equally well provided at home or on a day basis.
Article 2 Children in hospital shall have the right to have their parents or parent substitute with them at all times.
Article 3 Accommodation should be offered to all parents and they should be helped and encouraged to stay. Parents should not need to incur additional costs or suffer loss of income. In order to share in the care of their child, parents should be kept informed about ward routine and their active participation encouraged.
Article 4 Children and parents shall have the right to be informed in a manner appropriate to age and understanding. Steps should be taken to mitigate physical and emotional stress.
Article 5 Children and parents have the right to informed participation in all decisions involving their health care. Every child shall be protected from unnecessary medical treatment and investigation.
Article 6 Children shall be cared for together with children who have the same developmental needs and shall not be admitted to adult wards. There should be no age restrictions for visitors to children in hospital.
Article 7 Children shall have full opportunity for play, recreation and education suited to their age and condition and shall be in an environment designed, furnished, staffed and equipped to meet their needs.
Article 8 Children shall be cared for by staff whose training and skills enable them to respond to the physical, emotional and developmental needs of children and families.
Article 9 Continuity of care should be ensured by the team caring for children.
Article 10 Children shall be treated with tact and understanding and their privacy shall be respected at all times.
Highly specialized paediatric centres (tertiary care)
Expert specialist care is essential for the diagnosis and treatment of rare conditions and for children who require complex investigations and highly technical interventions, such as transplantation. This care typically requires sustained collaboration between different specialists and subspecialists to ensure optimal outcomes. However, while anecdotally it is known that there are different models of care, these have not, to our knowledge, been documented in detail.
Less well resourced countries in central and eastern Europe face the dilemma of how best to develop and fund specialist care in the future. Better resourced countries in western Europe face the problem of how best to rationalize and co-locate interdependent specialist services to improve outcomes. Small countries must find ways of developing effective cross-border care with larger countries, drawing on the many existing examples such as that between Malta and the United Kingdom (Reference SalibaSaliba et al., 2014).
One of the key questions facing those organizing specialized paediatric services is how best to balance centralization and decentralization. There are various arguments for creating a small number of large centres that can concentrate expertise and equipment, can create multidisciplinary teams, and can provide 24-hour services where necessary. The last of these is particularly important as it typically requires about 10 individuals to provide round-the-clock service, a number that can only be justified if there is sufficient caseload. The question of whether concentration of services achieves better outcomes has been debated extensively. There is clear evidence to support this for some services, such as neonatal intensive care and cardiac surgery. However, the evidence is rather more limited in other areas. It is also important to recognize that concentration of services in large centres, especially in countries with low population densities, can create a significant barrier to access, although it may be possible to compensate for this by the development of outreach services, whereby specialists travel from tertiary centres to other facilities. These decisions about how to provide highly specialized services are complex and require many, often competing, objectives to be balanced (Reference EhrichEhrich et al., 2015a).
These decisions must also be informed by considerations of which specialties should be co-located. The current situation is characterized by significant differences in care across European countries. One fact is the absence of consistent European definitions of either specialist care or specialist centres. There are also differences in training programmes and assessment, both within and between specialties, with 38 different accredited paediatric subspecialties reported in a European Paediatric Association survey in 2014 (Table 2.2), which exceeded the 22 recognized in the USA in 2012. Individual European countries recognize between 0 and 20 separate paediatric subspecialties (Reference EhrichEhrich et al., 2015a). This also poses challenges to those organizing training programmes, especially where the numbers of physicians in particular subspecialties are very low. However, the situation is further complicated by the scarcity of data on the numbers and qualifications of specialists in most countries. There is also little information on scope of practice and required competencies.
Table 2.2 Paediatric subspecialties in child health and the number of European countries in which each is recognized
| Adolescent medicine | 1 | Neonatology | 16 |
| Allergology | 8 | Nephrology | 12 |
| Anaesthesiology | 2 | Neurology | 14 |
| Cardiology | 14 | Neuro disability | 1 |
| Community paediatrics | 1 | Neuropsychiatry | 5 |
| Dermatology | 2 | Oncology | 12 |
| Developmental paediatrics | 1 | Ophthalmology | 3 |
| Emergency paediatrics | 5 | Orthopaedics | 2 |
| Endocrinology | 13 | Otorhinolaryngology | 3 |
| Gastroenterology | 13 | Palliative paediatrics | 1 |
| Genetics | 2 | Pharmacology | 1 |
| Gynaecology | 2 | Pneumonology | 12 |
| Haematology | 8 | Primary care paediatrics | 5 |
| Hepatology | 2 | Radiology | 3 |
| Immunology | 3 | Rehabilitation | 3 |
| Infectious diseases | 4 | Rheumatology | 8 |
| Intensive care | 9 | Stomatology | 2 |
| Mental health | 1 | Surgery | 6 |
| Metabolic diseases | 5 | Urology | 5 |
Note: Those in bold are also recognized by the American Council of Pediatric Subspecialties in Pediatrics 2012.
While recognizing these difficulties, it is possible to suggest the most important interdependencies among specialties (Figure 2.2). Where possible, those services with clear interdependency should be co-located. For example, a centre undertaking organ transplantation should also have the expertise necessary to provide care in haemato-oncology, cardiology, nephrology, metabolic medicine, paediatric surgery, and a paediatric intensive care unit (grey squares), while subspecialties such as endocrinology (white squares) are not required. Tertiary care children’s hospitals should have departments of child psychiatry and psychosomatic care in the same building. Transition and transfer of adolescents from paediatric to adult care should also take place in the same hospital, where possible (Reference CrowleyCrowley et al., 2011). Finally, the teams caring for children in hospital include teachers, who face particular challenges in meeting the needs of children who may divide their time between hospital and home over a prolonged period.
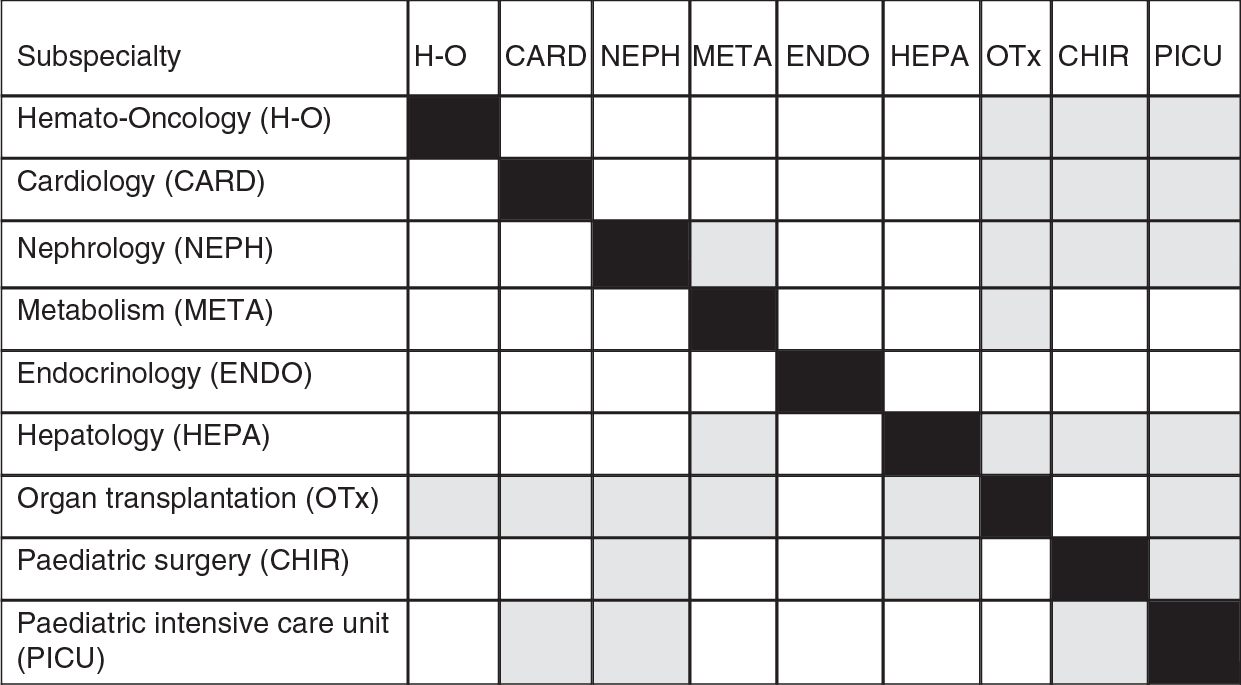
Figure 2.2 Appropriate co-location of paediatric subspecialties
The organization of services should be viewed from the perspective of both the child and their family and the health professionals working in the service. For the child and their family, it is important that all parts of the system should be in place and working well together with specialist advice easily accessible, but delivery should be as close to home as is safe and sustainable. This suggests the need to develop networked solutions where all those involved actively collaborate and constantly strive to improve safety and experienced outcomes. From the perspective
of the health professional, the specialist centre should not be seen as a “stand-alone” institution but as part of a well managed clinical network that promptly refers the most appropriate children and simultaneously receives children back into the local system for rehabilitation after specialist care. Clinical leadership for specialist care resides with the centre which organizes shared care with clear clinical care plans, with training and joint clinics for local teams. The local team should organize routine health and social care and education as appropriate. This model, based on good two-way communication, has already been achieved in some cancer and neonatal networks. The ideal system can be summarized with the phrase “centralized specialization and decision-making, but decentralized provision of treatment whenever possible”.
It is important to recognize that there is a risk that highly specialized paediatric subspecialty care may lead to fragmentation (Reference EhrichEhrich et al., 2015b). Consequently, especially where a child has multiple health problems, there is a strong argument for oversight of their care being undertaken by a general paediatrician, who can work closely with the child and their family. As Reference VohraVohra et al. (2012) state, “paediatric integrative medicine should be the paediatricians’ new subspecialty” to bring specialist care together.
In summary, there are many challenges in providing highly specialized paediatric care in Europe but there is a limited evidence base to inform the decision-making process. The most urgent questions needing answers include:
how best to plan an adequate number of specialist centres, where appropriate, taking account of possibilities created by the European Union Directive on Cross Border Care, so as to avoid both under-provision and over-supply;
how to develop a sustainable workforce to meet the medical needs of children; many different factors must be taken into account, including geography, population distribution, transport links, and relationships between centres;
how to balance any benefits from centralization with problems of access, recognizing that while most families will accept travelling long distances to receive episodes of specialist investigations or treatment, it is desirable that regular visits, for example for administration of treatment or follow-up, should take place as close to their homes as possible.
Typical patient pathways
We now look at the health system as seen through the eyes of the child. We do this by describing a series of journeys undertaken by children with four common conditions: an acute infectious disease, a chronic illness, a critical condition, and an illness requiring new technologies.
A child with acute infectious disease: acute lower respiratory tract infection (LRTI)
Acute LRTI in children – bronchiolitis and pneumonia – are normally managed in primary care by the first contact care giver. Should treatment fail, or in cases of atypical or recurrent pneumonias, the infant or child is referred to a secondary paediatric care setting (either ambulatory or inpatient facility) for further investigations, parenteral antibiotic therapy, oxygen and supportive treatment. Occasionally, a child with an LRTI might be found to have an underlying disorder, such as cystic fibrosis. In such cases, the child will be referred to a specialist team, normally at a tertiary care facility. The clinician treating the sick child must answer two questions. Is the infection due to a virus or bacteria? Antibiotic treatment is only indicated for the latter and unnecessary prescriptions increase the risk of antimicrobial resistance. Second, how likely is the child to respond to treatment, recognizing that small children in particular can deteriorate rapidly. On the one hand, it is necessary to ensure that further action is taken if there is such a deterioration. On the other hand, unnecessary admission to hospital should be avoided as far as possible. Advances in technology do, however, offer a possible solution as instruments based on evaluating gene expression profiles of leukocytes have demonstrated the ability to differentiate viral from bacterial infections, and scoring systems based on whole gene expression analyses may offer scope to assess severity in children with LRTI (Reference Wallihan and RamiloWallihan & Ramilo, 2014). In the future, these novel strategies may be able to identify rapidly those children who need antibiotics and those who should be promptly hospitalized.
A child with a chronic illness: asthma
If the asthma is moderate or severe, or if the diagnosis is uncertain, the child/adolescent is referred to a competent paediatric team either in an ambulatory paediatric setting or at the ambulatory clinic in a hospital paediatric department. Current recommendations are that such children should be referred to a specialist team at a regional centre if there are uncertainties about the diagnosis, or when children do not respond to recommended treatments. The paediatric teams should consist of specialized paediatricians and paediatric nurses. These teams will have access to lung function tests, blood tests and allergy testing. Ideally, the child will have a personalized treatment plan that is clearly documented, linked to a written asthma home management plan that is reviewed at every visit. Continued monitoring of asthma therapy is essential. Some centres offer group training (asthma schools). In case of an emergency, the child should be admitted to a paediatric department in a hospital. New biomarkers in blood, such as chitinases and periostin, as well as new means of diagnosing allergies, including component resolved diagnosis, which can identify the allergens involved, and basophil allergen threshold sensitivity, offer scope for more precise diagnosis of asthma and its triggers, and predict its severity. Novel treatment possibilities may include macrolide antibiotics and individualized cytokine antagonist therapies (Reference HedlinHedlin, 2014). In-home monitoring using telemedicine also offers future potential (Reference StarmerStarmer et al., 2010).
A child in a critical condition
When a child has an overtly critical condition, such as foreign body aspiration or ingestion, head injury, poisoning, or serious respiratory or cardiac failure, then immediate provision of ambulance services, contactable by phone, must be guaranteed. Ideally, paramedics in the ambulance will be able to initiate immediate life-saving treatment, if that has not already been provided by first-aid. The ambulance should take the child to the emergency unit at the nearest hospital. Ideally, this will have separate provision for emergency care of children.
Countries should have poison control centres offering haemodialysis/adsorption, plasma exchange, and blood exchange at regional or national level, open for consultation by phone 24/7 for medical staff and the public.
Continuous medical education and professional development, including practical training of parents and care givers in emergency situations at both community and hospital level, are essential. It is also important that the equipment provided for emergency responses should cover the entire age spectrum of children. All those staff involved in the emergency response should also be trained in the specific health problems of children. This includes paramedical staff in ambulances but also those who staff emergency telephone services. Teleconsultations can facilitate the emergency care delivered to children in remote areas (Reference Burke and HallBurke & Hall, 2015).
A child with a chronic illness requiring new technologies – type I diabetes mellitus (DM)
Immediate admission of children with diabetic ketoacidosis to hospital is warranted. Insulin, fluids and electrolytes are given while closely monitoring the patient. After stabilization is reached, a well planned preparation of the families for home treatment should be initiated in the paediatric department. Children with type 1 diabetes mellitus are increasingly utilizing continuous subcutaneous insulin infusions (insulin pumps) and continuous glucose monitoring systems (CGMS), both of which have been shown to improve glycaemic control and quality of life if the families are well trained. Proper education for families and providers should be provided to promote successful use of high-tech equipment and will reduce the number of adverse events (Reference ErnstErnst et al., 2016). These children should be closely followed in diabetes outpatient clinics of hospitals or in community centres providing appropriate staff and expertise. Future technology will focus on the new generation of pumps and monitoring (Reference CarchidiCarchidi et al., 2011). Telemedicine applications can facilitate monitoring and adherence to therapy (Reference Burke and HallBurke & Hall, 2015).
Future trends
The care of children has changed remarkably in the past few decades and will continue to do so. Many of the same factors that drove changes in the past will remain important, including advances in technology, models of care, and professional roles. However, it is likely that there will be particularly important advances in information technology, enabling care to be coordinated across many different settings. A number of these key drivers for change are set out in Table 2.3. Beyond these individual drivers, it is clear that there will be a need for much greater integration of services, with child-oriented care delivered jointly by child health professionals working in different locations.
Table 2.3 Future trends influencing the delivery of integrated care to children on five different levels
| Trend | Impact on the future hospital | Example | Challenges |
|---|---|---|---|
| Ongoing medical advances |
|
| Funding of new devices and drugs |
| Health information technology | Paper free children’s hospital | e.g. One record for all health care providers caring for a child with type 1 diabetes mellitus: the primary care taker, hospital paediatric department, subspecialists and community services | Interoperability of electronic health recording systems in hospital and community |
| Innovative models of care (Reference StarmerStarmer et al., 2010) |
| e.g. A child with a neuro-developmental syndrome and epilepsy | Coordination of care across service settings |
| Telemedicine |
| e.g. Monitoring a child with inflammatory bowel disease in a rural area (Reference Burke and HallBurke & Hall, 2015) | Infrastructure |
| Skill mix | Task shifting | e.g. Nurse practitioners in intensive care units (Reference KotzerKotzer, 2005) | New professional hierarchies, team working, quality assurance |
Conclusions and key messages
Existing systems providing secondary health care for children are facing several major challenges. First, there is enormous variation in the quality and nature of care provided for children across the European region, including differences in financial resources, organization of health care, and access to skilled health professionals and advanced technology. Second, the care of sick children has undergone a process of fragmentation, largely reflecting new opportunities to intervene, driven by scientific and technological advances. However, this fragmentation risks being exacerbated by organizational changes in some health systems. Third, although the sick child is on a journey that moves between different levels of care, they and their parents will often be challenged by structural and organizational barriers between primary, secondary, and tertiary care. Finally, all health systems are facing upward pressure on costs, with some aspects of paediatric care, including neonatal intensive care and highly specialized tertiary care, being especially vulnerable.
Many of these challenges apply equally to the provision of hospital care for adults. However, there are some specificities (Box 2.4). All hospitalized children should be admitted to children’s wards and not to adult wards, and those caring for children in hospital face some additional challenges. For example, while it is the child who is being treated in hospital, it is important to find ways to include other members of the family in the process, for example by the creation of family-friendly facilities. It is also the case that children are not simply small adults and many can find the clinical environment frightening, so it is important to incorporate elements of design that create a child-friendly environment (Boxes 2.1 and 2.2). As with any patient with a complex chronic disease, care is increasingly being delivered by multidisciplinary teams but, in the case of children, these teams extend beyond the health sector to the education sector. There are many opportunities for learning from the different models of care seen in Europe but this will require considerable effort to overcome the scarcity of comparative information and of health services research focusing on children’s services.
Box 2.4 Ten rules for the care of children in hospitals
1. The interests of the child should come first, with policies based on an understanding of the importance, and the life course model, of development.
2. Children should be cared for by a team of competent care givers who have been trained in communicating with children and in treating children of all ages.
3. Sick children should be treated in special age-appropriate units and not in adult units.
4. Priority should be given to non-invasive and ambulatory care for children as far as possible.
5. Care givers must have adequate time to communicate with children to strengthen their engagement in the clinical process. Hospital care for children includes support for patients, families and care givers, including the provision of relevant training, a process facilitated by enabling parents to stay with their children.
6. Hospital care for children must be adequately financed and staffed.
7. Child care at all levels should be integrated, taking account of lessons from whole systems thinking.
8. Competition between different care givers and between different institutions is unhelpful and can create unnecessary barriers to the seamless provision of care for children with complex needs.
9. Those providing hospital care for children should participate in research that advances knowledge on the care of children, including both scientific and organizational interventions, and should develop mechanisms to ensure that these advances are incorporated into practice to continuously improve quality of care.
10. Hospital care means a combination of inpatient and outpatient care to avoid fragmented care.
We conclude with a series of challenges for those responsible for the organization and delivery of health care for Europe’s children.
1. How can health systems prepare for the “unknown unknowns” in meeting the health needs of children? There are still many questions about how to translate the explosion in knowledge of the molecular basis of disease, including genomics, proteomics, and metabolomics, into practical solutions that can be applied widely and at scale, and in ways that are affordable.
2. Do stand-alone children’s hospitals have any future? Ageing societies and reduced demands for inpatient care of children, coupled with payment systems that often fail to cover the costs of paediatric care, suggest that most will struggle to survive, with the possible exception of some highly specialized facilities, such as those linked to academic health centres. The challenge facing those stand-alone children’s hospitals that are privately owned are especially great.
3. The motto “sick child in and healthy child out” simplifies the current problems of child health care. Prior to and following a stay in hospital there must be well developed pathways for long-term care, involving a wide range of child health care providers in a variety of settings.
4. How can models of care based on networks and integration across settings be delivered where there is choice of provider? Experience in Germany, for example, suggests that hospitals in such a setting have no incentive to develop initiatives designed to respond to the needs of chronically ill children in and outside hospitals (Reference BusseBusse, 2004).
The burden of stroke
Stroke is one of the leading causes of acute medical admissions to hospitals. It is among the most common causes of death and is a major cause of disability and poor health outcomes (Box 3.1). Worldwide, 17million people suffer a stroke each year and stroke is the third most common cause of death around the globe, accounting for 12% of all deaths, and exceeded only by heart disease and cancer (Reference FeiginFeigin et al., 2009; Reference ThriftThrift et al., 2014; GBD 2013 Mortality and Causes of Death Collaborators, 2015). While its management involves a series of specific responses by the hospital, the principles underlying them – including the importance of coordinated multispeciality and multiprofessional care, speed of response in the acute episode, the importance of prevention (of both the initial episode and any recurrence), and a model of care that follows the patient along the entire pathway, from the onset of illness to recovery and rehabilitation – apply equally to many other common medical conditions, such as acute myocardial infarction, gastrointestinal haemorrhage, or the acute and chronic complications of diabetes.
Box 3.1 What is stroke?
A stroke is an episode of neurological dysfunction caused by disruption of blood circulation (ischaemic stroke) or bleeding (haemorrhagic stroke) in an area of the central nervous system: the brain, spinal cord or retina. Approximately 90% of strokes are ischaemic and 10% are due to haemorrhage, although there is variation between populations in the relative proportions of ischaemic and haemorrhagic stroke. There are two main types of haemorrhagic stroke: primary intracerebral haemorrhage and subarachnoid haemorrhage. This chapter will address the health care needs of patients with the most frequent types of stroke: ischaemic stroke and primary intracerebral haemorrhage. Subarachnoid haemorrhage, although important in its own right, is less common and patients typically follow different patient pathways than those with ischaemic stroke or primary intracerebral haemorrhage – subarachnoid haemorrhage will therefore not be covered in this chapter.
A transient ischaemic attack (TIA) is caused by a temporary disruption of blood supply to the central nervous system – the symptoms are short-lived but it is a warning sign that an ischaemic stroke may be about to occur. The symptoms of stroke and TIA depend on the area of the nervous system affected, but commonly include muscular paralysis, loss of sensation, loss of vision and speech and language problems. The main risk factors for stroke and TIA are hypertension, physical inactivity, tobacco smoking, other cardiovascular disease (such as diabetes or ischaemic heart disease), atrial fibrillation (AF) and increasing age.
to 26 million (Reference FeiginFeigin et al., 2015). Essentially, changes in population demographics are outpacing improvements in stroke prevention, resulting in an increasing burden of stroke on populations and health systems, particularly in lower and middle income countries.
In addition to mortality, stroke causes a wide range of disabilities and impairments and has long-term implications for the health and well-being of survivors. These include neurological impairments such as muscle weakness or paralysis, impaired vision and impairments of speech and language skills. Up to 50% of patients will develop major depression in the years after stroke (Reference AyerbeAyerbe et al., 2013). Cognitive impairment is common and cerebrovascular disease is a major risk factor for dementia. More subtle cognitive problems, such as perceptual impairments, a change in personality, and profound fatigue are common, often lasting for years after the stroke (Reference WolfeWolfe et al., 2011). These problems are often referred to as “hidden deficits” but they account for a significant proportion of the suffering and costs that stroke causes. Effective risk reduction and high quality treatment should not only result in improvements in physical health but also reduce the future burden of dementia and mental health problems.
The financial costs of stroke are large and diverse: to the individual and their family in terms of health care and time off work; to governments in terms of medical and social care; and to wider society in terms of lost productivity. Other non-monetary costs are harder to calculate but are equally important – such as the emotional cost to family and friends of caring for a loved one who can no longer live independently.
Stroke accounts for between 2% and 4% of the total health care expenditure in developed countries. Moreover, stroke incurs substantial costs outside the health care system, reflecting survivors’ high rates of disability and dependence. In 2008 the total direct and indirect costs associated with stroke were approximately £8.9 (€9.7)billion per year in the United Kingdom (Reference SakaSaka et al., 2009). Most costs are incurred in the initial months and years after the patient has been discharged from hospital (Reference SakaSaka et al., 2009). Studies from Italy (Reference BottacchiBottachi et al., 2012), Denmark (Reference JennumJennum et al., 2015) and France (Reference SchmidtSchmidt et al., 2015) have produced similar estimates of the costs of stroke in Europe, at €7000–20000 per stroke. Almost any intervention that reduces the incidence of stroke or reduces the likelihood of long-term disability will be cost-effective in countries with expensive health and social care systems.
Age-adjusted incidence and mortality rates for stroke have fallen significantly over recent decades, thought to be due to improvements in stroke prevention through improved management of risk factors for stroke, especially hypertension and tobacco control, and, in some places, improved acute care. Between 1990 and 2013 the age-adjusted mortality rate for stroke in developed countries fell from 113 to 67 per 100000 (Reference FeiginFeigin et al., 2015). However, because of increasing longevity and the strong association between stroke risk and age, the absolute numbers of people having stroke are rising year on year, from an estimated 4.3 million globally in 1990 to 6.9million by 2013 (Reference FeiginFeigin et al., 2015). This is leading to increasing numbers of people dying from stroke (2.1 million in 1990 to 3.3million deaths in 2013 from ischaemic stroke), increasing disability adjusted life years and an almost doubling in the prevalence of stroke between 1990 and 2013, from 14million
Evidence-based stroke care
Historically, stroke was considered a condition for which little could be done, but there is now an extensive evidence base for interventions that are effective in improving outcomes after stroke: reducing disability, improving survival and reducing the risk of stroke recurrence. Some of these interventions (such as stroke unit based care) are applicable to almost all patients with stroke, while others are limited to selected patient groups (Figure 3.1).
Figure 3.1 Number needed to treat for the main evidence-based interventions in acute ischaemic stroke
| Proportion of patients with ischaemic stroke applicable | Outcome | Number needed to treat to benefit | Estimated number with improved outcomes per 1000 patients with ischaemic stroke if intervention was given to all applicable patients | |
|---|---|---|---|---|
| Stroke unit care | 90–100% | Death or long-term institutionalization | 19 | 50 |
| Antiplatelet therapy | 85–95% | Death or dependency | 79 | 11 |
| Early supported discharge | Up to 50% | Death or dependency | 20 | 25 |
| Thrombolysis | Up to 20% | Death or dependency | 25 | 8 |
| Thrombectomy | Up to 10% | Dependency | 3 | 33 |
Organized multidisciplinary stroke care
In the past 25 years the medical care of patients with stroke has changed enormously in high income countries. The central change has been the development of organized systems of stroke care, characterized by a move away from general medicine towards specialized multidisciplinary models of care based on the stroke unit model. Randomized controlled trials have shown that being admitted to a stroke unit improves survival and reduces long-term dependency: in the most recently updated Cochrane review of organized stroke care summarizing the results of 31 trials, the odds of death or death or dependency at one year were reduced by 14% and 18% respectively (Stroke Unit Triallists’ Collaboration, 2007). Stroke units have been evaluated in many different countries and settings, and found to be effective in all types of stroke patient and in both high and middle/low income countries (Reference Langhorne, de Villiers and PandianLanghorne, de Villiers & Pandian, 2012). Organized models of rehabilitation care after stroke have also been found to improve outcomes. In particular, early supported discharge (ESD) services, where multidisciplinary care and therapy are provided in the patient’s own home at a similar intensity to inpatient rehabilitation, improve long-term recovery and shorten length of hospital stay (discussed in detail later in this chapter) (Reference Langhorne and BaylanLanghorne & Baylan, 2017).
Acute re-perfusion
For patients with ischaemic stroke, early treatment to restore blood flow to the affected area of the brain can limit the extent of damage and increase the patient’s chance of making a recovery. Re-perfusion can be achieved either through administration of a clot-busting drug or through a procedure. The first landmark trial to demonstrate the effectiveness of thrombolysis was the National Institute of Neurological Disorders (NINDs) trial in 1995 (National Institute of Neurological Disorders, 1995), which found that the drug alteplase significantly reduced the rate of disability if given within 3 hours of stroke onset. As well as demonstrating the effectiveness of the therapy, the results also had the effect of highlighting the very poor quality of existing health care systems for patients with acute stroke, since the effectiveness of the drug was entirely dependent on very rapid recognition, triage and diagnosis. In the United States the results of this trial prompted in 1995 the first national effort to define standards about how to organize acute stroke care (National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995) and the subsequent development of the Joint Commission’s Comprehensive Stroke Centre hospital certification scheme. Thrombolysis has since been evaluated in a number of trials, and has been shown to improve recovery if provided to suitable patients within 4.5 hours of stroke onset (Emberson, 2014).
More recently, evidence has emerged that early physical removal of the blood clot causing ischaemic stroke (a procedure called mechanical thrombectomy) improves outcomes. If provided within 6 hours to suitable patients, thrombectomy is very effective in increasing the chance of patients regaining functional independence after stroke (Goyal, 2016).
The impact of both these approaches is, however, limited by being suitable only for a minority of patients: up to 20% of patients with ischaemic stroke may be eligible for thrombolysis and approximately 10% (Reference McMeekinMcMeekin, 2017) for thrombectomy.
Prevention of complications and stroke recurrence
Evidence-based secondary prevention for stroke includes antiplatelet therapy (Reference SandercockSandercock et al., 2008), anticoagulation in people with AF, blood pressure-lowering therapy, and treatment with statins to lower cholesterol (American Heart Association/American Stroke Association, 2014; Intercollegiate Stroke Working Party, 2016). The risk of recurrent stroke can also be reduced in some patients by early vascular surgery (carotid endarterectomy) to the carotid arteries (North American Symptomatic Carotid Endarterectomy Trial Collaborators, 1991). Early initiation of these therapies is also effective in reducing the risk of stroke in patients with TIA (Reference RothwellRothwell et al., 2007). Identifying the specific cause of the stroke for each patient is an important part of stroke care so that appropriate secondary prevention can be initiated, such as long-term treatment with anticoagulation in patients with AF. As discussed later in this chapter, this involves an increasingly sophisticated array of diagnostic tests and technologies.
Most patients dying of acute stroke do not die directly from brain injury, but from the complications of immobility and impairment. Preventing complications through, for example, screening patients for swallowing problems after stroke to reduce the risk of pneumonia, and using intermittent pneumatic compression (IPC) devices (CLOTS Trials Collaboration et al., 2013) to prevent venous thromboembolism, contribute to preventing the complications of acute stroke and improving survival after stroke.
The great majority of patients with stroke do not require any surgical intervention, but early neurosurgery can improve outcomes in selected patients with very extensive ischaemic strokes (Reference Cruz-Flores, Berge and WhittleCruz-Flores, Berge & Whittle, 2012) and in patients with certain types of intracerebral haemorrhage (Reference MendelowMendelow et al., 2013).
Rehabilitation
Helping people to recover, regain function and return to doing the activities and work they were doing before their stroke is an essential component of stroke care. Compared to other areas of stroke care, however, there have been very few large clinical trials of stroke rehabilitation and a relatively weak evidence base exists. There is evidence that very early mobilization with high intensity therapy after stroke may actually lead to poorer outcomes (AVERT Trial Collaboration Group et al., 2015) than physiotherapy protocols that use more frequent but less intense spells of activity.
The stroke care pathway
Hospitals are central to stroke care but exist as components of a pathway of care that spans pre-hospital emergency medical services, acute hospital care, rehabilitation and primary care (Figure 3.2). For many of the elements of the patient pathway, there is now good quality evidence about how to organize health services to optimize patient outcomes. At the same time, we also know that in the real world there are wide variations both across Europe and within individual health economies in how stroke care is delivered.

Figure 3.2 An example of a typical stroke care pathway in high income settings
Pre-hospital
Most people develop acute stroke out of hospital, although hospital inpatients are at high risk of stroke (particularly people undergoing cardiothoracic surgery or angioplasty) and approximately 5% of strokes occur in people who are already in hospital for another reason. Both stroke and TIA are medical emergencies, and delays in presentation to hospital are associated with worse outcomes. Delays in the assessment and treatment of patients presenting with TIA increase the risk of their going on to have a stroke (Reference RothwellRothwell et al., 2007), while pre-hospital delays in patients with ischaemic stroke reduce the chance that they will benefit from re-perfusion therapy. Patients with acute stroke should be admitted directly to hospital and patients with TIA may be managed as outpatients provided that clinics provide urgent (e.g. within 24 hours) assessment, diagnostics (brain and vascular imaging, blood tests, cardiac tests) and treatment (Reference Luengo-Fernandez, Gray and RothwellLuengo-Fernandez, Gray & Rothwell, 2009).
Public awareness of stroke symptoms is poor (Reference ReevesReeves et al., 2008) and contributes to the significant number of people presenting late after stroke onset. The FAST (Face Arm Speech Time) test was developed to improve recognition of stroke by the general public and has now been adopted worldwide into public campaigns as part of efforts to inform the general population about how to respond to a stroke (Public Health England, 2015). Promotion of this test as part of a mass media campaign in the United Kingdom appeared to be successful in increasing the proportion of people attending hospital rather than primary care with stroke symptoms (Reference FlynnFlynn et al., 2014), but evidence of effectiveness in other countries has been variable (Reference MellonMellon et al., 2013).
Pre-hospital delays in care can also be reduced by having effective systems to triage patients with possible stroke and alert the receiving hospital that a patient with stroke is on their way to hospital (Reference FassbenderFassbender et al., 2013). Tools have been developed (e.g. the ROSIER tool) that allow for the rapid triage of patients with probable stroke pre-hospital or in the emergency department (Reference NorNor, 2005).
Acute hospital care
Upon arrival at hospital, the key diagnostic test is brain imaging with CT and/or MRI. Most patients receive a CT scan acutely in order to distinguish between ischaemic stroke and primary intracerebral haemorrhage, and the main role of MRI is in follow-up imaging, in the assessment of patients with TIA or in cases of diagnostic uncertainty (Intercollegiate Stroke Working Party, 2016). For patients who are potentially eligible for thrombolysis or thrombectomy, more advanced types of imaging (such as CT angiography) may be used to identify appropriate patients and reducing the time from admission to brain imaging is critical in achieving delivery of these treatments quickly: co-location of scanning suites in or adjacent to the “front door” can help in reducing delays in scanning (Reference MeretojaMeretoja et al., 2013). With the development of thrombectomy as an established therapy, it is essential to rapidly develop robust stroke imaging protocols that include CT or MR angiography as well as CT (or MRI).
For patients suitable for thrombolysis, the sooner it is administered (“door to needle time”), the greater is the likely benefit. Some single centres routinely achieve extraordinarily fast times for treatment, with thrombolysis being administered in just 20 minutes after arrival at hospital (Reference MeretojaMeretoja et al., 2013), and there is evidence from the United Kingdom that treatment is fastest in larger/higher volume centres (Reference BrayBray et al., 2013b). Typical door to needle times in Europe are approximately 1 hour, although there is significant international variation in times between countries (for example, a median door to needle time of 56 minutes in England and Wales compared to 45 minutes in Sweden (RiksStroke, 2018; Sentinel Stroke National Audit Programme, 2018).
Inpatient care for stroke patients should be on a specialist stroke unit (Intercollegiate Stroke Working Party, 2016). A stroke unit consists of a discrete area of a hospital ward that exclusively or nearly exclusively takes care of stroke patients and is staffed by a specialist MDT (Cochrane Stroke Reference GroupGroup, 2013). A small proportion (less than 5%) of patients will require ICU care or surgical interventions as part of their stroke management – such as neurosurgical management of very large ischaemic strokes and intracerebral haemorrhages, or vascular surgery. However, most patients should spend the majority of their inpatient stay on either an acute stroke unit or a rehabilitation stroke unit.
Acute care in the stroke unit involves (Reference Langhorne and PollockLanghorne, Pollock & Stroke Unit Triallists’ Collaboration, 2002):
medical assessment and diagnosis
early assessment of nursing and therapy needs
monitoring of physiological and neurological status
screening and prevention of complications
mobilization
rehabilitation therapy (physiotherapy, occupational therapy, speech and language therapy).
Priorities in the first few hours after admission are physiological monitoring, correction of problems such as dehydration, fever and high blood sugar, and managing the complications of stroke. For patients with primary intracerebral haemorrhage, there is some evidence that rapid blood pressure lowering may improve functional outcomes (Reference AndersonAnderson et al., 2013). Swallowing problems are common after stroke and place patients at increased risk of pneumonia if they eat or drink; patients need to be screened for swallowing problems and may be temporarily fed through a feeding tube during this time to reduce the risk of pneumonia. Delivering these interventions as a care bundle has been shown to improve patient outcomes (Reference MiddletonMiddleton et al., 2011) and delays in carrying out swallow screening are associated with higher rates of stroke-associated pneumonia (Reference BrayBray et al., 2017). Careful nursing care in this early period is especially important in preventing complications of stroke (Reference Middleton, Grimley and AlexandrovMiddleton, Grimley & Alexandrov, 2015), since most of the early deaths after stroke are caused not directly by the stroke itself, but by complications such as pneumonia, sepsis or venous thromboembolism.
Many patients with stroke are immobile and so are at high risk of pressure ulcers and venous thromboembolism (VTE). Managing VTE risk is complicated in patients with stroke because the risk of intracranial bleeding is increased by the anticoagulants typically used for VTE prevention. VTE risk can, however, be reduced by the use of IPC devices in patients who are unable to mobilize (CLOTS Trial Collaboration et al., 2013).
End of life care
Approximately one in six patients admitted to hospital with stroke will die in the next 30 days, and the risk of death is particularly high in older people, those with more severe stroke and patients with intracerebral haemorrhage. Providing good quality palliative and end of life care is therefore an essential component of all stroke services. This requires health care professionals on stroke units to have the relevant knowledge and skills to provide palliative care, and the availability of specialist palliative care services for patients with complex or hard to manage symptoms. Palliative care for patients with stroke is complex, and requires not only the provision of symptom control and compassionate and dignified end of life care, but also complicated decision-making about treatment withdrawal, artificial nutrition and feeding, and goal setting (Reference HollowayHolloway et al., 2014).
Rehabilitation
Most patients with stroke will require a period of rehabilitation and assessment of their impairments and needs. This usually involves physiotherapy, occupational therapy, and speech and language therapy. Care models for this vary considerably between health economies. In some settings the stroke unit will provide both acute care and rehabilitation, whereas in other settings these functions are separated, with patients being transferred to a dedicated rehabilitation ward. Models commonly used in Europe include:
inpatient rehabilitation on a stroke unit which also provides acute care
inpatient rehabilitation on a stroke unit dedicated to providing rehabilitation
inpatient rehabilitation in a generic rehabilitation ward or facility
discharge home, with community-based rehabilitation provided in outpatient facilities
discharge home, with community-based rehabilitation provided in the patient’s place of residence.
In contrast to the strong evidence base concerning the organization of acute stroke care (see Figure 3.2), there is relatively scant evidence about the clinical cost and cost-effectiveness of the later stages of the stroke care pathway. One of the models of care that has been well studied is ESD. In this model, stroke patients are discharged home when medically stable, and continue to receive rehabilitation in their own home at the same intensity as they would do as an inpatient. This has several potential advantages: patients recover and learn to adapt to impairments in their own environment, leave hospital sooner and may be less exposed to hospital-related harms.
A strong body of research has shown that ESD provides better outcomes in terms of mortality, disability, institutionalization, patient satisfaction, and length of hospital stay (Reference Langhorne and BaylanLanghorne & Baylan, 2017). These improved outcomes are achieved at a reasonable additional cost. The incremental cost-effectiveness ratio of stroke unit care followed by early community rehabilitation is £10 661, compared with the general medical ward without such care, and £17 721 compared with the stroke unit without early community rehabilitation (Reference SakaSaka et al., 2009). Despite this evidence, there has been limited uptake of this model of care. For example, although the service is applicable to up to 40% of stroke discharges in the United Kingdom, 25% of the regions of the country have not commissioned an ESD service and overall only 20% of patients receive the services of a dedicated team (Sentinel Stroke National Audit Programme, 2018). ESD has also been slower to develop in other high income countries in Europe, which have traditionally focused more on inpatient or clinic-based models of rehabilitation (Reference Douw, Nielsen and PedersenDouw, Nielsen & Pedersen, 2015).
Recovery and long-term management
The final stage of the pathway is long-term care, management and support. This includes maintaining and monitoring secondary prevention therapy, identifying and managing the longer-term consequences of stroke, and providing support and information provision to patients and their families. In contrast with the acute phase of this pathway, it is arguable that this is an area of stroke care that has been relatively neglected by health care systems. Certainly, many stroke survivors express dissatisfaction about the quality of this longer-term support and many patients have a high burden of unmet needs after stroke (Reference McKevittMcKevitt et al., 2011). An additional challenge comes from managing multimorbidity, which is common in people with stroke (Reference GallacherGallacher et al., 2014) and adds to disease burden, increases the complexity of treatment decisions, and places patients at risk of the harmful effects of polypharmacy.
Workforce
Optimal stroke care is highly multidisciplinary, with a core stroke service requiring specialist doctors, nurses, physiotherapists, occupational therapists, speech and language therapists, dieticians and psychologists. Coordinating the work of the team is essential and formal MDT working (such as regular MDT meetings to discuss individual cases) is one of the components of stroke unit care (Reference Langhorne and PollockLanghorne, Pollock & Stroke Unit Triallists Collaboration, 2002).
Stroke medicine has traditionally not existed as a medical speciality in its own right, and as a result there is variation between countries in the specialty background of the lead physician. In most countries acute stroke care is largely provided by neurologists, but in some countries (such as the United Kingdom) stroke care is mainly led by stroke specialist physicians with a background in geriatric medicine.
Other medical specialties with important roles in the stroke pathway include neuroradiology (both diagnostic and interventional), neurosurgery, vascular surgery, intensive care, emergency medicine, rehabilitation medicine, and primary care.
Nursing care is an essential aspect of acute stroke care and it is likely that good quality nursing is one of the key mechanisms for the beneficial effect of stroke units (Reference Middleton, Grimley and AlexandrovMiddleton, Grimley & Alexandrov, 2015). In addition to general nursing skills, nurses need specific skills and knowledge in managing patients with stroke, such as screening and managing dysphagia, the positioning and mobilization of patients with muscle weakness or paralysis, prevention of pressure sores, and communicating with patients with language impairment after stroke (aphasia). Because many patients with stroke die as inpatients, nurses also need skills in providing end of life and palliative care. In some countries (such as the United Kingdom) nurses have taken on extended roles in prescribing, diagnostics, and assessing patients for thrombolysis.
In addition to the general evidence concerning nurse staffing levels and patient outcome (Reference NeedlemanNeedleman et al., 2011), there is specific evidence in stroke care that nursing-to-patient staffing ratios are associated with patient outcomes, with higher mortality rates for patients admitted at weekends to units with lower numbers of trained nursing staff (Reference BrayBray et al., 2014).
Rehabilitation is typically carried out by physiotherapists, occupational therapists, and speech and language therapists (speech pathologists). This includes carrying out assessments of the extent of a patient’s impairments and the impact of these on functioning, and planning treatment goals. Describing the full range of assessments and therapies provided by stroke therapists is beyond the scope of this chapter, but a wide range of methods may be used, from relatively simple mobilization techniques to more sophisticated interventions requiring the use of specialized equipment and aids. Therapists may also carry out additional diagnostic tests requiring additional skills and equipment. Therapists are central in planning patients’ discharge from hospital and implementing adaptations or the installation of equipment in patients’ homes, and have a key role in communicating with and providing psychological support for patients and their families and carers.
Stroke services also involve a variety of other allied health professionals. Dietetics is a core component of an acute stroke service, since many patients require nutritional support or assisted feeding. Problems with cognition, memory, mood or executive functioning are common after stroke and access to a clinical psychologist enables more detailed neuropsychological assessments to be carried out and appropriate information, support and therapy to be provided. Some patients with persistent physical or visual impairments may also require the provision of support aids from prosthetics and orthoptics specialists. As most patients will be discharged home on new or changed medications, pharmacists have an important role in ensuring safe prescribing, medicines reconciliation and in providing information to patients and family members about medications and side effects.
Networks of stroke care
In parallel with the development of organized stroke care in individual hospitals, many health systems have developed regional and network models of stroke care. In much of Europe and the USA a distinction is made between primary stroke centres and comprehensive stroke centres. Primary stroke centres are those with the necessary staffing, infrastructure and expertise to provide treatment for most stroke patients, but which may not have the capability to manage patients with more complex problems. Comprehensive stroke centres provide the same core stroke service but also the high technology and resource-intensive elements of care, such as interventional neuroradiology or neurosurgery, and play a greater role as centres for research and education. The European Stroke Organisation has produced guidelines setting out in detail the facilities and staffing required by comprehensive stroke centres (“ESO Stroke Centre”) in Europe (Ringelstein et al., 2013), which includes 24/7 provision of advanced imaging and interventional neuroradiology. In many countries these levels of care are formally accredited through certification schemes (for example, in the USA and Germany) or through quality registers (for example, in the United Kingdom and Sweden). There is evidence from the USA (Reference XianXian et al., 2011), Japan (Reference IiharaIihara et al., 2014), Finland (Reference MeretojaMeretoja et al., 2013) and the United Kingdom (Reference BrayBray et al., 2013a) that hospitals with higher levels of organized stroke care provide better outcomes for patients, suggesting that formal mechanisms to ensure stroke quality standards are important.
Networks of hospitals are frequently used in stroke care to provide access to the higher technology care offered in comprehensive stroke centres. These may act as the central referral centre for “hub and spoke” networks of hospitals, taking referrals from a number of primary stroke centres. Such networks have become increasingly important with the advent of more sophisticated diagnostic and interventional innovations, such as advanced brain imaging and thrombectomy, which would not be feasible or cost-effective to provide in smaller hospitals. In the United Kingdom the concept of the comprehensive stroke centre and primary stroke centre is more frequently defined in terms of hyperacute stroke units (HASUs) (providing acute care for the first 72 hours after stroke) and stroke units (for post-72 hour care).
There is evidence that these types of network can lead to better patient outcomes. In 2010 health care providers in London carried out a major reorganization of stroke services, reducing the number of acute admitting hospitals from 28 to 8 centres, each serving a population of approximately 1–1.5 million people. These eight hospitals were designated as HASUs and formal pre-hospital protocols were established so that all patients with suspected stroke would be transferred to a HASU. These HASUs provide acute care for up to 72 hours, and patients requiring ongoing inpatient treatment and rehabilitation are then transferred to a stroke unit closer to their home. The network is supported by agreed protocols for patient transfers, minimum standards for training, facilities and staffing, a common framework for payment and reimbursement from funders, and regular audit of quality and performance. Since these changes were established, there have been large improvements in the quality of stroke care in London, with stroke case fatality rates falling faster in London than in other urban areas in England (Reference MorrisMorris et al., 2014). One of the key aspects of stroke care in London that is different from many other “hub and spoke” models of care is the concept of providing higher level acute care to all patients and not just to selected patients; the majority of patients therefore have the opportunity to benefit from early intensive acute stroke care in an HASU.
Some models of care have emerged to help tackle the issue of providing specialist stroke care at scale by providing regional systems for transferring patients to specialist centres or providing specialist input remotely. Telemedicine is widely used in stroke care and many areas have implemented telemedicine systems to transmit video, audio and imaging data so that stroke specialists at home or working in another hospital can help in assessing patients presenting with acute stroke (Reference Hess and AudebertHess & Audebert, 2013). This has particular uses in delivering thrombolysis in rural areas where it may not be feasible to provide specialist stroke services in areas of low population density.
Telemedicine models may also be augmented by pathways that provide initial triage and assessment of patients in local hospitals, initiate thrombolysis if appropriate, and then transfer the patient to a hospital with specialist stroke care provision. These “drip and ship” models have been used particularly in the USA, where one in four patients treated with thrombolysis is now managed this way (Reference ShethSheth et al., 2015).
Variation in quality
Within Europe there is wide variation in the organization of stroke services and in the use of policies aimed at increasing care quality, such as clinical audit, financial incentives, clinical guidelines, accreditation and regulations (Reference Di CarloDi Carlo et al., 2015). The quality of care delivery across Europe is hard to measure consistently, since even when data are available, differences in the choice and definition of quality indicators make comparisons difficult (Reference WiedmannWiedmann et al., 2012). Nonetheless, wide variation exists even for aspects of stroke care with the strongest evidence base and most consistent inclusion in guidelines and audits, such as admission to a stroke unit or treatment with thrombolysis (Reference AyisAyis et al., 2013). For example, in 2011 only 33% of stroke patients in France were admitted to a stroke unit (Reference SchmidtSchmidt et al., 2015), compared with 62% in Scotland (Reference TurnerTurner et al., 2016). A survey of 25 European countries in 2005 found evidence of extremely wide variation in the provision of acute stroke care, with particularly poor provision of stroke unit care in Estonia, France, Greece and Portugal (Reference LeysLeys et al., 2007). Only 49% of the 886 hospitals included in the survey provided the minimum level of care to be considered a primary or comprehensive stroke centre. Stroke outcomes also vary significantly across Europe. For example, one comparative study of stroke outcomes between six European cities (in France, Italy, Lithuania, the United Kingdom, Spain and Poland) found three-fold variation in the risk of death after stroke (Reference HeuschmannHeuschmann et al., 2011).
Poor provision of acute stroke care occurs even in higher income European countries. For example, a survey of neurology services in Italian hospitals found that only 28% provided stroke unit based care, and large numbers of patients with stroke were admitted to hospitals without stroke units (Reference de Falco, Leone and Beghide Falco, Leone & Beghi, 2009). Organized stroke care was also relatively slow to develop in France, with only two hospitals out of 121 surveyed in 2005 providing stroke unit care (Reference LeysLeys et al., 2009). Policy-makers in France have subsequently prioritized stroke care and developed a national strategy for improvements, with a particular focus on developing stroke care networks (Reference LebrunLebrun et al., 2011).
Information on stroke care quality is collected systematically in some European countries (Reference WiedmannWiedmann et al., 2012), but most countries in Europe lack or have only fragmentary systems of data collection for quality improvement (Reference Di CarloDi Carlo et al., 2015). Where data are available, there is evidence of widespread variation in care quality within countries, not just between countries. For example, stroke care quality is measured in England and Wales by the Sentinel Stroke National Audit Programme (SSNAP) (Sentinel Stroke National Audit Programme, 2018) and there are wide geographical variations in a variety of care quality indicators (Figure 3.3). National clinical audits and registries in other countries show similar variation in stroke care quality, including Scotland (Scottish Stroke Care Audit; SSCA), Sweden (RiksStroke) and Germany (Reference WiedmannWiedmann et al., 2014). Some of these variations reflect broader geographical inequalities in the provision of health care services, such as the relative under-provision of stroke units in southern Italy compared to northern Italy (Reference GuidettiGuidetti et al., 2013).

Figure 3.3 Geographical variation in admission to a stroke unit within four hours of admission in England and Wales
Specialist resources
The main essential component of a stroke service is a stroke unit (Figure 3.4), and stroke unit beds should be available 24 hours a day for new admissions. Stroke units are more defined by staffing than by physical infrastructure, but stroke units do have a few specialist environmental and equipment considerations. Acute stroke units need to have the equipment to provide continuous (or regular) physiological monitoring. There is evidence that care bundles of nursing interventions that focus on physiological monitoring and detecting complications are effective in improving outcomes after stroke (Reference Middleton, Grimley and AlexandrovMiddleton, Grimley & Alexandrov, 2015). Appropriate facilities and space need to be available to mobilize patients and provide rehabilitation. This might include a gym for physiotherapy and areas (such as a therapy kitchen) for occupational therapy assessments.
Figure 3.4 Specialist resources for a stroke service
| Specialist resources required for a core stroke service | Specialist resources required for subgroups of patients |
|---|---|
| Brain imaging | Specialist diagnostics |
| Stroke unit | Neuroradiology/thrombectomy |
| Diagnostics (blood tests, ECG) | Neurosurgery |
| Rehabilitation | Vascular surgery |
| Pharmacotherapy for secondary prevention & thrombolysis | Critical care |
| Long-term care (follow-up, primary care) |
Brain imaging is the core diagnostic requirement of a stroke service. CT imaging needs to be available 24 hours a day for the assessment of new patients and in carrying out imaging on patients who develop neurological deterioration after stroke. Although non-contrast CT imaging is adequate for most acute treatment decisions, increasing use is being made of more advanced CT imaging modalities (CT angiography and perfusion) and MRI.
A small proportion (less than 5%) of patients will require neurosurgical intervention (Reference VahediVahedi et al., 2007). Managing these patients requires access to neurosurgical infrastructure (theatres, specialist surgical, anaesthetic and nurse staffing, critical care facilities) either on site or after transfer to a referral centre. Similarly, some patients with stroke or TIA require vascular surgery for carotid endarterectomy. Patients with strokes that result in reduced consciousness may need to be managed in an intensive care unit as part of their admission. As thrombectomy services become more widely available, stroke care in high income countries will increasingly require access to neurointerventional facilities and the staffing required to provide them for patients suitable for this treatment.
Vascular ultrasound and echocardiography are also recommended as part of the diagnostic work-up for many patients (European Stroke Organisation Guidelines). All of these investigations require appropriate equipment and staff skilled in carrying out and interpreting these tests.
The requirements from stroke services of laboratory and pathology services are limited largely to common blood tests and in the diagnostic work-up of rarer causes of stroke. POCT is widely used in the emergency room for patients being assessed for thrombolysis, where rapid results are important in achieving fast door to needle times (Reference RizosRizos et al., 2009).
Stroke is not an area with a high use of specialist pharmaceuticals. The main pharmaceuticals that are required for an acute stroke service are alteplase (the only agent licensed for stroke thrombolysis in Europe) and secondary prevention agents (anticoagulants, antiplatelets, statins, anti-hypertensives).
Barriers to delivering optimal care
Training sufficient numbers of specialist staff has been a challenge in many countries. In the United Kingdom, for example, despite training pathways for physicians developing a specialty interest in stroke and the existence of stroke-specific professional organizations to support trainees and specialists, there is a shortage of physicians specialized in stroke medicine, with 25% of consultant posts remaining unfilled (Sentinel Stroke National Audit Programme, 2018). The reasons for this are unclear but might include the increasing requirement for out-of-office-hours working as stroke care has become higher in intensity. There are also fewer options for private practice for stroke specialist physicians than many other procedure-based or office-based specialties, which may affect its perceived attractiveness as a specialty. Lack of staffing resources, particularly of therapists and nursing home staff, was also identified as being one of the main barriers to improving stroke care in France (Reference GacheGache et al., 2014). There is potential for tackling staff shortages by expanding the roles and skills of existing clinical staff, such as empowering nurses to manage thrombolysis calls and take on leadership roles in stroke services.
One of the key barriers to providing optimum care has been the difficulty of closing the gap between evidence and widespread implementation into practice. Here the barriers are not merely lack of financial or other resources, but also contextual and behavioural factors such as culture, organization and leadership. Indeed, implementation gaps in stroke care involve not only the high technology and resource-intensive elements of stroke care, but also the key evidence-based components of care (such as stroke units and secondary prevention): even “getting the basics right” can be difficult. For example, even 20 years after the publication of the first trial to demonstrate the effectiveness of thrombolysis for ischaemic stroke, rates of use of thrombolysis vary widely even in highly developed health economies. In particular, the uptake of thrombolysis was initially very poor in the United Kingdom: when the National Audit Office reported on the quality of stroke care in England in 2005 it found that fewer than 1% of stroke patients were receiving thrombolysis. By contrast, during the same period 3–4% of stroke patients were treated with thrombolysis in Sweden (Reference ErikssonEriksson et al., 2010). The low rate of implementation in the United Kingdom occurred in the context of underdeveloped stroke services and highly variable care between centres, with only 60% of stroke patients being cared for in a stroke unit and many patients waiting more than two days for a brain scan (National Audit Office, 2005). This report prompted the development of a national improvement strategy, financial investment in stroke care, an expansion in training for stroke specialists and new resources allocated to quality improvement and audit. National clinical audits have since demonstrated significant improvements in the quality of stroke care in England and an acceleration in the uptake of thrombolysis, with 11–12% of patients (of all ages) now treated with thrombolysis: rates that are comparable with other high performing health systems in Europe (Sentinel Stroke National Audit Programme, 2018).
Although stroke care has been transformed by evidence-based medicine, there are still many areas of stroke care with little evidence to guide practice. One of the reasons for this may be relative underfunding of stroke research: in the United Kingdom for every £10 of health and social care costs attributable to stroke, it received only £0.19 in funding, compared to £1.08 for cancer and £0.65 for coronary heart disease (Reference Luengo-Fernandez, Leal and GrayLuengo-Fernandez, Leal & Gray, 2015). The areas of stroke care with the poorest evidence base are generally the less acute components of care, such as therapy and rehabilitation. Even fundamental questions about rehabilitation, such as when physiotherapy should commence after stroke, and at what intensity, are only now being addressed in randomized controlled trials (AVERT Trial Collaboration Group et al., 2015). Lack of evidence makes it difficult to define what optimal care in these areas should be, contributing to variations in practice. For example, there are wide variations across Europe in the amount of therapy provided to patients after stroke, which are not explained by differences in patient characteristics and likely reflect variation in access and availability (Reference WolfeWolfe et al., 2004; Reference WellwoodWellwood et al., 2009).
One of the biggest challenges for stroke medicine in high income countries in the next few years will be in implementing access to thrombectomy. Current provision is largely concentrated in relatively small numbers of specialist hospitals, and even in these hospitals there may not be round-the-clock access. The main barrier to implementation is insufficient numbers of trained neurointerventionists; increasing capacity will take time and there are likely to be resource challenges in maintaining a 24/7 acute thrombectomy service that may only be used relatively infrequently, with only a minority of acute stroke patients being appropriate for this treatment. Another risk is that a focus on developing thrombectomy services will distract attention and resources away from the wider challenge of implementing good quality stroke unit based care and post-stroke rehabilitation.
The future
The challenge for the future involves the twin tasks of implementing an ever-growing evidence base on new interventions and innovations and in improving the availability and quality of the elements of stroke care that we already know to work. As has already been described, wide variations in care quality exist both across and within European countries and these will not be reduced if the focus of clinicians, funders and managers is solely on implementing the “new”. Indeed, it is worth emphasizing that from a global perspective most people with stroke do not even receive the most core elements of stroke care such as stroke unit based care. By far the greatest reduction in the future burden of stroke on populations will come about not through new technologies but as a result of public health efforts to reduce stroke incidence through tobacco control, public health programmes to reduce cardiovascular risk factors (such as hypertension, obesity, alcohol and physical inactivity), increasing access to stroke unit based care and rehabilitation, and effective use of secondary prevention.
There are also examples of interventions that are still in use, despite evidence of ineffectiveness or even harm. One of the most prevalent of these is the use of antiplatelet agents in patients with AF. Historically, antiplatelet agents such as aspirin were often used as an alternative to anticoagulants to reduce the risk of stroke in people with AF (particularly in older people), but it is now known that antiplatelets provide much less benefit and are no safer than oral anticoagulants (Reference Aguilar, Hart and PearceAguilar, Hart & Pearce, 2007). Current guidelines therefore recommend that antiplatelet agents are not used for stroke prophylaxis in AF. However, many patients with AF are still prescribed antiplatelets, and oral anticoagulants remain underused. In England, for example, 31% of eligible patients known to be in AF in primary care were not prescribed an oral anticoagulant in 2013/2014 (NHS Quality and Outcome Framework, 2015), resulting in many thousands of avoidable strokes per year. Newer oral anticoagulants have become available in recent years that offer similar reductions in stroke risk but may have reduced risks of major complications than treatment with warfarin (Reference Gómez-OutesGómez-Outes et al., 2013).
As already discussed, the innovation most likely to change stroke care in the next five years in high income countries is thrombectomy for ischaemic stroke. The challenges of implementing this at scale, though, are significant and it is not certain how quickly this will become widely available experience from thrombolysis suggests that it is likely to be slow and highly variable between settings. There are other emerging areas of research that are still at early stages but may lead to significant impacts in the future. One of the most intriguing ideas is of reducing delays in thrombolysis by installing brain CT scanners in ambulances, allowing pre-hospital diagnosis of stroke type and administration of thrombolysis if appropriate. The concept has been demonstrated in a small number of centres (Reference WalterWalter et al., 2012; Reference ParkerParker et al., 2015), and although the real-world feasibility and cost-effectiveness of this model of care remain unproven, using new diagnostic technologies to facilitate pre-hospital stroke diagnosis could transform stroke care pathways. Imaging is an area of fast-moving innovation and development – for example, it is now possible to non-invasively image areas of unstable atherosclerotic plaque that are the source of the majority of strokes, and identify at an early stage the patients at highest risk of new or recurrent stroke (Reference Tarkin, Joshi and RuddTarkin, Joshi & Rudd, 2014). Further off, there is the prospect that stem cell technologies may allow the repair of established brain damage occurring as a result of stroke; early-stage clinical trials in stroke patients are already ongoing (Reference BanerjeeBanerjee et al., 2014). Rehabilitation is also increasingly making use of new advances in robotics to provide therapy and augment motor functioning in patients with limb paralysis after stroke (Reference BurgarBurgar et al., 2000).
Although exciting, most of these innovations are likely to be applicable only to a minority of stroke patients. The implementation of these new resource-intensive interventions therefore needs to be linked to efforts to develop models of delivery that can provide these in the most clinically and cost-effective way: for many aspects of acute stroke care this is likely to mean further development of networks of care and centralization of specialist services into hub hospitals.
Perhaps of greater medium-term significance to population health will be innovations that are applicable to all patients with stroke: the shift towards increased engagement of patients in managing their own health through shared decision-making and self-management, and in the increasingly sophisticated use of data to support research, quality improvement and new models of care. For example, there is good evidence that helping patients to manage their own blood pressure leads to better blood pressure control than the typical model of clinic-based management (Reference McManusMcManus et al., 2014); it is likely that health care services will increasingly take the role of supporting stroke survivors (and their carers) in managing stroke as a long-term condition. Similarly, health care in the future will make much more sophisticated use of real-world data such as electronic health records and clinical registries (Reference KrumholzKrumholz, 2014). For example, use of such data to generate more accurate predictions of prognosis, or to generate patient-specific estimates of the harms and benefits of interventions, can help in making better decisions about treatment and support patients in shared decision-making (Reference SpertusSpertus et al., 2015).
Stroke care in the hospital of the mid-21st century
Stroke care has changed dramatically over recent decades, driven by the development of organized multidisciplinary care and an increasing emphasis on acute intervention. The dependency on advanced medical imaging, resource-intensive multidisciplinary care and acute treatments that can only feasibly be administered in large hospitals means than hospitals are likely to remain the cornerstone of acute stroke care with most patients being admitted for inpatient care. The hospital of the future, if it is to provide comprehensive care for patients with stroke, will therefore need to be organized and designed to deliver:
round-the-clock access to advanced imaging, diagnostics and neurointervention facilities that are geographically located within the hospital to optimize speed of access;
stroke units to which patients with acute stroke are admitted without delay and which are the setting for multidisciplinary stroke specialist care;
the appropriate environment and equipment to enable optimal provision of therapy and to support rehabilitation and recovery; and
organized pathways of care that reduce treatment delays and support the provision of good quality therapy not just in hospital but also in the community.
In many places this means that some acute hospitals should no longer attempt to treat stroke. Rather, there is a need to find alternative models of care whereby those suffering a stroke will be taken, at least for definitive treatment, to a hospital that can provide a comprehensive care package, including rapid diagnosis and intervention where required. This will often not be the nearest facility. This could have profound implications for the organization of hospitals in a defined area, especially where they have had a high degree of autonomy. It will often be extremely challenging, politically and legally, to tackle this and each solution must be tailored to the particular context.
The critical component of stroke care services will remain not physical assets and medical devices but the MDTs of people that are the core of organized stroke unit care. Maintaining and developing this resource will require long-term investment in the training of the stroke workforce (medical, nursing and allied health professions). Providing ongoing education and training will be of increasing importance in helping clinicians keep up to date with the accelerating pace of new medical knowledge and evidence.
It will be disappointing if the next few decades do not see the development of new, high-impact drugs and devices that improve recovery after stroke, reduce complications, or help survivors manage the long-term consequences of stroke. The development of therapies that facilitate brain repair (for example, through stem cells) could be a real paradigm shift, but the brain is vastly complex and still contains many mysteries; progress in the development of new “brain regeneration” therapies is hard to predict. For patients with permanent impairments after stroke, assistive technologies (such as robotics) are likely to become much more mainstream and sophisticated, and allow more stroke survivors to live independent lives. The challenge for the future will be providing these innovations at scale in a cost-effective way and in speeding up the diffusion of new evidence into widespread clinical practice.
The evidence of current variation in care quality and outcome points to the importance of prioritizing and developing quality improvement in stroke care. This includes supporting and developing current systems of clinical audit (SSNAP, SSCA, Riks-Stroke, Danish Stroke Register) and increasing the capacity of health care systems to deliver continuous quality improvement. As the sophistication and scope of health care data collection increase, this is likely to lead to a growing emphasis on the development of new ways of using data in stroke care as part of clinical care, research and quality improvement. It is hard to foresee in much detail what this new, data-aware world of health care will look like in practice, but it may have a transformative effect on the delivery and organization of stroke care in the next few decades.
Introduction
The current challenge
All European countries are experiencing rapid demographic transitions, with an increase in the proportion of over 65-year-olds and the most rapid increase in people over 80 years of age (Reference CreightonCreighton, 2014). This means that, increasingly, the business of acute hospitals is the care of older people, often with frailty, dementia or multiple long-term conditions complicating their acute illness. Without a radical shift in care models, at scale and surpassing anything we have yet seen, this will continue to be the case for the foreseeable future. There has been a general reduction in hospital beds and increases in ambulatory and community treatment but there remain gaps in services that fail to meet the needs of frail older people, which often result in hospital attendances (NHS Benchmarking, 2013; Reference CowlingCowling et al., 2014; Reference RadvanskyRadvansky, 2014; Reference MelzerMelzer et al., 2015). Particular challenges arise for those with frailty, chronic multiple conditions, and those with dementia, adding to the complexity of treatment and care needs of older people (Reference MelzerMelzer etv al., 2015).
Some of the key challenges facing hospitals caring for older people with frailty include unmet care needs, health inequalities, and a lack of quality service models and integration between services (European Institute, 2012). There is wide variation in the nature and scope of services addressing the needs of frail older people, with some countries such as Austria having recognized geriatric medicine as a subspecialty of internal medicine only from 2011 (Reference EkdahlEkdahl et al., 2012). While countries are acknowledging the need for better integration of services, implementation of more integrated care models has been slow (Reference Curry and HamCurry & Ham, 2010; Reference ShahShah et al., 2010).
The challenge of frailty
Frailty is defined as a state of increased vulnerability and a disturbance in homoeostasis where a stressor event can lead to dramatic changes to the health status of an individual, which result in increased dependency levels, mobility problems, a change in cognition, such as delirium, and marked levels of functional decline (Reference CleggClegg et al., 2013) (Figure 4.1; Box 4.1). Frailty is also associated with increased mortality and morbidity, and it is a strong predictor of care home utilization and death (Reference CesariClegg et al., 2016). There are two common models for defining frailty as a clinical entity and these are increasingly important as ways of segmenting and addressing the needs of hospital patients (Reference OliverOliver, 2016c). These rest on an identifiable “frailty phenotype” (Fried model) based on the presence of three or more characteristics or a “frailty index” (Reference Rockwood and MitnitskiRockwood & Mitnitski, 2011; Reference CesariClegg et al., 2016) based on an accumulation of deficits. There is an overlap between frailty, multiple co-morbidity and age-related disability (World Health Organization, 2015; National Institute for Health and Care Excellence, 2016), although it is now possible to identify people with frailty in community settings using existing primary care data (Reference CleggClegg et al., 2016) (Table 4.1), at the hospital emergency front door or on the inpatient wards, where simple pragmatic case-finding tools are often employed (Royal College of Physicians, 2013; British Geriatrics Society, 2014a; Health Improvement Scotland, 2015).

Figure 4.1 Vulnerability of frail older people to a sudden change in health status following a minor illness
Note: The top line represents a fit older person who, following a minor stress such as a urinary tract infection, experiences a relatively small deterioration in function and then returns to homoeostasis. The lower line represents a frail older person who, following a similar stress, experiences a larger deterioration which may manifest as functional dependency and who does not return to baseline homoeostasis.
Box 4.1 Common presentations of frail older people
Frailty syndromes (how people with frailty present to services)
“Non-specific” – e.g. fatigue, weight loss, recurrent infection
Falls/collapse
Immobility/worsening mobility
Delirium (“acute confusion”)
Incontinence (new or worsening)
Fluctuating disability
Increased susceptibility to medication side effects – e.g hypotension, delirium
Table 4.1 Adjusted 1, 3 and 5 year hazard ratios for outcomes of mortality, unplanned hospitalization and nursing home admission for older people with mild, moderate and severe frailty
| Outcome | Mild frailty (HR, 95% CI) | Moderate frailty (HR, 95% CI) | Severe frailty (HR, 95% CI) |
|---|---|---|---|
| 1 year mortality | 1.92 (1.81–2.04) | 3.10 (2.91–3.31) | 4.52 (4.16–4.91) |
| 3 year mortality | 1.77 (1.71–1.83) | 2.78 (2.68–2.89) | 3.99 (3.79–4.20) |
| 5 year mortality | 1.72 (1.68–1.77) | 2.64 (2.57–2.72) | 3.83 (3.68–3.99) |
| 1 year unplanned hospitalization | 1.93 (1.86–2.01) | 3.04 (2.90–3.19) | 4.73 (4.43–5.06) |
| 3 year unplanned hospitalization | 1.78 (1.74–1.82) | 2.63 (2.55–2.71) | 3.76 (3.60–3.94) |
| 5 year unplanned hospitalization | 1.71 (1.68–1.74) | 2.50 (2.44–2.56) | 3.43 (3.31–3.58) |
| 1 year nursing home admission | 1.89 (1.63–2.15) | 3.19 (2.73–3.73) | 4.76 (3.92–5.77) |
| 3 year nursing home admission | 1.67 (1.56–1.80) | 2.60 (2.40–2.82) | 3.55 (3.19–3.96) |
| 5 year nursing home admission | 1.59 (1.51–1.67) | 2.30 (2.18–2.44) | 3.12 (2.88–3.38) |
Note: For all outcomes the comparator is fit older people. All data adjusted for age and sex. NB: Hospitalization outcome for external validation cohort includes only those practices (n = 158) with Hospital Episode Statistics (HES) linked data. CI: confidence interval; HR: hazard ratio.
Adequate assessments and interventions for frailty are important. Survival plots using primary care data in England suggest that those with severe frailty are at higher risk of dying by a factor of five (Reference BatesBates et al., 2014). A ten-year prospective cohort study involving community-dwelling older people identified frailty to be the leading cause of death, accounting for 28% of deaths compared to organ failure (21%), cancer (19%), dementia (14%) and other causes (15%) (Reference CleggClegg et al., 2013).
Falls are a common reason for admission to hospital and have come to the attention of policy-makers and payers. Frailty is known to be an independent predictor of falls, and figures over the last five years show that Ireland had spent an estimated €520 million on falls, while in the Netherlands fractures were estimated to have led to 80% of fall-related costs, amounting to approximately €540 million between 2007 and 2009. In England alone, falls in older people have been estimated to cost the National Health Service £2 billion annually (Reference FentonFenton, 2014). Across Europe and other high income countries, the estimated costs of falls to health care services are significant.
Minimizing harm in frail older people in hospital
Older people are at significant risk of “harm” often associated with their care and medicalization of their illnesses. Polypharmacy, falls, hospital-acquired infections, malnutrition and immobility are some of the common problems that arise, which can lead to increased morbidity and mortality of older patients admitted to hospital (Reference BarberBarber et al., 2009; Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). Bed rest in itself has also been associated with a range of harms where 10 days of bed rest in healthy older adults can lead to a 14% reduction in leg and hip muscle strength, and a 12% reduction in aerobic capacity (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). Older people often already have decreased physical function which may be negatively affected by hospitalization. They also have poorer functional outcomes and are less likely to recover from their problems in hospital (Reference CovinskyCovinsky et al., 2003; Reference Kleinpell, Fletcher and JenningsKleinpell, Fletcher & Jennings, 2008; Reference Mudge, O’Rourke and DenaroMudge, O’Rourke & Denaro, 2010).
Models of pre-hospital care
The role of primary care in care coordination and urgent access
Frail older people pose a challenge to primary care although family physicians are ideally posed to incorporate the identification and management of frailty in their practice (Reference Lacas and RockwoodLacas & Rockwood, 2012). Countries are increasingly implementing more proactive personalized care planning, care coordination and case management to enhance primary care services for (frail older) people with one or more long-term conditions (Reference CoulterCoulter et al., 2015). These care provisions are often provided by specialist nurses and therapists as well as volunteers in the care sector (Reference KringosKringos et al., 2013; Reference Bienkowska-GibbsBienkowska-Gibbs et al., 2015). At the same time, when the health or independence of older people rapidly deteriorates, it is important to ensure rapid access to urgent care, including effective alternatives to hospital (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014; NHS Benchmarking, 2015). The following sections describe selected models that are being implemented in different settings to meet this need in particular.
Rapid community response teams
Older people with frailty are at a higher risk of unplanned hospital admissions (Reference BoutsioliBoutsioli, 2012; Reference SonaSona et al., 2012; Reference WittenbergWittenberg et al., 2014). In England alone, up to 42% of emergency admissions in 2011 came from care homes with older people who were within the last six months of their life; these patients also often had multiple admissions in the year leading up to death (Reference SmithSmith et al., 2015).
Rapid response teams can offer specialist advice and improve the care for frail older people with long-term conditions (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014; Reference WittenbergWittenberg et al., 2014; NHS Benchmarking, 2015). These services may variously include geriatricians, GPs, specialist nurses, physiotherapists, occupational therapists, and sometimes others such as pharmacists, social workers, personal care assistants or generic rehabilitation assistants to address the complex medical needs of frail older people where time is of the essence, and without which the episode may result in hospital admission. These teams can also collaborate with ambulance organizations to divert patients to the community rapid response team and towards the community team. They are most likely to be effective when able to see patients within hours and when they have a range of skills within the team (NHS Benchmarking, 2016; Reference ShepperdShepperd et al., 2016).
Ambulatory care clinics
Ambulatory care clinics are defined as units that provide preventative intervention and chronic disease management services for frail older people who may be at risk of future hospital admission (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). Ambulatory care clinics can be located in hospital outpatient settings or in primary care, and led by GPs or specialist clinicians with a range of multidisciplinary staff that can include pharmacists and social workers to optimize care of the frail older patient. Ambulatory care clinics or ‘‘one-stop’’ frailty clinics are an emerging service in France that provides assessment, management and support for older people with the aim of preventing and minimizing disability among those who are fit enough to attend such services (Reference TavassoliTavassoli et al., 2014) and uses a collaborative approach with primary care and other allied professionals (Box 4.2). The overall evidence for impact of such service models remains weak, although a recent randomized controlled trial in Sweden demonstrated improved survival, and reduced length of stay in hospital without increasing cost up to three years after assessment (Reference Ekdahl, Alwin and EckerbladEkdahl, Alwin & Eckerblad, 2016). In the United Kingdom, rapid access ambulatory care clinics in community hospitals have shown that frail older patients who are referred from primary care, ambulance services and community teams can be seen more quickly and closer to home, with a MDT addressing complex care needs effectively, and figures show that only up to 20% of those referred are transferred on to the nearest acute centre, with 56% discharged home or to their previous care setting (Reference KoduahKoduah et al., 2014).
Box 4.2 Gerontopole frailty clinic
A geriatric frailty clinic (structured as a day hospital unit) was established in 2011 in Toulouse, France, for frail people above the age of 65 years, who were referred by their GP, geriatrician or specialist to undergo a multidisciplinary evaluation to assess frailty and underlying risk factors for disability. During a two-year period the clinic assessed over 1000 people and a personalized prevention plan was developed to optimize their care in the community. The unit was led by a physician with ad hoc training in geriatrics at the university hospital outpatient clinic. The physician coordinating the evaluation was supported by other health care professionals (in particular, nurses, nutritionists, neuropsychologists and physical therapists) in the development of a personalized plan of intervention. This was then shared with the person’s GP in order to make them aware of the recommendations and promote adherence to the preventive programme. A month after the assessment at the clinic a nurse would make a follow-up call to the patient to ensure that the interventions agreed had been undertaken; if a further deterioration in health was detected at this time, further action/plans were put in place to remedy the situation, where possible through the local GP responsible. This service focuses on secondary prevention for frail older people still completely autonomous in their basic activities of daily living. It was found that almost 94% of patients referred to the service were either frail or pre-frail, according to Fried’s definition of frailty.
Community hospitals and intermediate care units
In Europe, community hospitals are increasingly being (re)considered as a means to address the care needs of older people in particular and are predominantly staffed by GPs and nurses, with some specialist input (Reference WinpennyWinpenny et al., 2016). These often provide pre- and post-hospital care and so bridge the gap between care received for an acute illness prior to discharge to home (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). One recent example is the introduction, in 2012, of municipal acute care beds in Norway, which are organized as part of the municipal health services together with GPs, local emergency services, long-term care services and other parts of social care (Reference Swanson and HagenSwanson & Hagen, 2016). They are targeted at stable patients who need monitoring or close follow-up from acute illnesses, often exacerbated by chronic medical conditions. Evidence from community hospital-type set-ups are mainly observational in nature, and evaluation of their effectiveness is still lacking (World Health Organization, 2015).
Models of hospital care
Across Europe frail older people account for approximately 20% of total attendance to emergency departments (Reference SonaSona et al., 2012). The majority of people over 50 years old attending emergency departments have multiple long-term conditions (Quality Watch, 2015). One important response is hospital care based on comprehensive geriatric assessment as the underpinning tenet of assessment and management. The models described in this section can be found in different European countries, although we also consider successful models from other developed health care systems such as the United States.
Comprehensive geriatric assessment for frail older inpatients
Comprehensive geriatric assessment (CGA) is a process of assessing an older person’s medical, psychological, physical and social functioning to inform the use of specific interventions and then develop and implement a plan for ongoing treatment and follow-up. There is good evidence from meta-analysis of numerous studies from several European countries that comprehensive interdisciplinary assessment of older people presenting to hospital delivers long-term benefits in terms of survival and the ability to remain in their own homes with less cognitive decline (Reference EllisEllis et al., 2011). Because this is an iterative process rather than a discrete event, a CGA initiated in hospital can be continued in a person’s own home to fully assess the need for support and so enable the frail older person to remain within their own environment (Reference EllisEllis et al., 2011).
CGA is multidisciplinary, although outcomes are best with a specialist geriatrician leadership or input team on admission of the patient (Reference EllisEllis et al., 2011; Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). In the United Kingdom standards of care and assessment have been set out by national leadership bodies (British Geriatrics Society, 2014b; Health Improvement Scotland, 2015). Across Europe CGA is gaining momentum and is being used to assess and optimize frailty for a variety of medical and surgical conditions, and as a predictor of adverse outcomes. For examples, see Reference KristjanssonKristjansson et al. (2010) and Reference Molina-Garrido and Guillén-PonceMolina-Garrido & Guillén-Ponce (2011).
A recent review of the practice of CGA in high income countries in Europe, North America and Taiwan showed that only 32% of interdisciplinary geriatric consultation teams had used any formal CGA screening aid in intervention decisions. Also, while nurses formed key members of teams, their roles and responsibilities tended not to be clearly identified (Reference DeschodtDeschodt et al., 2016). There is a need to place implementation barriers of CGA into local contexts and so effectively address its effectiveness, culture change, educational needs of practitioners, research and evolving requirements of service provision (Reference GladmanGladman et al., 2016).
Specialist models of acute hospital care for people with frailty
Acute frailty services are specialist units that focus on specialized and tailored care for complex frail older people at, or close to, the hospital front door and with a focus on older people in the first phase of hospital admission. Where possible, they aim to assess and stabilize patients with a view to early discharge before they move to “deeper” wards within the hospital (Acute Frailty Clinical Network, 2015; Royal College of Physicians, 2015; Reference OliverOliver, 2016c). There are different models in various acute settings but the most common models include: emergency department-based models and acute frailty units (Reference Van CraenVan Craen et al., 2010; Reference DeschodtDeschodt et al., 2013; Reference ConroyConroy et al., 2014).
Emergency department-based geriatrics and frailty services provide specialist geriatric input in decision-making for frail older people who attend the emergency department; other objectives include providing a multidisciplinary assessment using CGA and initiatives to reduce admission rates (Reference BlakemoreBlakemore, 2012). Specialized nurses, who are experienced in falls, dementia, mental health and continence, are often available within these teams to provide support in hospital and coordinate better specialist care in the community at discharge. The overall evidence for emergency frailty units remains weak, with the majority of care models being trials of transitional care, which is a relatively novel concept of providing care for older people (Reference Conroy and ChikuraConroy & Chikura, 2015). Implementation of such care models in the emergency department remains challenging because of the complexity involved in identifying frailty, including the lack of standardized frailty instruments and poor understanding of frailty and the absence of clinical guidelines of frailty management in the emergency setting (Reference DentDent et al., 2016).
Acute frailty units, also referred to as “acute geriatric evaluation and management units”, are inpatient wards at or close to the hospital “front door” that admit frail older people for assessments, treatment, review and rehabilitation through the use of CGA (Reference Van CraenVan Craen et al., 2010). A meta-analysis by Reference Van CraenVan Craen et al. (2010) of American, Austrian, German and Norwegian studies found that acute frailty units showed a significant positive impact on functional decline at discharge and institutionalization at one year. It also demonstrated that multidisciplinary CGA added value to those who were admitted to hospital by meeting the specific needs of frail older people and resulting in higher satisfaction of care provided to the patient.
European countries are at different stages in the development of acute frailty or acute geriatric units, which tend to be concentrated in larger cities, mainly because of the uneven distribution of geriatricians (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011; Reference EkdahlEkdahl et al., 2012) and poor availability outside major centres. Whereas geriatric medicine is the largest internal medical speciality in the United Kingdom (Royal College of Physicians of London, 2015) and acute frailty units are found in small and medium-sized hospitals (Acute Frailty Clinical Network, 2015; NHS Benchmarking, 2016), it is not as well established in many European countries (EUGMS Survey, in press). For example, in Denmark and Sweden specialist geriatric units tend to be based at tertiary hospitals where frail older people undergo assessments that aid further planning of care. In smaller hospitals, geriatric care is embedded within general internal medicine departments on the whole (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011; Reference EkdahlEkdahl et al., 2012).
Delirium units and teams
Delirium units often coexist with dementia wards because of the common coexistence of the two conditions and similar management strategies that are employed to support patients (Reference LamLam et al., 2014). Delirium is so widely prevalent among older hospital inpatients that it is unlikely that specialist delirium units could ever look after all patients or that it is possible or desirable to cohort them all in one clinical area (National Institute for Health and Care Excellence, 2010; Reference OliverOliver, 2016a). And so, it is equally important to ensure that all staff caring for frail older people are able to recognize, prevent and manage delirium and that specialist teams are able to provide support and training of other staff.
One example is the Hospital Elder Life Programme (HELP), which was developed in the USA in 1993; it involves the use of a “multicomponent strategy” with multidisciplinary specialist teams who provide structured support to older people with delirium (Reference Young and InouyeYoung & Inouye, 2007). HELP has been implemented in more than 11 countries across more than 100 sites (Reference SteelfisherSteelfisher et al., 2013). It has been shown to be cost- and clinically effective, with reduced rates of delirium and functional decline, including the prevention and exacerbation of chronic medical conditions, with improved satisfaction among providers, patients and family (National Institute for Health and Care Excellence, 2010). Health Improvement Scotland is driving a national programme to prevent, recognize and improve outcomes in people with delirium and to share best practice (Health Improvement Scotland, 2016). The European Delirium Association now also has a network to share best practice and research across Europe.
Geriatric–surgical collaboration and liaison for frail older people
Not all frail older people are admitted under geriatric medicine and therefore it is crucial to provide CGAs where possible to optimize the care and health of older people admitted under different specialties. The more familiar and most widely developed liaison service across Europe is orthogeriatric collaboration with available evidence demonstrating cost-effectiveness and significant associations with reduced mortality rates in frail older people with fragility fractures (Reference Sabharwal and WilsonSabharwal & Wilson, 2015; Reference Knobe and PapeKnobe & Pape, 2016; Reference Ozalp and AsprayOzalp & Aspray, 2016). In Germany and Austria the explicit implementation of geriatric trauma centres has been developed where hip fracture patients are co-managed with common ward rounds between geriatricians, orthopaedic surgeons and specialized nurses (Reference KammerlanderKammerlander et al., 2011; Reference PapePape et al., 2014). In the United Kingdom the development of a fracture liaison service has been promoted as a “model of best practice” to provide optimum care to frail older people with hip fractures; a recent analysis of data from 11 hospitals in England points to significant improvements in mortality post surgery (British Orthopaedic Association, 2007; Reference HawleyHawley et al., 2016).
General surgical liaison is now a growing field in the United Kingdom, after its initial liaison model was developed specifically to address the needs of older people undergoing surgery, known as the proactive geriatric liaison with older people undergoing surgery (POPS) model (Reference HarariHarari et al., 2007). A survey of 161 hospitals in the United Kingdom showed that there are varying levels of geriatric-led perioperative services being provided across the country, with a combination of preoperative and postoperative services being offered that covers both acute and elective surgery, although barriers include funding, workforce issues, and a lack of inter-specialty collaboration (Reference PartridgePartridge et al., 2014).
Other European countries are at different stages of developing medical liaison services as there is clear recognition of the value of geriatric input into the management of complex medical issues. Belgium introduced the “Geriatric Health Care Programme” in 2007 by adopting the development of a geriatric unit that also provides internal and external liaison services to frail older people on non-geriatric wards through similar tenets of CGA and MDT working (Reference Van Den Noortgate and PetrovicVan Den Noortgate & Petrovic, 2009; Reference BaitarBaitar et al., 2015). In Ireland the older person assessment and liaison service (OPAL) showed that the service model provided timely CGA, and facilitated effective discharges from hospital, which may be further enhanced by efficient referrals and assessment processes through the use of clinical nurse managers (Reference HayesHayes et al., 2016).
The role of outpatient clinics
Outpatient clinics in secondary care serve to bridge the gap between hospital care and the community once a patient has been discharged from hospital. They may also assume the role of “specialist” clinics that assess and treat specific conditions such as Parkinson’s disease, respiratory or cardiology conditions, as well as falls and syncope clinics. Falls (prevention) clinics have been shown to reduce the incidence of injurious falls among older people by providing specific interventions around falls prevention with the support of physiotherapists and occupational therapists (Reference MooreMoore et al., 2010; Reference PalvanenPalvanen et al., 2014). Outpatient falls and syncope clinics are sometimes defined as day clinics, where assessments involve addressing underlying medical conditions to be treated, which are followed by further assessments by the physiotherapist and occupational therapists before an intervention is put into place (Reference Lamb, Gates and FisherLamb, Gates & Fisher, 2007). Outpatient clinics may also provide day services such as blood transfusion and chemotherapy where appropriate to enable patients to return home without needing inpatient admissions for such procedures. However, the provision of such assessment and follow-up does not necessarily have to happen on hospital sites, especially when travel and repeat attendances could be disruptive and distressing to older people with frailty. In some cases, hospital specialists and skilled MDTs can provide outpatient services in community and primary care settings closer to patients’ homes, often in collaboration with primary care teams (British Geriatrics Society et al., 2012; King’s Fund, 2014; Reference GordonGordon, 2015).
End of life care
A study examining the place of death in older people with dementia-related diseases across 14 countries showed that in Europe the proportion of deaths in hospital ranged from 1.6% in the Netherlands to 62.3% in Hungary (31.7% in England, 35.9% in France, 32% in Italy, 33.6% in Spain, 21.6% in Belgium) (Reference ReyniersReyniers et al., 2015). A qualitative systematic review of integrated palliative care in Europe found that a palliative care framework is necessary to improve symptom control, lessen care-giver burden, improve continuity and coordination of care, reduce admissions, increase cost-effectiveness and enable patients to die in their preferred place of care (Reference SioutaSiouta et al., 2016). In 2010 the National Gold Standards Framework in End of Life Care Centre, a volunteer sector organization in the United Kingdom, was formed to provide support, training, and innovation in delivering better end of life care through advance care planning, with the goal of improving the quality and coordination of care, reducing hospitalization, and enabling more people to live and die at home (Gold Standards Framework, 2012). A 2015 audit on death and dying by the Royal College of Physicians of London (2016) found that of the 93% of patients whose death was predictable and documented, only 54% of case records showed that the needs of the person were asked about, with only 24% of patients having clinically assisted (artificial) hydration; 34% of cases had documented evidence about the need for clinically assisted (artificial) nutrition. Only 67% of hospitals reported that they implemented change to their service by taking into account bereaved family and friends’ requests about patient care in their final days (Royal College of Physicians of London, 2016). Between 2005 and 2012 improvements in coverage of palliative care services had been made mostly in western European countries compared to central and eastern European countries, with still significant gaps across services (Reference Centeno-CortesCenteno-Cortes et al., 2016). There is only one chance to get end of life care right and often this is unfortunately not the case. With a limited number of hospice beds and palliative care specialists available, advance care planning and addressing end of life issues earlier is pivotal, and if the patient does end up in hospital in their final days, then every effort should be made to get it right from the start (Reference OliverOliver, 2016d; Royal College of Physicians of London, 2016).
Care of older patients with dementia and mental health problems in general hospitals
Dementia encompasses a group of organic brain diseases and the most common forms are Alzheimer’s dementia, vascular dementia, mixed dementia (having Alzheimer’s and vascular components), Lewy bodies and fronto-temporal dementia (Reference HackmanHackman et al., 2013). The personal, social, and economic costs of dementia are substantial, often complicated by multiple co-morbidities or frailty. The global estimate of older people living with dementia is expected to increase to 81 million by 2040, of whom 30% will be living in Europe (Reference Kaplan and BerkmanKaplan & Berkman, 2011). Hospital patients with dementia are typically more frail, and at risk of significant complications of hospital-acquired infections, delirium, loss of function and unplanned readmissions (Reference Hermann, Muck and NehenHermann, Muck & Nehen, 2015). They can find hospital admission confusing, which can have a negative impact on their health and well-being both physically and mentally. Many who present with delirium are subsequently found to have dementia after discharge from hospital, with the two often coexisting (Reference JacksonJackson et al., 2016).
Countries across Europe have developed national strategies towards the diagnosis and management of dementia in hospitals and the community (Royal College of Psychiatrists et al., 2013). Specialized and appropriate care in hospital is vital for diagnosis and for supporting frail older people with dementia and their families towards a life that is disability-free and productive as far as possible. The main models of care delivered in acute hospital settings include specialist dementia wards, liaison psychiatry teams who provide diagnosis and support to patients, and dementia specialist nurses who work both in the hospital and in the community setting. The following discusses each approach in turn.
Specialist dementia wards
Specialist dementia wards have been in development across European hospitals to cater for the needs of older people with dementia (Reference Wilkinson and HendriksWilkinson & Hendriks, 2015; Reference O’ConnorO’Connor et al., 2016). Although such specialist units have not demonstrated measurable impact on hospital and primary care utilization, mainly because patients tend to be at the end of life, the experience of patients and their carers were reported to be significantly better compared to care received on general wards (Reference GoldbergGoldberg et al., 2013). Reference GoldbergGoldberg et al. (2013) also demonstrated, in a randomized controlled trial of specialist and mental health units, that patients had more positive interactions and engagement with the staff, families perceived the management of confused patients to be more empathetic, and discharge planning was seen to be more efficient. There is variability in terms of the number of beds available in these facilities and length of stay. Components of care include therapy involvement, spaces for patient interaction, a routine that meets the needs of patients with cognitive deficits, volunteer workers, and specialist staff who provide care and tailored plans for individual patients that take into account their social and cultural backgrounds (Reference Hermann, Muck and NehenHermann, Muck & Nehen, 2015).
Liaison psychiatry for older people
With so many older hospital patients having dementia or mental problems accompanying their other complaints, there is no prospect of all patients being admitted to specialist units, so other models of specialist input matter. Liaison psychiatry or liaison psychological medicine is defined as a specialty that manages people who present with mental and physical symptoms concerned with the interplay between physiological, psychological and social determinants that cause ill health. Liaison psychiatry teams often consist of a MDT which includes psychiatrists, nurses, support workers and therapists (Royal College of Psychiatrists et al., 2013), and liaison psychiatry for older adults is provided either by psychiatrists with an interest in old age psychiatry or by specialist nurses. Liaison psychiatry for older adults (LPOA) has become embedded in many European hospital settings as part of the routine assessment to improve the quality of life of older people (Reference Mukaetova-LadinskaMukaetova-Ladinska, 2006). For example, an LPOA service in a tertiary hospital in Portugal found that delirium and dementia accounted for more than 60% of the diagnoses and although the referring complaint was mostly “mood disturbances”, it was found that only 24% of these patients had depression, highlighting the poor diagnostic experience of referring clinicians (Reference NogueiraNogueira et al., 2013).
Evidence on liaison mental health services points to some benefits for people with dementia, for example increased referral rates for cognitive assessment, better detection and diagnosis, and greater staff confidence in caring for patients with dementia. However, a literature review of dementia care in general hospitals showed that, despite individual case studies demonstrating local benefit, trial evidence around mental health liaison is lacking. Quality of inpatient care improves as a result of these services, but the impact on cost-effectiveness and length of stay remains uncertain (Reference Dewing and DijkDewing & Dijk, 2014).
Specialist dementia nurses
The care delivered by specialist nurses has been identified to be of key importance in supporting people with dementia. There has been increasing interest in many settings in developing specialist nurse roles as one approach to improving the care of people with dementia in hospital (Reference Griffiths, Bridges and SheldonGriffiths, Bridges & Sheldon, 2013), and across European countries specialist nurses are being widely used to support frail older people with dementia in acute hospitals and the community (Reference Hermann, Muck and NehenHermann, Muck & Nehen, 2015). A scoping review of the role of the dementia specialist nurse in acute care working directly with people with dementia and their families for a significant period of time found this model to benefit older people with dementia in hospital and their families (Reference Griffiths, Bridges and SheldonGriffiths, Bridges & Sheldon, 2013).
Models of post-hospital care
Transitional care arrangements that constitute post-hospital care can put pressures on frail older people, and need to be timely and safe to ensure effective and efficient transfers (Reference AllenAllen et al., 2014). Across high income countries various models of post-hospital care are emerging to bridge the gap between hospital and people’s homes, with core elements including anticipatory care targeting older people, MDTs, and enhanced interagency working to promote improved outcomes (Reference PhilpPhilp et al., 2013). These services aim to allow people to leave hospital sooner, reduce the chance of readmission and improve their short- and medium-term health outcomes. This section focuses on a range of models that have been implemented across European countries and describes discharge-to-assess and early discharge approaches, while also considering the role of community geriatricians and of primary care in promoting and supporting post-hospital care in the community. It is sometimes the same teams or referral hubs providing pre-hospital or “step up” care and admission prevention (see Section 2) that are able to provide this transitional or “step down” care and such an arrangement allows for simplicity and continuity of care.
Discharge-to-assess models and early supported discharge
In a discharge-to-assess model, a patient whose acute health needs have been stabilized is subsequently discharged home for rapid assessment of their needs in their own home environment and follow-up of ongoing care by community-based clinicians (Reference Andrew and RockwoodAndrew & Rockwood, 2014). An older person who is deemed medically stable for discharge from the emergency department or acute medical unit ward but still requires ongoing support is discharged home with a team of multidisciplinary therapy staff for assessment. A plan of support is put in place immediately; should the patient fail the assessment at home, they would then return to hospital (Reference SilvesterSilvester et al., 2014). In the United Kingdom a number of local quality improvement studies have shown the benefits of early senior review linked to these models in terms of reduced admission rates, reduced bed occupancy, and higher rates of discharge home within 24 hours of presentation (Reference FoxFox et al., 2013; Health Reference FoundationFoundation, 2013). However, the majority of studies are single case based and there is little robust evidence from controlled trials. Data from such quality studies suggest that effective discharge-to-assess models require timely expert assessment on initial acute presentation to hospital and adequate capacity for medical and nursing care, therapy support, and social care for providing assessment and support at home (Reference SilvesterSilvester et al., 2014).
Early supported discharge (ESD) enables patients to return home earlier and receive rehabilitation within their own homes. Unlike discharge-to-assess, it tends to rely on more traditional assessment of needs in the hospital setting as the basis for defining ongoing clinical and care needs after discharge. This service is more commonly provided for people who have physical disabilities such as post-acute stroke (Reference Fearon and LanghorneFearon & Langhorne, 2012). In contrast to discharge-to-assess models of care, ESD follows after completion of assessments in the hospital and the patient is found to have met the minimum criteria for transfer back to their own home (Reference KirkKirk, 2013). EDS is comparatively widely implemented across European countries, with much of the evidence originating from northern Europe and a 2012 Cochrane review concluded that among older patients following stroke, those who were discharged with an ESD service had improved physical outcomes, reduced lengths of stay in hospital, lower dependency rates and reported higher satisfaction with services compared to those receiving conventional services (Reference Fearon and LanghorneFearon & Langhorne, 2012; Reference Mas and InzitariMas & Inzitari, 2015).
Hospital at home schemes
A number of countries in Europe have developed innovative models of care in the community to bridge the gap between hospital and home, or to provide extra support at home without hospital admission (Reference Jones and CarrollJones & Carroll, 2014; Reference VilàVilà et al., 2015). Examples include the “hospital at home” model and the “virtual community ward”, which enable frail older people to continue to be treated within their familiar environments.
In a hospital at home setting, care is provided within a patient’s home, with services similar to those provided in hospital but delivered by a community-based team or hospital-resourced outreach staff through domiciliary visits (Reference ShepperdShepperd et al., 2010). There is mixed evidence about the effectiveness of hospital at home services. Systematic reviews of single chronic disease management, such as COPD and heart failure, suggest that patients seem to benefit from the service as the readmission rate is reduced and the system is proving to be more cost-effective. In contrast, frail older people with multiple co-morbidities seem to have an increased rate of readmission (Reference ShepperdShepperd et al., 2010; Reference Jeppesen and JaeJeppesen & Jae, 2012; Reference QaddouraQaddoura et al., 2015).
End life care seems to be better managed using hospital at home type models. For example, a programme in Barcelona, Spain, found such a service to improve end of life care in patients with terminal illnesses, with up to 72% choosing to remain at home in their final days with support from the community teams (Reference VilàVilà et al., 2015). A recent systematic review of home-based end of life care found this to significantly increase the likelihood of dying at home compared with usual care, with some evidence of improved patient satisfaction at one-month follow-up (Reference ShepperdShepperd et al., 2016).
Virtual and community wards
Virtual wards also replicate a hospital ward. However, contrary to the hospital at home model, which provides acute clinical care, the virtual ward places emphasis on the integration of medical teams, nursing, therapists and social care to provide a proactive approach of care to people at risk of hospital admission (Reference Jones and CarrollJones & Carroll, 2014). They can be used to support discharge (“step down”) as well as preventing admission. The evidence of the effectiveness of virtual wards in frail older people with complex multimorbidity remains mixed (Reference BardsleyBardsley et al., 2013; National Institute for Health and Care Excellence, 2016). Recent evaluations of virtual wards in four parts of England were unable to demonstrate reductions in cost or hospital bed utilization, although there were some reductions in elective activity (Reference LewisLewis et al., 2013). Similarly, a randomized controlled trial of a virtual ward for high-risk adult hospital discharge patients in Toronto, Canada, did not find a statistically significant effect of a virtual ward model of care on readmissions or death at different points of time after hospital discharge (Reference DhallaDhalla et al., 2014). Reference LewisLewis et al. (2013) commented, based on the English experience, that where virtual or community wards are developed locally, this should be motivated by patients’ needs and the need to provide care closer to home for those at highest risk, rather than because they will deliver savings (Box 4.3).
Box 4.3 ‘‘virtual ward hub” services for older patients in Bradford, England
In order to improve integration of services, and because of the need to reduce readmission rates, a virtual ward hub was developed by Bradford Teaching Hospitals NHS Trust in 2012 to provide support to frail older patients who were discharged from the elderly admissions unit and general geriatric wards. The service is geriatrician-led, with typical support involving daily nurse visits and therapy staff depending on the needs of the patient and a shared electronic health records system enabling cross-boundary sharing of information and skills to manage a patient within their home. The team consists of 3 advanced nurse practitioners, 4 physiotherapists, 6 nursing sisters, 19 nurses, 18 rehabilitation support workers, and 2 geriatricians. A typical monthly caseload is approximately 40 patients, with multidisciplinary discussions held three times a week. Bed occupancy across geriatric medicine has reduced by 6% (compared to 1.5% across the rest of the hospital), and there has been a perceived reduction of pressure on the acute hospital. This service is continuing to expand with further development of the hub to take on more patients, co-location with social services, and embedding CGA in all their assessments of frail older patients.
The role of community geriatricians
We have discussed the role of geriatricians in hospital care but their role in the community is just as important in providing support to community services for frail older people. In some European countries this is the major part of their work, with acute hospital care being more the province of internal medicine physicians (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011; Reference EkdahlEkdahl et al., 2012; Reference GordonGordon, 2015). The role of community geriatricians includes support for people in nursing and residential facilities, support for community case management teams or virtual wards or discharge teams, and close work with primary care teams to support high-risk patients with frailty (Reference Oliver and BurnsOliver & Burns, 2016). For example, in the Netherlands and Norway community geriatricians provide specialist care to frail older people residing in nursing homes through CGA with a network of multidisciplinary professionals to optimize care (Verenso, 2015). Community geriatrician involvement in care homes has been linked to a reduction in medications prescribed and optimizing drug treatment, thereby reducing risks of readmission associated with adverse drug reactions (Reference Burns and McQuillanBurns & McQuillan, 2011). Evidence suggests that adverse drug reactions are common in the post-hospitalization period and this needs to be addressed effectively across transitions of care in order to prevent harm and inconvenience.
Intermediate care rehabilitation services
There are several definitions of intermediate care but the common thread underlying them is the provision of health care services to those who require support in the transitions between acute care, primary care and social care. They vary in their provision of support depending on the needs of the patient to optimize and achieve their baseline function where possible or to provide an environment for further assessments such as CGA to take place (Reference Woodford and GeorgeWoodford & George, 2010). The following section discusses each category in turn.
Crisis response teams can take the shape of rapid response teams (see above) that provide step up or step down services. Step up services are targeted at older people who require support in their home or in an intermediate care facility, with the aim of avoiding hospital admission where possible and appropriate. Step down services provide a bridge service for transitions from the emergency department or post discharge from hospital (NHS Benchmarking, 2015).
Home-based intermediate care services are provided within a person’s home by a multidisciplinary professional team. In Finland such services are provided by a nurse and home-care aid worker, depending on individualized plans devised by the case manager for a period of time until independence has been restored or regular home care has been put in place (Reference Hammar, Rissanen and PeräläHammar, Rissanen & Perälä, 2009).
Bed-based intermediate care services overlap with other community-based facilities that are situated within nursing homes or local community hospitals and more commonly accommodate frail older people who have been admitted to hospital and require a period of convalescence and rehabilitation prior to discharge to their home.
Rehabilitation services outside hospital focus on providing a suitable environment to promote functional recovery. Delivered by a MDT, these services aim to meet the rehabilitative goals of service users by concentrating on activities that are important to the individual but which may have been missed in a clinical environment (Reference PearsonPearson et al., 2015). Rehabilitation primarily includes physical therapy and occupational therapy to prevent admission to an acute hospital or facilitate a stepped pathway out of hospital.
Workforce planning in caring for frail older adults
One of the key workforce challenges in the care of frail older adults is a shortage of medical and nursing staff within geriatric care (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011; Reference HeinenHeinen et al., 2013). In the United Kingdom geriatrics is the largest internal medicine speciality with the highest number of training posts. But demand for both geriatric medicine posts and acute internal medicine posts is so high that not all posts are filled currently (Royal College of Physicians of London, 2015). Guidance from the Royal College of Physicians recommends a minimum of one consultant geriatrician per 50 000 population for effective facilitation of geriatric care (Reference FisherFisher et al., 2014). France, Spain and Ireland have a lower number of geriatricians per capita compared to Belgium, Germany and Switzerland, with vast differences in recruitment and structured training programmes (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011).
With shortages of geriatrics specialist doctors, nurses and allied health professionals, those in other adult clinical areas all commonly encounter older patients with complex co-morbidities, dementia and frailty as a big part of their core role (British Geriatrics Society, 2014a; Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014; Quality Watch, 2015). Non-geriatric trained health care professionals do not always have the competence or confidence to manage frail older people (Alzheimer’s Society, 2009). Unfortunately, ageist attitudes persist in parts of the workforce, leading to age discriminatory treatment and service models (Centre for Policy on Ageing, 2009; Economist Intelligence Unit, 2012; British Geriatrics Society, 2014b; World Health Organization, 2015; National Institute for Health and Care Excellence, 2016).
Surveys from North America and Europe have shown that there are shortcomings in the undergraduate curriculum of geriatric medicine for doctors in training, and as a result there are initiatives in place to ensure that resources are allocated towards specialist teaching around geriatric medicine, focusing on attitudes towards older patients, and trying to engage these patients in teaching to enable a broader view of managing frail older patients in practice (Reference OakleyOakley et al., 2014). There are now toolkits available that define core requirements for postgraduate training across Europe in geriatric medicine that can help inform a structured curriculum at the European Union level (Reference SinglerSingler et al., 2016).
Nursing staff shortages and issues such as attitudes to older people and the lack of training to work with them are also significant problems (Reference CapezutiCapezuti et al., 2012; Reference HeinenHeinen et al., 2013). Reference CapezutiCapezuti et al. (2012) found that geriatric-specific nurse training can contribute to successful recruitment of nurses and provide the high level nursing input required for geriatric patients. Staff development in specialist areas such as dementia is needed to improve their knowledge and competence (Reference Page and HopePage & Hope, 2013; Reference Hermann, Muck and NehenHermann, Muck & Nehen, 2015). NHS Education for Scotland in partnership with the Scottish Social Services Council developed a framework for all health and social services staff working with people with dementia, their families and carers in 2011, with four levels of training depending on the amount of contact staff had with the patients (Reference BanksBanks et al., 2014).
Advanced nurse practitioners (ANPs) may carry out CGAs and provide advice about acute care, including managing mental health illnesses, as well as playing a part in rehabilitative medicine and supporting clinical governance, education and innovation (Reference GoldbergGoldberg et al., 2016). A systematic review of the role of ANPs in long-term residential care concluded that they play a positive role in reducing mental health illnesses, improving urinary continence and pressure ulcer care, improving residents’ abilities to meet personal goals and in family satisfaction with medical services (Reference DonaldDonald et al., 2013).
Geriatricians are unable to look after all patients with frailty and, with an ageing population, frail older people are seen in all specialties such as surgery, general medicine, and mental health (Reference BagnallBagnall et al., 2013; Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). European countries clearly need an increase in the specialist geriatric medicine workforce as increasingly the core business of acute internal medicine, emergency medicine and general internal medicine is geriatric medicine (Reference CesariCesari et al., 2016). At the same time, there will never be enough geriatricians or specialist nurses and allied health professionals to look after all older people with frailty and so other specialists will need greater competencies (Reference Oliver and BurnsOliver & Burns, 2016). This has been recognized in plans for European training curriculums by the European Federation of Internal Medicine (2016 and ongoing).
The challenges facing geriatric medicine call for a new way of collaborative and integrated working across disciplines, and key elements to inform this should include: definition of roles of those managing the patient, goal setting with the patient, team communication between geriatricians and the treating team, care planning with relevant guidelines in place, and leadership to oversee that the overall care is safe, effective and deliverable (Reference TsakitzidisTsakitzidis et al., 2016). The National Institute for Health and Care Excellence guidelines on managing patients with multimorbidity has set out similar messages (Reference FarmerFarmer et al., 2016).
Barriers to delivering optimal and integrated hospital and acute care
The quality of geriatric care depends on available resources, structures and a specialized workforce to deliver acute care, rehabilitation, long-term care and palliative care services; however, countries across Europe vary in terms of how well established their geriatric systems are, with some countries having more developed services compared to others (Reference Kolb, Topinkova and MichelKolb, Topinkova & Michel, 2011; EUGMS, 2016). But all doctors, nurses and allied health professionals working in acute internal medical and surgical specialties will care for older people with frailty (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014; Reference Oliver and BurnsOliver & Burns, 2016). Geriatric training curriculums need to change and evolve to reflect the complexities that surround frail older people and the European Union Geriatric Medicine Society (EUGMS) has now set plans in place to develop a curriculum for “geriatric emergency medicine” for this specific purpose (Reference Bellou and ConroyBellou & Conroy, 2016).
Care for older people is still very much divided into primary care, secondary care and social care, often with a lack of continuity throughout the process of an older person’s journey as they transition through any of these systems (Reference Oliver, Foot and HumphriesOliver, Foot & Humphries, 2014). One restructuring process that distributed funding and developed an integrated model of health care provision in New Zealand transformed the way older people were cared for, which subsequently improved waiting times, reduced unplanned readmissions and increased the availability of social care to the older population through their “one budget, one system” philosophy (Reference Timmins and HamTimmins & Ham, 2013). The province of Quebec in Canada has also been successful in integrating health and social care through structural organizations, contractual agreements, and the sharing of informatics between these systems of care (Reference VedelVedel et al., 2011).
Improved information systems will be increasingly important but a systematic review (Reference LluchLluch, 2011) demonstrated that health information technologies are difficult to implement even though evidence suggests that this does improve exchange of data, and subsequently improves the safety and quality of care provided to older people and those with multiple co-morbidities.
System-level changes are required to deliver quality, coordinated and economically viable care to an ageing population that have co-morbidities as the norm rather than the exception (Reference JesteJeste, 2011). Some authors have suggested that evidence-based practices need to change from traditional randomized controlled trials that are costly and time-consuming to a more pragmatic approach; with quality improvement gaining momentum, implementation research can add great value to innovation and transferability across systems (Reference BalasubramanianBalasubramanian et al., 2015; Reference McGrathMcGrath et al., 2016; Reference Thompson and JonesThompson & Jones, 2016). Some countries in Europe are more advanced on their journey to integrated care than others, with governments prioritizing it on national agendas over the last 20 years, and others are less worried and more confident about future challenges (Economist Intelligence Unit, 2012). Finland, for example, has spent the last 30 years developing centralized integrated care approaches aimed at optimizing care for older people, as well as those with issues with mental health or substance misuse and younger children (Reference Mur-Veeman, van Raak and PaulusMur-Veeman, van Raak & Paulus, 2008). Integration of long-term care to meet the needs of ageing populations will remain challenging but will be an important area for organizational development, training and research in the future (Reference LeichsenringLeichsenring, 2012).
Geriatricians and other staff groups specializing in coordinated care for older people need to lead the way by using their expertise to enter leadership positions and work in partnership with physicians, researchers, and other health care professionals, which is crucial to achieving a critical mass. Together, they can lead a comprehensive national health agenda for frail older people and advocate ground-breaking policy changes (Reference Nikolich-ZugichNikolich-Zugich et al., 2015).
Conclusion
Older people are increasingly the main focus of much of hospital care. Older people with frailty are at high risk of hospital admissions, increased mortality, and care home utilization, and there is much that the design of hospital services and their associated community and primary care services can do to reduce these issues. There are opportunities from a number of new approaches to the management of care for older people and from changes in how professionals work and how they come together in teams more effectively. The acute hospital remains a centre of care provision to the frail and the vulnerable, but it sits within the wider context of the community and social care arrangement, where integration of care is vital to the provision of holistic care to people with frailty. There are major challenges from workforce shortages and a need to equip a wide range of professionals with the skills to help them care for older people more effectively. A shift in focus is needed in managing the complex pathway of patients through the health care system and, in many parts of Europe, reducing their dependence on the hospital.
Introduction
With an estimated 1.75 million deaths from cancer in Europe in 2012 (Reference FerlayFerlay et al., 2013), cancer is the second leading cause of death in Europe (World Health Organization, 2012). Diagnosis, treatment, continuing care, and in many cases palliation account for a substantial volume of the work of the acute general hospital. Yet while the word “cancer” is widely used in popular discourse, it is important to recognize that it is not a single disease but rather a pathological process that can affect almost all organs of the body. This process involves uncontrolled tissue growth, based on changes related to genetic or acquired abnormalities of the DNA, or related processes in the cell. Genetic changes that cause cancer can be inherited or, more commonly, they arise during a person’s lifetime as a result of errors that occur by chance as cells divide or because of damage to genes caused by environmental exposures such as chemicals in tobacco smoke or ultraviolet radiation. The clinical management of cancer thus depends on both the nature of the pathological processes involved, increasingly being characterized at the molecular level, and the organs affected. An individual’s cancer is thus characterized by a unique combination of genetic changes, which can change over time, for instance as a result of developing resistance by selecting clones of therapy-resistant cells. As a consequence, there has been an important paradigm shift from organ-based interventions to a patient-centred and targeted treatment for which increasingly innovative biological therapies are being discovered. In addition, cancer shares certain risk factors with other diseases. Thus, patients with cancer may be at greater risk of those other conditions. For example, tobacco use, which is the leading preventable cause of cancer in Europe, is associated not only with cancers of the lung but also with many other cancers, while also contributing to other conditions, such as coronary heart disease (Reference PetoPeto et al., 2012).
Cancer diagnosis and treatment have changed substantially in the past decades, with, for example, the advent of new chemotherapeutic agents transforming many cancers from short-lasting fatal illnesses into long-term chronic disorders. With increased understanding of the underlying disease processes, there have been considerable advances in early detection and diagnostic imaging, genetic profiling, and increased treatment options, including the introduction of targeted drugs and multidisciplinary care in many settings. In addition, and related to improved survival rates, cancer care takes account of psychosocial aspects, quality of life, patients’ rights, and empowerment and survivorship. In this chapter we explore these shifts in cancer treatment and care, with a focus on oncological hospital care in Europe.
The burden of cancer in Europe
In 2008 one-quarter of the global cancer burden was observed in Europe, which is striking given that the total European population comprises only one-ninth of the world’s population (Reference FerlayFerlay et al., 2013). For 2012 the Globocan project predicted an incidence of 3 715 000 cases with a five-year prevalence of 9 701 000 cases. There were an estimated 3.45 million new cases of cancer (excluding non-melanoma skin cancer) and 1.75 million deaths from cancer in Europe in 2012 (Reference FerlayFerlay et al., 2013).
Female breast cancer (464 000 cases), colorectal cancer (447 000), prostate cancer (417 000) and lung cancer (410 000) were the most frequent cancers, together representing half of the overall cancer burden in Europe in 2012 (Reference FerlayFerlay et al., 2013). The most common cancer modalities leading to death in 2012 were lung cancer (353 000 deaths), colorectal cancer (215 000), breast cancer (131 000) and stomach cancer (107 000). Incidence varies across the region, however, with cancers resulting from external carcinogens and bacteria (e.g. stomach cancer) tending to be higher in eastern Europe and Portugal, while breast and prostate cancer are more common in western Europe. Data from the International Agency for Research on Cancer (IARC), an agency within the World Health Organization, found the incidence in western European countries to be over 244 per 100 000 in 2012, compared to between 177.3 and 244.2 per 100 000 in eastern Europe.
Overall, cancer incidence continues to rise, with growth rates of up to 2% or 3% per year across Europe. As survival is also gradually improving, prevalence is growing at an even quicker pace, leading to increased numbers of cancer patients (those still alive but in various stages of disease after primary treatment) and cancer survivors (those who have ended therapy and are in follow-up schedules).
Table 5.1 Summary indicators of cancer burden in selected high income regions, 2012
| North America | EU-28 | Western Europe | Northern Europe | Southern Europe | Australia and New Zealand | |
|---|---|---|---|---|---|---|
| New cancer cases | ||||||
| Age-standardized rate (per 100 000) | 315.6 | 273.5 | 298.7 | 277.4 | 253.6 | 318.5 |
| Risk of getting cancer before age 75 (%) | 30.9 | 27.3 | 29.6 | 27.5 | 25.3 | 30.7 |
| Cancer deaths | ||||||
| Age-standardized rate (per 100 000) | 105.5 | 109.4 | 105.0 | 108.0 | 105.2 | 97.6 |
| Risk of dying from cancer before age 75 (%) | 11.2 | 11.5 | 11.0 | 11.2 | 10.9 | 9.9 |
| Five-year prevalent cases, adults (per 100 000) | 1 888.2 | 1 690.4 | 2 018.6 | 1 658.6 | 1 585.3 | 1 901.8 |
| Five most frequent cancers (defined by total number of cases) | Prostate Breast Lung Colorectal Bladder | Breast Prostate Colorectal Lung Bladder | Prostate Breast Colorectal Lung Bladder | Prostate Breast Colorectal Lung Melanoma of skin | Colorectal Breast Lung Prostate Bladder | Prostate Colorectal Breast Melanoma of skin Lung |
Note: Estimates of worldwide age-standardized incidence and mortality as provided by GLOBOCAN use the World standard population, while EUCAN uses the European standard population. The World standard population presents a young population compared to the European standard population; EUCAN estimates for individual European countries or regions (such as those reported by Reference FerlayFerlay et al., 2013) are therefore higher than those provided by GLOBOCAN.
Cancer survival rates are typically used as an indicator of the quality of cancer care, from prevention and screening to treatment. In Europe the EUROCARE study has systematically collected survival data from national cancer registries to monitor trends in cancer survival in children and adults (Reference BerrinoBerrino et al., 2007; Reference AngelisDe Angelis et al., 2014). EUROCARE data show large differences in survival, with some analyses linking differences in survival to differences in spending on cancer care, with the Nordic countries and Switzerland scoring favourably compared to the rest of Europe (Reference Luengo-FernandezLuengo-Fernandez et al., 2013). However, interpretation of the data is challenging because of persisting variations in the quality of data available, and the challenges of adjusting for case mix-when interpreting observational studies (Reference LymanLyman, 2013), as well as how best to respond to this evidence (Reference WhalenWhalen, 2010). What is clear is that greater resources will be needed to respond to the combination of a projected rise in the number of cancer cases and technological innovations that could potentially improve outcomes (Reference Aggarwal, Ginsburg and FojoAggarwal, Ginsburg & Fojo, 2014).
The development of contemporary cancer care
Cancer diagnosis and treatment have progressed rapidly since the discovery of the cellular origins of cancer in the 1860s, but mainly in incremental steps. Important developments came about from the 1950s onwards, with advances in radiation technology and, in particular, cancer chemotherapy such as the treatment of childhood leukaemia and, in adults, Hodgkin’s disease from the mid-1960s, with success of adjuvant treatment of breast cancer since the 1970s (Reference DeVita and RosenbergDeVita & Rosenberg, 2012). At the same time, a greater understanding of the causes of cancer has increased scope for primary prevention, reducing the risk of developing disease. The most prominent example is perhaps the discovery of tobacco smoking as a cause of cancers of the lung and various other organs, with declines in the occurrence of lung cancer and subsequently mortality as a consequence of antismoking measures (Reference Jha and PetoJha & Peto, 2014). The discovery of certain viral infections as a cause of cancer has also led to the development of vaccines against, for example, certain types of human papilloma virus (HPV) for the prevention of cervical cancer or hepatitis B virus for liver cancer.
Recent advances in the understanding of biological functioning of the cell have increased our understanding of cellular mechanisms, enabling the development of targeted drugs that have been very successful in certain subgroups of patients. Among the most recent developments is immunotherapy, which provides for progression-free survival in tumours that were previously considered to be uniformly fatal, such as metastatic melanoma and lung cancer.
While advances in screening have enabled early detection of certain cancers, albeit at a risk of over diagnosis for some (Reference ViguierViguier, 2015), these changes have had considerable implications for the management of cancer, which increasingly involves a complex array of interventions that require different professionals working together in a coordinated fashion to enhance outcomes for people with cancer. As it can involve many disciplines, sequential and parallel process steps, different handovers and frequent patient contacts by different disciplines, cancer care is increasingly organized through MDTs and along cancer care pathways.
The cancer care pathway
The cancer care pathway describes the patient’s journey from the initial suspicion of cancer and symptom-based investigations, or through screening and early detection, to the various diagnostic procedures leading to a diagnosis of cancer, followed by treatment, which typically involves a selection of one or more interventions, such as surgery, radiotherapy, or chemotherapy. Depending on the outcome of the primary treatment, the patient will receive follow-up care and rehabilitation or, where the tumour remains active or is advanced, undergo further treatment or receive palliative and end life care when the tumour proves incurable (Figure 5.1).
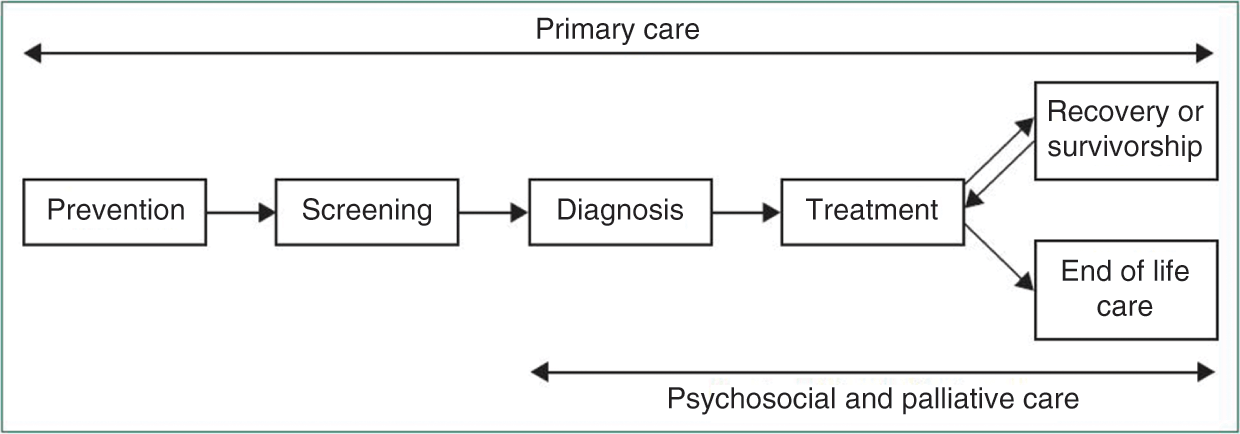
Figure 5.1 The cancer journey
The precise nature and scope of the cancer care pathway differs between cancer types and countries. Detailing cancer care pathways provides patients and professionals with a better understanding of the complex processes that are involved in treatment, while also contributing to enhancing the patient journey to strengthen high quality cancer care. Each pathway identifies the different steps and recommended care processes at each stage of the journey (Cancer Council Australia, 2016).
In countries where the general practitioner (GP) acts as gatekeeper to specialist care, such as the United Kingdom and the Netherlands, most cancers are diagnosed by a specialist after presentation of complaints or symptoms to the patient’s GP, while others are diagnosed upon emergency presentation (Reference RubinRubin et al., 2015). A proportion of cancers is detected through screening programmes such as for breast cancer, colorectal and cervical cancer, although percentages differ across cancer sites and coverage of related programmes in different health systems. Countries where patients can directly access specialist care enable direct and extensive diagnostics; this can lead to overuse of diagnostic procedures. On the other hand, there are some concerns that overly stringent primary care gatekeeping may introduce inappropriate delays. Clearly, it is difficult to get the right balance.
As we shall see below, the diagnosis and treatment of common tumour types is commonly provided by medical specialists within a hospital setting. Many countries have also established designated cancer centres for the delivery of specialized care for a large portfolio of common and rare tumours, serving also as tertiary referral centres for patients with rare tumours, late-stage disease or other difficult cases. Comprehensive cancer centres usually undertake a wide range of activities in translational cancer research, from basic scientific discovery, to the delivery of novel approaches, to care of patients with cancer, such as targeted therapies.
The precise nature of the cancer patient journey differs for different types of cancer. Figure 5.2 illustrates a typical pathway for a patient with breast cancer in a cancer centre in the Netherlands. In this example, the patient would typically consult with a range of specialists within the cancer centre, which would also be responsible for aftercare. In some countries therapy is provided in “shared care” arrangements involving office-based physicians or local hospitals, for instance in order to provide chemotherapy closer to the patient’s home.

Figure 5.2 Breast cancer patient pathway, the Netherlands
Although most types of cancer care require specialized equipment and staff, there is an increasing trend to move more parts of the cancer care pathway into the community. Care in the community takes several forms, including chemotherapy delivered in people’s own homes (Reference CorbettCorbett et al., 2015), rehabilitation in community settings, blood and other monitoring tests in general practices or local settings, and increased access to local services and support groups (Macmillan, 2014).
Patient-focused, integrated care initiatives can provide greater quality, efficiency and patient satisfaction (Reference LeutzLeutz, 1999; Reference Burns and PaulyBurns & Pauly, 2002; Reference Kodner and SpreeuwenbergKodner & Spreeuwenberg, 2002). Evidence emerging over the past 20 years suggests that the transition of cancer care from oncologist-led models to nurse-led models in cancer centres or primary care-led models in the community may improve cancer outcomes (Reference GrunfeldGrunfeld et al., 1999; Reference WattchowWattchow et al., 2006; Reference LewisLewis et al., 2009; Reference Grunfeld and EarleGrunfeld & Earle, 2010; Reference SussmanSussman et al., 2011). Primary care providers are often willing to assume follow-up care with appropriate guidance and a clear path for transition of care for their patients, and they are more likely than oncologists to provide preventive interventions directed at non-cancer conditions (Reference GiudiceDel Giudice et al., 2009; Reference Grunfeld and EarleGrunfeld & Earle, 2010).
Multidisciplinary teams
We have noted above that the management of cancer increasingly involves a complex array of interventions that require different professionals working together in a coordinated fashion to enhance outcomes for people with cancer. Figure 5.3 provides an illustration of the range of staff involved in the breast cancer care pathway introduced in Figure 5.2. Optimizing delivery of the care pathway and patient outcomes will require close coordination and communication among the different professionals involved. Health providers are increasingly drawing on MDT working to enhance decision-making between health care team members and patients (Reference FleissigFleissig et al., 2006; Reference BorrasBorras et al., 2014). MDTs usually address one type of cancer or a group of cancers. Oncology MDTs can include surgeons, diagnostic and therapeutic radiologists, pathologists, medical and clinical oncologists, nurse specialists, and palliative-care physicians, among others. Such a team will often collaborate closely with other supportive professionals, such as psychologists and psychiatrists (Reference FleissigFleissig et al., 2006).
Figure 5.3 Breast cancer pathway for staff, the Netherlands
The move towards MDT working in oncology has been supported by several expert groups and it is now considered the standard in cancer care in most countries in Europe and elsewhere (Reference BorrasBorras et al., 2014). Some countries require that the management of all patients with cancer within MDT conferences should be the norm, although some cases that are uncomplicated will be dealt with according to standard guidelines without being discussed by all involved.
Overall, the adoption of MDTs in cancer care has been rapid. For example, in England in the mid-1990s fewer than 20% of cancer patients were managed by an MDT compared with more than 80% in 2004 (Reference Griffith and TurnerGriffith & Turner, 2004). In the Netherlands the peer review system for hospital cancer services, which was introduced in 1994, provided a strong stimulus for the adoption of MDT working (Reference KilsdonkKilsdonk et al., 2015a, Reference Kilsdonk2015b). Although it is difficult to evaluate the exact mechanism through which an MDT exerts its effect and little direct evidence exists, MDT working has been linked to improved patient outcomes, increased recruitment into clinical trials, and better job satisfaction and psychological well-being among health professionals (Reference FleissigFleissig et al., 2006; Reference PillayPillay et al., 2016). For example, Reference PillayPillay et al. (2016) found, based on a systematic review, that MDT meetings impact positively upon the ways cancer patients are assessed and managed. This is consistent with a review by Reference TaplinTaplin et al. (2015), which suggests that using team-based approaches across the care continuum can improve access to and the quality of care processes and structures. However, robust evidence on the impact of MDTs on patient outcomes such as survival remains weak. Overall, while they appear intuitively to be beneficial, the current evidence base provides only a limited degree of support for their widespread use (Reference PillayPillay et al., 2016) and, at least for now, it may be more cost-effective to limit MDT meetings to the discussion of particularly complex or controversial patients.
Barriers to delivering optimal care and the sustainability of the oncological service system
Workforce
The hospital workforce more broadly, and the oncology workforce specifically, face several barriers regarding the delivery of optimal and sustainable cancer care. Key challenges include demographic changes in the composition of the workforce, including an ageing health workforce, leading to shortages due to retirement (European Commission, 2008), along with fewer younger generations entering the workforce due to the limited attractiveness of employment in the health sector (European Commission, 2008). Further challenges are related to the mobility of the workforce across the EU, in particular the movement of some health professionals from poorer to richer countries within the EU, as well as the health brain drain from third countries (European Commission, 2008).
Expensive biological drugs
A review of market access to cancer drugs in Europe found that reimbursement mechanisms, the use of cost-effectiveness analysis in decision-making, and the extent of pharmaceutical price regulation schemes vary considerably across countries (Reference PauwelsPauwels et al., 2014; Reference Hartenvan Harten et al., 2016). Most countries have some form of risk-sharing agreement for high value drugs, be it financial agreements where rebates are offered to third-party payers for the cost of increased expenditure over an annual subsidization cap, or performance or outcome-based agreements (Reference CheemaCheema et al., 2012).
Overall, there are marked differences in the availability and reimbursement of new and often expensive cancer drugs. For example, in Italy innovative new cancer drugs are classified as Class H, qualifying their use in the hospital setting. Class H drugs are bought directly by hospitals from the manufacturers, enabling them to benefit directly from cost sharing agreements and minimum discounts of 50%. This has enabled expansion of patient access to pharmaceuticals (Reference Folino-GalloFolino-Gallo et al., 2008). In the United Kingdom the National Institute of Health and Care Excellence (NICE) provides advice on whether or not to reimburse innovative drugs; in other countries there are comparable agencies although the implications of their decisions vary. Differences
in the availability of cancer medicines across countries, and the cost of cancer treatment, have prompted considerable public debate in a number of countries. However, the impacts of variation in access to innovative medicines on cancer outcomes at population level are difficult to ascertain.
Radiotherapy and radiology
Unlike cancer drugs, the evaluation of radiation technologies has attracted less attention although it is an area that has undergone significant development over the past 5 to 10 years. Radiotherapy is considered a necessary component of treatment in about half of all newly diagnosed cancers (Reference DelaneyDelaney et al., 2005). However, European countries are in the paradoxical situation where delivering affordable radiotherapy over the next 20 years is being compromised by both current under-capacity and under-investment in “standard” radiotherapy and also over-penetration of newer radiotherapy technologies that have far greater associated costs (Reference Van LoonVan Loon et al., 2012). A recent analysis of the Directory of Radiotherapy Centres (DIRAC) database demonstrated variation in radiotherapy capacity and quality across the EU (Reference RosenblattRosenblatt et al., 2013).
Imaging techniques and radiology play a major role in the management of many patients, including cancer patients (see Chapter 9). The quality of imaging has improved significantly over recent decades and the use of these new devices has increased, although often because of a belief – not always justified – that “newer is better” (Reference DeyoDeyo, 2002). However, the greater use of these techniques has created a larger problem. They often lead to diagnosis of lesions of dubious clinical significance (Reference LumbrerasLumbreras et al., 2010).
An unexpected finding can trigger additional medical care, including unnecessary tests and other diagnostic procedures and treatments which, in some cases, may pose an additional risk to the patient. This process has been called the “cascade effect” (Reference WhitingWhiting et al., 2003). A review by Reference LumbrerasLumbreras et al. (2010) found that a considerable percentage of patients in whom incidental findings were observed underwent further evaluation with additional expensive and often uncomfortable or risky imaging tests or other diagnostic tests and procedures. Radiologists and clinicians have to balance the diagnostic potential against unnecessary testing and treatment (Reference LumbrerasLumbreras et al., 2010). Some measures have been recommended to clarify the situation (Reference WhitingWhiting et al., 2003), including explicit assessment of the potential risk of the incidental finding for the patient or the availability of a beneficial treatment that justifies follow-up, although the optimum strategy will depend greatly on the particular circumstances.
General trends in oncology care
Looking ahead, there are some trends in cancer care that will have especially profound consequences for the hospital. These include precision medicine, targeted treatment, and immunotherapy; image guided interventions; and improved survivorship and survivorship care.
Precision medicine, targeted treatments, and immunotherapy
Greater understanding of the mechanisms by which cancers develop has provided important insights into interventions targeting under-
lying mechanisms and treating the condition (Reference SagerSager, 1997). The primary treatment option is removing the tumour through surgical or radiotherapeutic intervention (often accompanied), which remains by far the most common treatment by which patients are cured (World Health Organization, 2016). However, a considerable percentage of patients are not cured or experience relapse or metastatic disease. Here, chemotherapy and the more recently developed targeted treatments provide important therapeutic options.
Until recently, chemotherapy was given to a large number of patients on the understanding that only a certain percentage would benefit. A better understanding of the underlying pathways has helped to develop treatments that target mechanisms acting at cellular, subcellular or molecular levels. These targeted treatments rely on molecular diagnostics of underlying cell abnormalities, and expertise in genetic aberrations of tumours (Reference GingerasGingeras et al., 2005). Targeted therapies have shown promising results in a number of tumours, especially in advanced stages where so far very few therapeutic options were otherwise available, requiring genome sequencing and analytical techniques along with professional expertise to interpret and weigh findings. It is expected that this trend towards precision medicine, which includes health care innovations involving molecular diagnostics and pharmacogenomics, will continue to bring promising results. This is expected to generate a rapidly growing industry in which genetic markers of disease and treatment responses are searched on a larger scale (Reference DzauDzau et al., 2015), but which could come at a price that can threaten the financial sustainability of health systems.
In recent years immunotherapy (also referred to as biological therapy) has been shown to be promising in treating certain cancers (and other diseases). This was informed by the observation of “spontaneous” cures in some patients, stimulating research into immune reactions around and inside tumours (as, for instance, observed by white blood cell activity). Immunotherapeutic drug options are available for (metastasized) lung and renal cancer and melanoma, with further experimentation under way with other tumour types. DNA vaccination, stimulating antitumour cell reaction, is also being tested (Reference StockwellStockwell, 2015; Reference BlankBlank et al., 2016) . It is estimated that up to 20% of tumours may benefit from some form of immunotherapy in future. This method has the potential to provide treatment for patients who until recently have had no curative options. However, the costs involved have meant that there is considerable variation in access to new immunotherapeutic drugs and treatments across countries, generating debate on pricing levels and sustainability of the financing of cancer treatment. Recent studies have shown marked differences in list prices and actual prices in a number of European countries; overall access to innovative drug treatment in cancer seems especially difficult in the less developed economies in Europe (Reference JohnssonJohnsson et al., 2016; Reference HartenVan Harten et al., 2016; Reference Vogler, Vitry and BabarVogler, Vitry & Babar, 2016).
Image guided interventions
Surgical and radiotherapeutic removal of the tumour (bulk) tissue are the primary treatment options if curative treatment is considered. Complete removal is essential but is not always successful. For example, a study on prostatectomy showed that 38% of patients had not had the tumour tissue completely removed (Reference RetelRetel et al., 2014). Here, imaging guidance, a technique available in fields such as cardiology and neurosurgery, has become an important aide to distinguish normal from malignant tissue, or where the tumour is hard to delineate from the surrounding environment. These include perioperative CT scanning, smart needles with optical features and navigation technology combined with image integration. These methods require expertise and infrastructure in imaging modalities as well as biomedical technology expertise within the operating theatre (see Chapter 9). The investments related to these developments can lead to the gradual concentration of diagnostic and intervention infrastructure and expertise. Most technologies are in “proof of principle” or early phased clinical studies and in oncology in innovator locations, such as the Netherlands Cancer Institute in Amsterdam and the Institut Gustaphe Roussy in Paris.
Improved survivorship and survivorship care
Improved survival rates (Reference StockwellStockwell, 2015) have led to a continuously growing number of cancer patients who have survived primary treatment but require ongoing treatment, including cancer survivors treated with curative intent, and who require follow-up and symptom-related aftercare (Reference Van HartenVan Harten et al., 2013).
Improved cancer survivorship poses challenges for health services, requiring a rethink of how services should be reconfigured to enhance care for cancer survivors (Reference StovallStovall et al., 2006). The increasingly chronic nature of cancer means that survivors require ongoing support and care in specialist settings and the community. Guidelines for follow-up and survivorship care are being developed in many countries, and research and development into interventions and service development are on-going. Cancer and cancer treatments are associated with a wide range of physical and psychological challenges, some of which may even appear only years after the initial treatment. Person-centred and stepped care approaches are considered to be the most appropriate way forward, but evidence remains weak on the best ways of providing care that optimizes symptom treatment and problem solving for different cancer sites. For example, Reference TsianakasTsianakas et al. (2012), in a study of patient experience of cancer services, found that while those with breast and lung cancer reported broadly similar experiences, they differed in the nature of information they required and the priorities they attached to service improvement activities. New care models are emerging that emphasize the importance of supporting patients to engage in self-management activities and to enable them to make informed choices about the type of support they need. It is apparent that cancer survivors must become more effective coproducers in their own care, with Reference TsianakasTsianakas et al. (2012) proposing experience-based codesign as an approach to ensure that patients will be involved as active partners in the care process.
Patient empowerment will be at the core of new approaches to cancer care in hospitals, with Reference GroenGroen et al. (2015) identifying five key attributes: (1) being autonomous and respected; (2) having knowledge; (3) having psychosocial and behavioural skills; (4) perceiving support from community, family, and friends; and (5) perceiving oneself to be useful. The latter two are essential in the cancer setting. Information and communication technology (ICT) and eHealth initiatives are playing an increasing role in supporting cancer patients (Reference McAlpineMcAlpine et al., 2015). Technology ranges from electronic patient portals to electronic decision aids or online cognitive behavioural therapy programmes. The majority of eHealth initiatives in cancer care tends to focus on providing patients with information about their disease and treatments to enable shared decision-making, with only a minority aimed at patients to build the skills necessary to cope with the symptoms they experience (Reference GroenGroen et al., 2015).
The fragmentation of ICT services poses a major challenge to realizing their potential benefits. In the Netherlands, for example, many hospitals have their own patient portal system and do not easily connect to other systems in the cancer care trajectory. If they are to increase patient-centredness and shared care, IT services need to be linked in a way that makes information available for every health care provider in the chain (“shared care”). This most probably will lead to a medical record that is owned by the patient and has connections to multiple health care providers.
Organizational trends in cancer care and hospital-based oncology across the EU
Networks
The development of advanced diagnostics in nuclear medicine, MRI, molecular pathology, and genetic sequencing requires specialist staff and investments in infrastructure and equipment (see Chapter 9). In combination with a growing awareness that in some areas hospitals with a greater volume of patients with certain conditions achieve better outcomes, we see a gradual trend towards cooperation between hospitals in networks, with centralization, especially of complex low volume interventions. This requires providers to formalize agreements on cooperation, division of labour and handovers, and to discuss pathways across organizational boundaries. In 2018 the Organisation of European Cancer Institutes (OECI) started the development of Patient-centred Quality Standards for Cancer Networks (Organisation of European Cancer Institutes, 2018). We here use the examples of the Netherlands (Box 5.1) and Italy (Box 5.2) to illustrate these trends.
Box 5.1 The emergence of cancer networks in the Netherlands
In the Netherlands most hospitals provide cancer care, although there has been a steady trend towards the centralization of, in particular, low volume and complex treatments. University centres specialize in rare cancers and large hospitals have a leading role in the treatment of high volume tumours such as breast, lung, colorectal and prostate cancer. This trend was initially stimulated by the setting of minimum volume standards for various procedures by government and health insurance companies. This role has now been taken on by professional associations, which are defining norms and quality criteria; they have also introduced quality registries.
The Netherlands Comprehensive Cancer Organization (IKNL) is the quality institute for oncological and palliative research and practice in the Netherlands. It collaborates with health care professionals and managers and patients on the continuous improvement of oncological and palliative care, encourages knowledge exchange and organizes consultation service between centres of expertise and regional hospitals. IKNL also coordinates the issuing and maintenance of care guidelines (Volksgezondheid en zorg, 2016).
In response to the centralization of cancer services, and to clarify the role of various types of hospital and university medical centres (UMCs), regional cancer centre networks (CCNs) are being established. The CCNs will focus on improving treatment, care and clinical research in oncology across the network. It remains challenging to implement CCNs, however, as not all UMCs cover all relevant high volume tumours. Importantly, so far the Netherlands has only one comprehensive cancer centre – the Netherlands Cancer Institute – that has received formal accreditation by the OECI. Also, it was only recently that it was decided to concentrate all paediatric oncology in one national centre (construction started in 2016). As the total number of new cases amounts to around 500 per year, this guarantees state-of-the-art expertise for every individual case (Prinses Maxima Centrum, 2016).
Box 5.2 The emergence of cancer networks in Italy
The Italian National Cancer Plan is increasingly focusing on the concept of regional oncology networks, according to a federal model, of which the Lombard Oncology Network (Regione Reference LombardiaLombardia, 2006) is the most representative example in the national network Alleanza Contro il Cancro. Locally, the network provides significant benefits in terms of resources and information optimization; at a national and international level it helps to maximize the collaboration and the sharing of best practices.
The EU has established European reference networks (ERNs) for rare diseases, with networks for paediatric, haematological, and solid tumours approved in December 2016. These are intended to improve the quality of care, to coordinate knowledge dissemination and to facilitate cross-border care. By early 2017 the first European reference networks became operational (European Commission, 2018). Reference PalmPalm et al. (2013) highlighted various challenges in the implementation of European reference networks. For example, for Denmark it was shown to be challenging to identify the right national balance in terms of geographical coverage and capacity of “good clinics”, and for monitoring and evaluating the system. There remain questions about how the concept of reference centres and networks should be defined, the management of the process of identifying centres, and the implications of the establishment of such networks for funding of services and coverage of the population.
Organization
There is an increasing trend to centralize cancer services through the formation of cancer centres (outside or within the hospital structure), with a growing number of hospitals and cancer centres in a range of EU countries entering the Accreditation and Designation (A&D) programme of the OECI (Organisation of European Cancer Institutes, 2018).
There remains debate about the optimal model of organizing cancer care; the added value of different forms is difficult to establish, with little robust evidence on the best way of delivering cancer services.
Germany provides an example of a distinct organizational model, which involves a three-tier approach (DKG German Cancer Society, 2014). The first tier comprises comprehensive cancer centres (Onkologische Spitzenzentren), which are the leading oncology centres holding a major research portfolio. The cancer centres focus primarily on rare cancers and specialized aspects of care, with a specific programme by the Deutsche Krebshilfe periodically designating 14 centres as comprehensive cancer centres, which will receive considerable additional funding for their translational research. The second tier includes oncology centres, which cover several cancer sites or specialties, particularly rare cancers. The designation as an oncology centre is led by the German Cancer Society (GCS) and aims to guarantee high quality of services for payers, the public and government. The third tier includes organ cancer centres, which specialize in one organ or specialty (e.g. breast, bowel, lung, prostate, skin, and gynaecological tumours). The organ cancer centres are also covered by the GCS programme (DKG German Cancer Society, 2014).
At the European level there are various professional and institutional oncology societies. Professional societies include the European Society of Medical Oncologists (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO). An example is the European Cancer Organization (ECCO), a multidisciplinary organization that connects all stakeholders in oncology across Europe. ECCO is a not-for-profit federation that aims to uphold the right of all European cancer patients to the best possible treatment and care, promoting interaction between all organizations involved in cancer at European level (ECCO, 2016). Another is the Organization of European Cancer Institutes (OECI). The OECI is a non-governmental, non-profit organization with the primary objective to improve communication and bringing together cancer research and care institutions across the European Union, in order to create a critical mass of expertise and competence (Organisation of European Cancer Institutes, 2016).
Patient registries
Patient registries (to be distinguished from the more traditional cancer registries) that collect and enable the monitoring of data on treatments and tumour characteristics have been established in Norway, Sweden and, more recently, the Netherlands. The population-based cancer registries in the Nordic countries include more comprehensive disease-specific quality registries covering treatment data and detailed outcomes data (Reference MøllerMøller et al., 2002).
In Italy the Italian Association of Cancer Registries (AIRTUM) established a network of registries which gained international importance in contributing to European survival studies (Reference AngelisDe Angelis et al., 2014), international prevalence comparisons (Reference CrocettiCrocetti et al., 2013) and the consolidation of partnerships within the network of cancer registries in the Mediterranean area, including the southern coast (Reference Hamdi CherifHamdi Cherif et al., 2015), through the Euromed (EEAS, 2016) project.
Cancer Care in the hospital of the mid-21st century
Cancer will account for a growing volume of hospital activity in coming years. Not only does this mean that more patients will be offered innovative and promising treatments, but the sustainability of the health system, in terms of human and financial resources, will remain under continuous strain.
Perhaps the most important message from this chapter is that the care of cancer in hospitals has become vastly more complex than in the past, in terms of both the technical ability to characterize and understand tumours and to individualize their treatment, and the organizational responses that bring together staff with differing types of expertise. This will require close coordination between clinicians and managers. Further concentration and specialization of services will be inevitable, and this will require continuing networking, increasingly using innovative information technology, telehealth, and telemedicine to ensure that concentration of services does not undermine geographical access to services. It is very likely that regional networks of hospitals will organize cancer care among themselves, concentrate and share expensive diagnostics and intervention capacity, and establish liaisons with referral centres of expertise for rare tumours. Networks of reference centres are established in the EU and offer considerable potential for promoting research and innovative treatments for patients with some very rare tumours, or tumour subtypes.
Introduction
Studies suggest that around 25% of the European population receive treatment for a chronic condition. As the population ages, the prevalence of chronic diseases increases, with an average of two per person in their mid-60s and three for those surviving to their mid-70s (Reference BarnettBarnett et al., 2012). People with chronic diseases now form a sizeable proportion of all hospital admissions both elective and emergency. Once admitted to hospital, people with multiple complex conditions may require a long length of stay and place a significant demand on acute hospital services.
Chronic obstructive pulmonary disease (COPD) is such a condition which affects between 3% and 10% of Europe’s adult population and accounts for 1.1 million hospital admissions per year (Reference GibsonGibson et al., 2013). While it is a preventable condition, once contracted it is not curable and management strategies aim to reduce the burden of disease both on the individual and on society, which is currently estimated to cost the EU €200 billion per year (Reference GibsonGibson et al., 2013). Managing COPD and other long-term conditions effectively is critical not only for patients and carers but for the effective functioning of the health system itself.
In this chapter we use COPD as an exemplar of a chronic condition whose management depends on the work of the acute general hospital. As noted in Chapter 1, while the care of patients with COPD involves many specific features, it also raises issues of more general relevance to many common chronic disorders. Here we describe the burden of the disease in detail, the current management of the condition within the hospital system, and options for future care pathways illustrated by innovations that have already been implemented across a range of European health systems.
What is COPD?
Chronic obstructive pulmonary disease is an overarching term for the clinical and patho-physiological manifestations of the inflammatory response of the lungs to the repeated inhalation of noxious particles and fumes. This inflammation over time results in damage to both the airways, causing narrowing, and to the alveoli, manifesting as emphysema. People with COPD will characteristically exhibit the symptoms of cough, often with sputum production and usually worse over the winter months, with breathlessness being the most prevalent symptom (Reference Aitsi-Selmi and HopkinsonAitsi-Selmi & Hopkinson, 2015) that tends to be progressive over time and may be accompanied by wheeze. The condition results in airflow limitation in both the small and large airways that is detected by lung function tests, notably spirometry, which are used to confirm the diagnosis (Reference BarnesBarnes et al., 2015). People who develop COPD probably have a genetic predisposition so that when exposed to noxious inhaled substances, most commonly cigarette smoke but also occupational dusts and, especially in low income countries, biomass fumes in poorly ventilated housing, they react with an increased inflammatory response that causes intrinsic lung damage.
The clinical course once COPD develops is variable but overall is progressive and may lead to death from respiratory failure or as a result of respiratory infection, which may cause intermittent acute exacerbations of the condition. There are a number of identifiable phenotypical expressions of the condition that provide an opportunity for delivering more personalized interventions to individuals. The one intervention that would make most difference to all those with COPD, however, is to remove the exposure to the noxious substances provoking the lung inflammation (Reference VestboVestbo et al., 2013).
Additionally, co-morbidities may have a significant impact on clinical presentation and prognosis (Reference LaforestLaforest et al., 2016) and reduced physical activity is a well recognized consequence of the condition (Reference Hopkinson and PolkeyHopkinson & Polkey, 2010). Accordingly, there is a need for early intervention to prevent later more severe and expensive disease.
The burden of COPD
It is difficult to provide reliable estimates about the population health burden that can be associated with COPD. This is in part because the disease is often under-diagnosed as it is not usually recognized until it is clinically apparent and moderately advanced (Reference LamprechtLamprecht et al., 2015). Furthermore, where estimates are available, these frequently draw on varying definitions and diagnostic criteria. For example, studies of COPD prevalence have variously used self-reported respiratory symptoms, physician diagnosis of COPD, or the presence of airflow limitation with or without spirometric tests as criteria. As a consequence, available estimates vary by study design.
The recent 2010 update of the Global Burden of Disease (GBD) study revisited previous estimates on respiratory diseases and estimated the number of people to have COPD at 328 million globally (Reference VosVos et al., 2012). Worldwide the prevalence of COPD is rising, with the highest rise in the eastern Mediterranean region (119% between 1990 and 2010) and the lowest rise in Europe (22.5%), both, however, being substantial. Overall prevalence among men is around twice that of women but there are significant national variations (Reference AdeloyeAdeloye et al., 2015). More recently there is evidence of falls in COPD prevalence within some western European countries, for example in Spain (Reference SorianoSoriano et al., 2010) and Finland (Reference PelkonenPelkonen et al., 2014), thought to be as a result of tighter tobacco controls.
COPD is one of the major causes of mortality worldwide (Figure 6.1). There has been a steady increase in mortality over time (Reference JemalJemal et al., 2005) and it was estimated that COPD would become the fourth leading cause of death globally by the year 2030 (Reference Mathers and LoncarMathers & Loncar, 2006), a projection that was confirmed by the 2010 GBD study, when COPD became the third leading cause of death globally (Reference LozanoLozano et al., 2012). Within Europe it is estimated to have caused 150 000 deaths in 2010, potentially rising to 338 000 a year by 2030 (Reference GibsonGibson et al., 2013).

Figure 6.1 Age-standardized death rate from COPD per 100 000, both sexes, 2016
Economic costs that can be associated with the burden of COPD
As noted, COPD has been associated with considerable economic costs to the health system (Reference KhakbanKhakban et al., 2015) and projections suggest a significant further increase in direct costs by the year 2030 because of population ageing (Reference Herse, Kiljander and LehtimakiHerse, Kilijander & Lehtimaki, 2015). COPD also poses a substantial burden at individual level in terms of activity limitation and disability and to society more broadly because of lost productivity and associated costs (Reference Patel, Nagar and DalalPatel, Nagar & Dalal, 2014).
The predominant health care cost item is hospital utilization for exacerbations, which, in the United States in the early 2000s, was estimated to account for $18 billion (€14 billion) annually (Reference Anzueto, Sethi and MartinezAnzueto, Sethi & Martinez, 2007). In southern Spain the annual cost of hospital admissions for COPD exacerbation was estimated to be €27 million in 2000 (Reference López-Campos BodineauLópez-Campos Bodineau et al., 2002), with admissions to intensive care accounting for one-fifth of the total costs for COPD management (Reference DalalDalal et al., 2011). Estimates of the mean actual cost per severe exacerbation range from €1711 in Greece (2006–07) (Reference GeitonaGeitona et al., 2011) to €3985 in Italy (2006) (Reference BlasiBlasi et al., 2014). Co-morbidities such as cardiovascular disease, diabetes, asthma and anaemia (Reference ManninoMannino et al., 2015) were shown to further increase the economic burden that can be associated with COPD (Reference HuberHuber et al., 2015), as they drive increased service utilization among people with COPD (Reference Simon-TuvalSimon-Tuval et al., 2011). With the advent of new pharmacological treatments for COPD (Reference Barjaktarevic, Arredondo and CooperBarjaktarevic, Arredondo & Cooper, 2015), it is reasonable to expect that health care costs that can be associated with COPD will rise further, despite the evidence that pharmacotherapy for COPD in ambulatory care is cost-effective (Reference SimoensSimoens, 2013). In summary, available data highlight the need to prioritize interventions aimed at delaying the progression of COPD, preventing exacerbations and reducing the risk of co-morbidities, in order to alleviate the clinical and economic burden of COPD (Reference WoutersWouters, 2003; Reference FosterFoster et al., 2006; Reference Anzueto, Sethi and MartinezAnzueto, Sethi & Martinez, 2007; Reference ManninoMannino et al., 2015).
The COPD care pathway
It is suggested that a high proportion of people with COPD remain undiagnosed either because they have few if any symptoms in the milder stages of the disease or because clinicians are slow to associate common symptoms of cough or breathlessness with the need to screen for COPD (Reference LlordesLlordes et al., 2015). People with diagnosed COPD present usually with symptoms on the background of an exposure history, most commonly to cigarette smoke, but in around 5–15% of cases to occupational fumes, with exposure to biomass fuels a particular challenge in low and middle income countries (Reference Smith, Mehta, Maeusezehal-Fauz and EzzatiSmith, Mehta & Maeusezehal-Fauz, 2004). The diagnosis is made clinically but by definition it must be confirmed by spirometry lung function testing.
Once a diagnosis is made, the underlying lung damage is largely permanent and the prognosis is of a slow decline in lung function and symptoms related to the continuing exposure to the causative agent. Thus in a cigarette smoker, stopping smoking will halt further decline but not resolve any existing disease (Box 6.1).
Box 6.1 Evidence-based interventions for the management of COPD: smoking cessation
Smoking is a major risk factor for the development of COPD and current smoking is also higher among people with COPD compared to the general population, up to 47% and 20%, respectively (Reference SchauerSchauer et al., 2014). Reference AnthonisenAnthonisen et al. (1994) demonstrated that among people with early-stage COPD annual lung function decline was reduced following a smoking intervention compared to people with COPD who did not receive the intervention. There is also evidence of improvements in the presence of respiratory symptoms and quality of life over time. Against this background, smoking cessation has been proposed as an intervention with the highest impact on the natural history of COPD (Reference VestboVestbo et al., 2013).
Evidence further suggests that even brief advice provided by physicians to quit smoking can significantly increase the likelihood of successfully quitting smoking (Reference Bao, Duan and FoxBao, Duan & Fox, 2006; Reference SteadStead et al., 2013). At the same time, while behavioural interventions (including simple advice) have modest efficacy in improving smoking quit rates among people with COPD, the combination of counselling and pharmacotherapy tends to be more effective and more cost-effective than either on its own (Reference HoogendoornHoogendoorn et al., 2010; Reference TashkinTashkin, 2015). International guidance recommends a five-step programme, involving brief strategies to help patients willing to quit smoking (Reference VestboVestbo et al., 2013), while recognizing that more complex interventions will increase quit rates. Smoking cessation has been identified to be a cost-effective intervention for patients with COPD independently of stage of disease and should therefore be offered to every single smoking COPD patient (Reference Buck, Richmond and MendelsohnBuck, Richmond & Mendelsohn, 2000; Reference WoutersWouters, 2003).
There are no interventions other than smoking cessation that impact the natural history of the disease and arrest the decline in COPD. Management outside of smoking cessation is therefore largely designed to improve symptoms and functional status, and interventions outside smoking cessation tend to be matched to the stage of the disease and the severity of symptoms. This is further illustrated in Figure 6.2, which provides an example of a care pathway to improve outcomes in COPD.

Figure 6.2 Example of a care pathway to improve outcomes in COPD
In the late stages of the disease, long-term oxygen therapy (LTOT) and non-invasive ventilatory (NIV) support may prolong life (Box 6.2) and optimizing palliative care interventions may also improve both quality and length of life. Lung transplantation in selected patients is an ultimate option in very severe COPDs, although this tends to be available to a small minority of end-stage patients only (Reference Lane and TonelliLane & Tonelli, 2015).
Box 6.2 Evidence-based interventions for the management of COPD: long-term oxygen therapy and non-invasive ventilatory support
Long-term oxygen therapy has been shown to prolong life in patients with COPD and chronic respiratory failure (hypoxia; deficiency of oxygen in the tissues) (Reference StollerStoller et al., 2010). Effects on survival are only achieved if LTOT is given for at least 15 hours per day. LTOT is usually provided in the home environment of people with COPD. Ambulatory devices can increase the mobility of the patient and provide longer oxygen usage with resultant patient benefit (Reference Bradley and O’NeillBradley & O’Neill, 2005). Ambulatory oxygen may in some cases also reduce breathlessness on exertion in some patients who do not fulfil the strict criteria for LTOT. Providing LTOT for citizens who wish to spend time across national borders is challenging as there is no established European oxygen prescribing system and using oxygen aboard commercial flights can also be difficult and expensive with each airline following its individual set of rules.
Non-invasive ventilation (NIV) has been shown to be effective in patients with stable but very severe COPD and chronic respiratory failure (hypercapnia; high concentration of carbon dioxide in the blood), with evidence of positive effects on health status and survival (Reference KohnleinKohnlein et al., 2014; Reference StruikStruik et al., 2014). NIV in acute respiratory failure in COPD due to exacerbation has been shown to positively impact respiratory acidosis, symptoms, prevalence of ventilator-associated pneumonia, and length of hospital stay (Reference RamRam et al., 2004b). Evidence suggests that NIV and invasive mechanical ventilation (IMV) are increasingly used in hospitals, primarily in emergency departments and intensive care units, and access to this therapy has been increased within recent years (Reference López-CamposLópez-Campos et al., 2014).
Progressive decline in lung function may lead to significant disability and quality of life impairment where palliative care interventions are most appropriate. For some people COPD leads to death; the condition accounts for around 2.5% of all deaths in Europe (Global Health Observatory, 2008). Death from respiratory causes is, however, not inevitable in COPD and a number of patients will die from linked conditions that share the same aetiology of cigarette smoking, for example heart disease and lung cancer (Reference ZielinskiZielinski et al., 1997; Reference McGarveyMcGarvey et al., 2007).There is growing recognition that older patients with COPD suffer with multiple morbidities, all of which contribute to the state of frailty, that must be factored into their management. Death will more likely result from one of these diseases than it will from COPD, which in itself will be a major driver in future hospital models of care.
The effect of social factors on the outcome determinants of this complex health picture will further motivate collaboration between social and health care providers. As with many chronic diseases, the care provided to people with COPD tends to be fragmented in most system contexts. Countries are experimenting with new models of care that are designed to better meet the needs of people with long-term conditions (Reference Nolte, Knai and SaltmanNolte, Knai & Saltman, 2015), including for COPD, based on the available evidence of the (cost-)effectiveness of structured disease management of COPD (Reference SteutenSteuten et al., 2009; Reference KruisKruis et al., 2013).
The level of interaction between the patient and the hospital will depend very much upon the stage of disease of the patient, the level of support available for out-of-hospital care and the complexity of the individual case. While stable patients with COPD are typically managed outside hospital, there are a number of indications for specialist input that will require hospital care, even in those stable patients. But again these are most often delivered in the outpatient or ambulatory care setting rather than resulting in an admission to hospital. In cases where the diagnosis remains unclear or where, despite optimal treatment in primary care, a patient remains symptomatic, referral for a hospital-based specialist opinion is appropriate. In complex cases at the more severe end of the spectrum some interventions such as endoscopic lung volume reduction and surgical techniques are only available within the hospital setting (Box 6.3).
Box 6.3 Evidence-based interventions for the management of COPD: surgical treatment
Stable COPD patients with severe emphysematous lung damage (hyperinflation) can benefit from surgical treatment such as lung volume reduction surgery (LVRS). This intervention has been shown to lead, in an appropriately selected subgroup of patients with COPD, to better functional outcomes and improved survival compared to standard medical therapy (Reference NaunheimNaunheim et al., 2006). Similar to other invasive procedures, surgical treatment carries an operative mortality risk compared with medical management. The cost per quality adjusted life year (QALY) in appropriately selected individuals is estimated to be between $40 000 and $55 000 (Reference RamseyRamsey et al., 2007).
Bronchoscopic lung volume reduction (BLVR) is a novel treatment option, with clinical trials showing improvements in symptoms, exercise capacity, and lung function (Reference DaveyDavey et al., 2015). Some countries in Europe, notably Germany and Switzerland, have now incorporated this intervention into usual clinical pathways (Reference Patel, Nagar and DalalPertl et al., 2014). Others are awaiting further evidence. Effective BLVR appears to be associated with a survival benefit in carefully selected patients (Reference HopkinsonHopkinson et al., 2011; Reference KloosterKlooster et al., 2015; Reference GarnerGarner et al., 2016; Reference HerthHerth et al., 2016). The cost per QALY for BLVR in that subgroup is around €25 000 (Reference Pietzsch, Garner and HerthPietzsch, Garner & Herth, 2014).
The acute exacerbation patient pathway
The main cause for a person with COPD to be admitted to hospital as an emergency will be as a result of an exacerbation of his/her condition. Exacerbations are characterized by increasing breathlessness and accompanied frequently by worsening cough and increased volume or discoloured sputum production. In many cases these acute attacks are caused by infection, while in other cases they represent a deterioration in the underlying condition worsened by atmospheric changes or other environmental factors. Exacerbations can be treated out of hospital but may also result in hospital admission; exacerbations constitute a common cause of hospitalization across Europe (Reference LibreroLibrero et al., 2016).
People admitted to European hospitals with acute COPD exacerbations have an inpatient mortality of around 4.9% and a 90 day readmission rate of 35% (Reference HartlHartl et al., 2016). It is against this background that much focus has recently been given to preventing hospital admissions (Reference Vestbo and LangeVestbo & Lange, 2015). A number of interventions including combination therapy with inhaled corticosteroids and broncho-dilating drugs (Reference SpencerSpencer et al., 2011), prophylactic antibiotics (Reference Herath and PooleHerath & Poole, 2013) and patient education with self-management (Reference ZwerinkZwerink et al., 2014) have been shown to effectively reduce exacerbation frequency and hospital admission, with evidence suggesting that these should be implemented for all patients identified to be at risk. There is less evidence that self-management with provision of “rescue packs” of antibiotics and steroid tablets in isolation of a robust education programme is effective at reducing hospital admission (Reference WaltersWalters et al., 2010).
While it may have previously been considered that hospitals and their teams should concentrate on hospital care, it is clear that if patients are to receive a more joined-up and consistent level of care, then the influence of the hospital must extend outside of the physical bounds of the buildings themselves. There is some evidence that early self-management and proactive community interventions may reduce hospital admissions for patients with COPD at risk of exacerbation by up to a third (Reference EffingEffing et al., 2007; Reference Suh, Mandal and HartSuh, Mandal & Hart, 2013). Supporting both clinicians and patients and carers to better manage conditions to avoid unscheduled care and emergency admissions can best be facilitated by collaborative care linking to the education resources now found in abundance on the world wide web provided by national and international patient support groups (European Lung Foundation, 2013).
Which organization takes responsibility for interventions designed to reduce unscheduled care and admission to hospital will depend upon local systems but the skills and resources found in hospitals can enrich such out-of-hospital services through a variety of models. One example is the Kings Health Partners (London) Integrated Respiratory Team, which involves a partnership between hospital, community and primary care clinicians who form a collaborative team to manage out-of-hospital patients (Box 6.4). In the Spanish Ribera Salud model, a more formal vertically integrated accountable care organization directly employs an integrated community and primary team (Ribera Reference SaludSalud, 2016). Such integrated teams tend to be nurse-led and often include multidisciplinary members such as a physiotherapist and a social care case worker who can address the social aspects and may prevent an otherwise unnecessary admission. MDTs have been shown to be more effective at reducing admissions than nurse mono-professional teams (Reference Wong, Carson and SmithWong, Carson & Smith, 2012; Reference KruisKruis et al., 2013).
Box 6.4 Integrated Respiratory Team (IRT), Kings Health Partners, London, UK
The Integrated Respiratory Team works across King’s College Hospital and Guy’s and St Thomas’ NHS Foundation Trusts and the community in London to deliver care to patients with COPD, including oxygen, pulmonary rehabilitation and supported discharge services. Key components include the IRT working in acute care hospitals to support accurate diagnosis and acute management, communication and post-discharge care, VCs in the community, a single point of referral to IRT from the community and optimizing respiratory prescribing. Respiratory virtual clinics (VCs) run twice a week in primary care. The focus of VCs is joint working between primary care teams and the IRT to systematically review the diagnosis and long-term management of the respiratory patient caseload. Since its launch in 2012 the service has seen a 34% reduction in COPD admissions and a 17% reduction in length of stay.
Intervention teams may be COPD specific or have a general remit to reduce hospital admissions across a range of patient diagnostic groups. Some are specifically targeted at reducing readmissions to hospital while others provide a prevention service for a broader range of at-risk patients identified through primary care and secondary care ambulatory services. A key enabler for effective team working, particularly across sites and organizations, and to link with the patients across a geography, is technology (see Box 6.5 below). While the evidence for primary technology-based interventions in COPD care is currently weak (Reference LundellLundell et al., 2015), it seems sensible to suggest that integrated electronic patient care records, web-based self-management programmes (Reference LuckettLuckett et al., 2016) and greater use of communication technologies to facilitate coordinated and specialist support to generalist care are to be of increasing importance in the future.
While promising, preventative services such as those described in Box 6.4 are not currently implemented widely across Europe and will therefore be available to only a minority of patients. Most patients will be evaluated by their community-based primary care or specialist doctor and either treated or referred to the hospital. The decision-making process may be supported by (national) guidelines for the diagnosis and management of COPD that have been established in many countries (Reference EffingEffing et al., 2007).
Frequently, however, an acute exacerbation requires assessment in an emergency department (ED) and hospitalization. Across Europe there will be on average 200 hospital admissions for acute COPD per 100 000 population but with a 10-fold difference between countries with high and low admission rates (Reference GibsonGibson et al., 2013). The reasons for such variation are not known but it is hypothesized that this reflects the maturity of primary and community services, prevalence of COPD and the availability of hospital beds (Reference GibsonGibson et al., 2013). While much of the variation may be attributed to “system and population factors”, it seems clear that if hospitals are to moderate admissions for long-term conditions, there will be a need to extend their influence outside the physical walls of their estate.
Hospital care for exacerbations of COPD
While efforts are made to prevent admission to hospital, there is a need for severe exacerbation cases to receive the kind of management that currently can only be provided in hospital. The ideal pathway for a COPD admission can be seen to involve early triage to a specialist unit and provision of appropriate care using a MDT, to include ventilatory support where appropriate, and then discharge once safe with entry to a rehabilitation programme at an early stage following discharge (Reference VestboVestbo et al., 2013). For the minority of end of life patients palliative care services should be provided (Reference VestboVestbo et al., 2013).
However, hospital services are currently organized very differently across Europe, both within and between countries, which will influence the pathway for the individual COPD patient into the hospital and upon discharge. Data from the 2010–2011 European Respiratory Society audit of hospital care of people with COPD admitted to hospital with exacerbations (European COPD Audit) highlighted this variation (Reference López-CamposLópez-Campos et al., 2014). It showed that, for example, triage was operated in only 7% of Belgian hospitals included in the audit compared to 67% in Slovakia and 60% in Croatia. Specialist respiratory wards were available in 93% of UK hospitals but only in 27% of hospitals in Austria. While all, or the majority, of patients in Belgium and Switzerland (90%) were seen by a nurse or physiotherapy respiratory specialist, this was only the case for 35% of patients in Poland and 20% in Turkey.
Around 5% of admissions will die in hospital, although there are now predictive tools that allow the identification of those with a much higher risk of death who are most likely to benefit from the potentially life-saving interventions of ventilator support. Respiratory acidosis is one such predictor that affects about 20% of COPD admissions and has a mortality of between 20% and 30% without assisted ventilation support. In contrast there is a significant cohort of admissions at very low risk of death who could safely be managed in the community by a MDT as described earlier. The European COPD Audit found that a considerable share of admissions is for people with mild disease (Global initiative for chronic Obstructive Lung Disease (GOLD) stage I or II), ranging from 54% admissions in Romania and 51% in Switzerland to only 35% in the United Kingdom and 30% in Turkey (Reference López-CamposLópez-Campos et al., 2014). This suggests that many people with COPD exacerbations currently admitted to hospital could potentially be managed in the community if appropriate services (such as MDTs) were available. In contrast, patients requiring ventilatory support, or who are at risk of developing ventilatory failure requiring such support, should be managed in hospital according to national and international management guidelines. Yet, as data from the European COPD Audit indicate, availability of high dependency units that deliver ventilatory support varies substantially across countries, from 95% of Swiss hospitals to only 22% in Greece and 10% in Romania. Non-invasive ventilation was provided in all hospitals in Switzerland, Ireland and Slovakia but only in 70% of Croatian and 60% of Romanian units. For some patients the key hospital intervention can be palliative and end of life care, yet in the audit this service was available in only 13% of Greek hospitals and 5% in Turkey compared with 91% in Ireland and 92% in the United Kingdom.
Furthermore, the European COPD Audit found that hospital adherence to the 2010 GOLD standards varied considerably both within and across countries (Reference RobertsRoberts et al., 2013). Spirometric confirmation of diagnosis was available in just 59% of cases, while even in patients with previous admissions with the same diagnosis 37% had no record of lung function confirmed diagnosis. Further more, of those with a spirometry result recorded, 13% had a result incompatible with the diagnosis of COPD. Taking arterial blood gases on admission, which provides essential information about prognosis and the need for key interventions, was performed in 91.5% cases with an interquartile range (IQR) between hospitals of 78.4% and 98.7% and an IQR between countries of 81.9% and 93.5% (Table 6.2).
As indicated above, diversity of pathways, if not quality of care, for people with COPD admitted to hospital with exacerbations across different health systems is in part the consequence of the different organizational structures that are based on medical models rather than population need. For example, the hospital infrastructure in many countries distinguishes smaller local units and larger regional institutions that are often associated with a university and thus include teaching and research functions. A small number of European countries operate a national respiratory centre of excellence, such as Romania and Slovakia, while elsewhere expertise is spread among several tertiary institutions, including in Spain and the United Kingdom (Reference López-CamposLópez-Campos et al., 2014). The resources and organization of care vary widely, with larger hospitals tending to have a higher number of specialist doctors and offering a wider range of specialist services while not necessarily providing better quality care to patients or improving patient outcomes (Reference López-CamposLópez-Campos et al., 2014) (Table 6.1).
Table 6.1 Selected characteristics of hospital centres participating in the 2010–11 European COPD Audit Variation
| Country | Number of hospital centres participating | Median number of beds per hospital (10th, 90th percentile) | Median catchment population (10 000s) per unit (10th, 90th percentile) | Number of respiratory specialist doctors per unit (10th, 90th percentile) |
|---|---|---|---|---|
| Austria | 47 | 377 (169–1 098) | 10.6 (2.87–25) | 7 (3–12) |
| Belgium | 21 | 450 (240–935) | 20 (6–100) | 5 (3–12) |
| Croatia | 8 | 461 (105–1 191) | 34 (11–75) | 7 (2–13) |
| Greece | 22 | 575 (200–700) | 32.5 (6–15) | 5 (2–6) |
| Republic of Ireland | 11 | 343 (131–851) | 25 (12–50.6) | 2 (1–6) |
| Malta | 1 | 850 | 41.8 | 5 |
| Poland | 38 | 400 (182–1 002) | 25 (5.8–21) | 6 (2–16) |
| Romania | 9 | 185 (118–517) | 47.5 (17–77) | 11 (7–21) |
| Slovakia | 3 | 644 (400–887) | 106 (12–200) | 7 (2–11) |
| Spain | 91 | 460 (150–1 023) | 25 (3.7–99.9) | 8 (3–16) |
| Switzerland | 18 | 245 (161–784) | 15 (3.5–40) | 3 (1–6) |
| Turkey | 20 | 610 (133–1 200) | 100 (9.6–1 000) | 6 (3–14) |
| United Kingdom | 112 | 527 (290–1 000) | 30 (17–55) | 4 (2–8) |
Care experiences and standards of care that people with COPD in European countries can expect when admitted with a COPD exacerbation will depend very much on the particular hospital they present to. Data from both the European and UK audits of hospital COPD care suggest that the number of specialists per 1000 beds is the single most important resource factor in determining outcomes for patients (Reference HartlHartl et al., 2016; Reference PricePrice et al., 2006).
Data further suggest that current service delivery often falls short of international guideline standards and that there is major variation in quality of care not just between countries but equally within them (Table 6.2).
Table 6.2 Quality of COPD care across European hospitals against recommendations of the GOLD strategy document
| Audit standard | Compliance at case level (%) | Absolute case numbers | Median by hospital (%) | IQR by hospital (%) | Median by country (%) | IQR by country (%) |
|---|---|---|---|---|---|---|
| Spirometry result available at admission | 59.4 | 9 513/16 018 | 63.1 | 43.4–83.3 | 64.7 | 49.3–69.9 |
| Arterial blood gas performed at admission | 82.4 | 13 191/16 018 | 91.5 | 78.4–98.7 | 88.1 | 81.9–93.5 |
| Chest radiograph performed at admission | 98.6 | 15 790/16 018 | 100 | 98.6–100 | 99.0 | 98.0–99.4 |
| Controlled oxygen therapy used | 84.9 | 13 602/16 018 | 89.7 | 76.9–97.9 | 85.7 | 79.8–88.5 |
| Short-acting bronchodilator use | 91.1 | 14 594/16 018 | 95.9 | 89.1–100 | 91.4 | 80.3–94.7 |
| Non-use of intravenous methylxanthines | 85.7 | 13 742/16 018 | 96.8 | 83.3–96. | 79.9 | 54.7–97.4 |
| Systemic corticosteroids given | 82.3 | 13 187/16 018 | 87.9 | 77.3–95.0 | 76.9 | 62.7–88.3 |
| Antibiotics given if sputum purulence or IMV | 90.5 | 8 457/9 347 | 93.5 | 85.7–100 | 89.5 | 86.3–93.6 |
| NIV given if pH <7.35 and PaCO2 >6 kPa | 51.0 | 1 133/2 222 | 58.6 | 40–77.8 | 47.0 | 40.9–66.6 |
| IMV given if pH <7.25 and PaCO2 >8 kPa | 15.4 | 73/473 | 50.0 | 33.3–100 | 31.6 | 22.2–44.4 |
| Fulfilled all 10 recommendations | 15.3 | 2 444/16 018 | 16.6 | 9.09–25.0 | 10.1 | 5.18–17.8 |
Post-acute care
There is growing recognition that the hospital has potential to influence out-of-hospital care not just to prevent admission but also to prevent readmission and there are excellent examples of where such influence has major benefits to the patient and to the system. Once a patient has recovered from their acute illness they are usually discharged back to the environment they came from, such as the community or their own home. In some cases, the deterioration in their condition will not have improved enough to allow this to happen and in some health systems a period of convalescence or rehabilitation may be arranged. In other cases this is not an option and a patient may be placed within institutional care, such as residential care or a nursing home. Available evidence supports the use of ESD for selected patients with acute exacerbation of COPD as an effective and safe intervention (Reference EchevarriaEchevarria et al., 2016). Such schemes aim to accelerate discharge from hospital with the provision of continued support in a community setting, typically at the same intensity that would have been provided had the patient remained in hospital, and involving MDTs to prevent (re)admissions. Although countries are increasingly introducing these type of programmes, their availability varies considerably. For example, the European COPD Audit found that 75% of participating UK hospitals offered early discharge support programmes compared to only 37% in Switzerland, the next most frequent user. In many of the participating countries there was no use of such programmes (Reference López-CamposLópez-Campos et al., 2014). This suggests that many patients may be receiving suboptimal care.
There are also concerns about the transition from hospital to community, with patient experience varying both within and between countries. This ranges from simple discharge from hospital without coordination of care post discharge to that of an integrated care system where there is seamless continuity of care with a single organization responsible for both secondary and primary care services with a shared electronic health record (Ribera Reference SaludSalud, 2016). Telehealth may offer opportunities to link the hospital to the patient after discharge and to provide monitoring to ensure clinical improvement but also to then provide early warning signs of a deterioration that initiates an early intervention to prevent readmission (Box 6.5), with telemedicine considered more broadly as an aid to the management of long-term conditions (Reference McKinstry, Pinnock and SheikhMcKinstry, Pinnock & Sheikh, 2009; Reference Hernandez, Mallow and NarsavageHernandez, Mallow & Narsavage, 2014). However, rigorous evaluation is required as in other areas of medicine it has often failed to live up to what has been promised.
Box 6.5 Telehealth teams for monitoring patients with COPD post discharge, Barcelona, Spain
As part of the EU-funded Supporting Healthier and Independent Living for Chronic Patients and Elderly (NEXES), a multidisciplinary telehealth team was established in one of the four health sectors of the city of Barcelona, Spain, to monitor post COPD exacerbation discharge patients. Patients were monitored remotely and had access to regular video conferencing, a dedicated call centre and an online patient management web portal. The call centre was managed by a health coach who might deal with problems directly or refer to the patient’s case manager who in turn could access other services as required, including the GP, other health care professionals or a respiratory specialist depending upon the issue identified. The intervention was associated with significantly fewer hospitalizations among patients with chronic respiratory diseases, reduced in-hospital days for patients in a Home Hospitalization/Early Discharge scheme, and increased quality of monitoring of patients receiving additional support.
Where care provision remains fragmented, alternative approaches to providing more joined-up care include a discharge bundle quality improvement tool (Box 6.6), which promotes a standardized set of processes designed to enhance optimal transition back to the community (Reference TurnerTurner, 2015) and which has been shown to reduce emergency readmission to hospital post discharge (Reference HopkinsonHopkinson et al., 2012).
Box 6.6 COPD discharge care bundle project, London, UK
A care bundle is a structured way of improving the processes of care and patient outcomes. It involves a small set of between three and five evidence-based practices that, when performed collectively and reliably, have been shown to improve patient outcomes. The project involved the design and implementation of a COPD discharge care bundle in northwest London. The bundle includes: (i) smoking cessation advice; (ii) assessment and referral for post-discharge pulmonary rehabilitation; (iii) patient education and self-management plans; (iv) medication review including inhaler technique checks; and (v) assured follow-up post discharge. Evidence from the initial implementation phase suggested that the introduction of the care bundle had reduced readmission rates and improved both staff and patient satisfaction with the discharge process. Further evaluation of the subsequent roll-out of the care bundle to other acute hospitals in London provided further evidence that the introduction of the bundle was associated with a reduction in readmission rates (Reference LavertyLaverty et al., 2015).
Other interventions that can reduce readmission rates and which lie within the influence of the hospital include early pulmonary rehabilitation (Reference PuhanPuhan et al., 2011), while for those with end-stage disease, and a high chance of relapse, advanced care planning may result in the avoidance of future admissions. Evidence suggests that in those cases care provided in the patient’s own home or in a community setting that is more suited to end of life care can be effective in reducing the symptom burden for patients (Reference GomesGomes et al., 2013).
Rehabilitation
As noted above, pulmonary rehabilitation has been shown to be a very cost-effective therapy in COPD (Reference SpruitSpruit et al., 2013; Reference McCarthyMcCarthy et al., 2015). Reported benefits include improved exercise capacity and quality of life, reduced symptoms, anxiety and depression, and enhanced medications effects. Rehabilitation has further been shown to reduce hospitalizations and length of hospital stay as well as improving the recovery after hospitalization because of COPD exacerbation (Reference PuhanPuhan et al., 2016). Components of pulmonary rehabilitation can vary but a comprehensive programme typically includes smoking cessation, exercise training, nutrition therapy, and patient education. Programmes are designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviours. The collaborative approach of multiple provider services working across organizational boundaries to provide rehabilitation at its best can have much wider system impacts as exemplified by the Copenhagen SIKS programme (Box 6.7), which has become a model for locality-based integrated care systems in Denmark (Reference JacobsenJacobsen et al., 2014).
Box 6.7 Integrated effort for people living with chronic diseases (SIKS) project, Copenhagen, Denmark
Set up as a research project for the period 2005–2007, the SIKS project focused on the implementation of rehabilitation programmes for people with type 2 diabetes, COPD, and heart disease or with balance problems following falls, requiring close collaboration between a local health care centre, a local hospital, and GPs. Standard packages of rehabilitation included disease-specific education and patient self-management sessions, a physical training session, nutritional consultation sessions and smoking cessation programmes. The programmes lasted 7–12 weeks depending on the specific disease. Patients were followed up upon completion of the programme. An evaluation of the impact of rehabilitation on health-care utilization found that compared with their matched controls, patients with COPD participating in the programme in the health care centre showed smaller increases in hospital admissions, bed days and outpatient visits over a two-year period that were statistically significant (at 18%, 34%, and 24%, respectively). The SIKS project is reported to have influenced the way integrated care has been conceptualized in Denmark. For example, after completion of the project, health care centres based on the SIKS model were established across Denmark and the experiences informed wider policy development for coordinated care approaches in Denmark.
Despite its demonstrable benefits, rehabilitation after an exacerbation is not widely offered in Europe and elsewhere. Data from the European COPD Audit showed that in 2010–11 pulmonary rehabilitation at discharge was available in just half of participating hospitals, ranging from 91% in Ireland and 88% in the United Kingdom to just 18% in Austria and 20% in Romania (Reference López-CamposLópez-Campos et al., 2014). Also drawing on the European COPD Audit and additional data, Reference SpruitSpruit et al. (2014) reported large differences among pulmonary rehabilitation programmes in mostly high income countries in Europe and North America as they relate to the setting, composition of the pulmonary rehabilitation team, methods of referral and types of reimbursement, among others. For example, in North America the majority of programmes (~70%) were delivered in outpatient settings whereas in European countries this was the case for half of the programmes while another 30% were offered in both inpatient and outpatient settings. There was also substantial heterogeneity in referral practices, in terms of the types of practitioners who refer patients and the types of patients referred, which was attributed, in part, to varying knowledge, attitudes, and perceptions of pulmonary rehabilitation within and across countries, and which may impact on patient outcomes. Importantly, the survey found that only a small number of patients were enrolled in pulmonary rehabilitation across the centres studied, highlighting that a potentially large number of people with potential to benefit from pulmonary rehabilitation are either not referred, not enrolled, lack access, or choose not to participate (Reference Rochester and SpanevelloRochester & Spanevello, 2014).
Workforce
The workforce required to staff the future European hospitals will need to meet the challenges posed by advances in medical innovation and technology, the changing population needs as reflected by older people with complex multiple chronic conditions, but most of all by the impending shortages of clinical staff. While there is no European standard for what constitutes an ideal hospital staffing level to make such a judgement, evidence from large-scale studies suggests that higher numbers of doctors and of nurses per hospital bed correlate with better outcomes for patients (Reference NeedlemanNeedleman et al., 2011; Reference HartlHartl et al., 2016). For example, using data from the European COPD Audit, Reference HartlHartl et al. (2016) found that a higher number of respiratory specialists per 1000 beds reduced the risk of post-discharge mortality for patients with COPD. As we have noted above, the European and UK/England and Wales COPD Audits highlight not only large variations in clinical staff per 1000 beds between countries but also within each country (Reference López-CamposLópez-Campos et al., 2014; Reference StoneStone et al., 2015). This suggests that workforce distribution is not necessarily based upon workload or patient need but is dependent upon other factors that might include local funding, hospital status or specialty and academic interest, geography and social factors, or simply historical models of care.
The optimal management of patients with COPD faces the same challenges as the health care sector does more widely in deploying an appropriately trained workforce, with shortages in some medical specialties, and especially nurses, alongside demographic changes. Countries are experimenting with extended and new roles for nurses in particular to support nurses and physicians working within the hospital system. Such roles include physician associates with a science-based first degree plus a vocational master’s degree who are trained to perform a number of duties, including taking medical histories, performing examinations, diagnosing illnesses, analysing test results, and developing management plans. They are supervised by a senior doctor but take on many of the more routine duties that a physician might otherwise fulfil. Respiratory nurses or physiotherapy specialists are independent practitioners with master’s level or equivalent training in respiratory medicine and often specifically in COPD care. They may be deployed as part of a hospital or joint community team bridging the gap between hospital and community care with in-reach or outreach connectivity. They may lead a multiprofessional team with or without medical input. Key roles are within supported discharge, admission prevention teams and pulmonary rehabilitation. The exercise physiologist is a professional role developed in the United States and now adapted in some European systems. They usually hold a biomedical sciences degree with an additional master’s qualification in exercise physiology, and specializing further in the management of people with chronic conditions, notably heart and lung disease. Exercise physiologists may prescribe and oversee a personalized exercise programme for patients with COPD and may also supervise a pulmonary rehabilitation programme for a larger number of patients with COPD. The ability to plan and oversee tailored exercise programmes raises the potential to extend rehabilitation to those with co-morbidities with perhaps greater confidence than staff trained purely in COPD or respiratory health care. The result is a blurring of traditional responsibilities in an attempt to provide a wider professional team contributing to a competencies-based workforce. The benefits of this trend include a refocusing of roles around the needs of the patient today rather than to continue a pattern of service delivery configured decades in the past, and to provide multiskilled staff who can meet most of the patient’s needs in a single episode of care rather than requiring multiple professionals to input multiple narrow specialized interactions.
COPD teams have been at the forefront of developing new professional roles but there is little consistency of adoption across Europe. The aforementioned European COPD Audit report found that at the time of the study participating hospitals in several countries did not have specialist respiratory trained physiotherapists (Romania, Spain, Turkey), or nurse specialists (Austria, Poland, Switzerland) and while all countries recognized respiratory function technicians as a team member, they were not employed in all hospitals (Reference RobertsRoberts et al., 2013). Even where there are roles with similar titles, their competencies and scope are often difficult to compare because of differences in training, and the clinical systems within which they operate.
Specialist resources
There is no equivalence across Europe in terms of function and size or resource level for hospitals that establishes either a minimum or optimum standard, although there are standards described within international COPD recommendations for the interventions that should be available to patients admitted to hospital with exacerbations of COPD (GOLD, 2017). The patient might reasonably expect to receive the same high quality care wherever they present, accepting that this might not be all provided in one location. A range of factors will determine what can be provided, ranging from geography and accessibility to workforce availability and financial pressures on resource allocation. Within any one country, however, systems that share data and promote real-time interaction between clinicians working separately have the potential to reduce the variation in quality of care that is currently the reality. Life-saving treatments can be administered if patients are appropriately diagnosed and triaged in terms of severity using history taking and clinical examination followed by basic blood tests, arterial blood gas measurement and a chest radiograph which should be available at all hospital sites. Severely ill patients with acute respiratory acidosis need to have access to ventilatory support within a period of 1 to 3 hours of presentation according to management guideline recommendations (Reference Celli and MacNeeCelli, MacNee & ATS/ERS Task Force, 2004; Reference VestboVestbo et al., 2013). This is one key and potentially life-saving intervention outside the basic level of care that can be provided in all locations, i.e. antibiotics, steroids, bronchodilators and oxygen therapy. NIV, while currently often delivered by respiratory specialists, is also managed in some countries by anaesthetists, and some services are led by specialist nurses or physiotherapists who could be supported remotely by specialist doctors if not available on site (Reference Bierer and So HooBierer & Soo Hoo, 2009; Reference PintoPinto et al., 2010; Reference CabriniCabrini et al., 2015; Reference AmbrosinoAmbrosino et al., 2016). Invasive ventilation required for those who fail on non-invasive support or where there are other factors making this the more appropriate intervention is more complex and is nearly always delivered by anaesthetic-trained staff in an intensive care unit setting. Ideally such facilities – i.e. the equipment, monitoring facilities, the staff and the specialist unit – should be available in every hospital admitting COPD exacerbations or be accessible by rapid site transfer. This is not the case at present (Reference RobertsRoberts et al., 2013; Reference López-CamposLópez-Campos et al., 2014).
For the subacute situation all hospitals should also provide diagnostic facilities available to hospital, primary care and community physicians that will ensure accurate diagnosis of COPD. These would include lung function testing and imaging, notably chest radiography and CT scanning. Advice from an expert clinician would be helpful in making the more difficult diagnostic cases where other conditions may exist as co-morbidities or as differential diagnoses.
Stable patients at the advanced stage of the disease will require more complex investigation and interventions that may include LVRS and potentially, in a very small number of cases, lung transplantation. Such patients would be referred to a specialist centre with specific expertise in these techniques and with the expensive equipment and clinical staff available. Once again the implementation of technological solutions would provide opportunities for patients in this situation to be considered regardless of their physical location by the transmission of images, electronic patient records and by video interviews between clinicians and patients. In this manner a hub and spoke model provides an efficient and effective use of resources.
In summary, a technological interconnectivity of hospitals provides an opportunity for all patients to access specialty opinions regardless of their location and situation. Critical to good patient care will be establishing the correct diagnosis at an early stage and, for patients admitted to hospital, early access to assisted ventilation if needed and then prior to discharge a comprehensive care package that will reduce the progression of the disease and risk of further admission. A small number of highly specialized units could provide nationally available expertise to all while networked to a larger number of more local provider units. These in turn, or through the national specialty units, could also provide networked support to both primary care health professionals and to patients and carers facilitated by technological solutions.
Barriers to delivering optimal care
Optimal care can be defined as a composite of evidence-based and consensus-based interventions that promote good outcomes for patients, and guidelines, or in a resource-constrained system might more productively be considered as the appropriate implementation of these interventions within a value-based hierarchy. Unlike for most chronic medical conditions, this value-based approach is well documented for COPD and it provides a useful reminder to clinicians of their responsibilities to the system as well as to the patient (Figure 6.3).

Figure 6.3 The pyramid of value for COPD interventions
Implementing optimal care is a multifaceted challenge with the need to modify clinical behaviours and a culture that requires a shift in the balance of control towards patients and away from clinicians. The European evidence relating to the quality of care offered by hospitals confirms that the complex interactions that constitute an organization account for the majority of the variation between units (Reference RuparelRuparel et al., 2016). The principle of value to the system is equally valid when applied to a hospital as it is to the care offered to an individual patient. The evidence that resource-rich organizations perform better in delivering high quality COPD care is relatively weak and although there are some associations between medical staffing levels and better care outcomes (Reference RobertsRoberts et al., 2013; Reference HartlHartl et al., 2016), there is no direct evidence of a link with other inputs. However, it is concerning that European audits reveal that some elements of treatment that have been shown to benefit patients with COPD are unavailable in many of the hospitals that care for these patients.
A useful example of the complex organizational interactions that account for some unwarranted variations in care is the degree to which specialist COPD care is offered to patients within any institution. Much care of patients with COPD in hospitals is delivered by non-respiratory specialists and the evidence is that generalists are less likely to deliver optimum COPD care to their patients with COPD (Reference HoskerHosker et al., 2007; Reference López-CamposLópez-Campos et al., 2015). At a population level, most COPD care is delivered out of hospital by generalists, while most of the expertise remains locked within hospital buildings. Providing greater access to that expertise both within and outside the hospital is an important facet of delivering optimal care. Sadly, patients themselves are unlikely to understand what good care looks like and are therefore unlikely to be able to negotiate high quality care with their health teams. Better-informed patients might drive better COPD care.
Until regular measurement of care quality becomes a routine element of clinical care it remains difficult to identify areas of excellence or those where improvement is required. Engaging clinicians in reviewing performance data is a key challenge but if successful promotes the improvement of clinical practice (Reference FlottorpFlottorp et al., 2010). Leadership is required if Europe is to move forward in redesigning hospital care for patients with COPD. That must come from the health professions and from politicians. At present, there is no functional European health profession voice to provide that leadership and little evidence of a united political will.
COPD and the future hospital – summary
The hospital of the future is likely over time to be admitting sicker and frailer patients with COPD exacerbations. The evidence for ambulatory care as an alternative to admission (Reference RamRam et al., 2004a) and early discharge once admitted is compelling (Reference EchevarriaEchevarria et al., 2016) but will not be appropriate for all individuals who require a greater level of support. Particularly in health systems with under-developed primary care, these measures offer huge potential benefits to hospitals in the future. Such a hospital would be a central hub, supported by technology that could provide a learning and education resource supporting patient self-management (Reference SmidthSmidth et al., 2013) for a large population, over a geographical area well beyond its historical area of influence. Patients at risk of acute deterioration and admission could be directly linked to a COPD clinical monitoring team to provide the opportunity for early interventions to improve patient well-being (Reference McLeanMcLean et al., 2011). Patients would be managed at distance, gaining specialist expertise without the need to regularly travel to hospital appointments (Reference D’AnconaD’Ancona et al., 2014). Clinicians too could be connected using digital communication, sharing patient clinical records, laboratory results, and imaging, and holding multidisciplinary discussions with colleagues via video conferencing and email to provide wider access to expertise extending well outside the physical buildings of the hospital itself. The challenge has always been how to provide equality of access to higher standards of care regardless of geography. Providing a technological network of the highest level of expertise available to all provides an opportunity to make progress towards that ideal while managing more people in an out-of-hospital setting.
Introduction
Emergency medicine specializes in acute illness and injury (Reference EdwardsEdwards, 1996; Reference TangTang et al., 2010). It has developed from the realization that these conditions can occur at any time and that dealing with many of these events within more traditional medical specialties led to suboptimal care. Recently the increasing relevance of time-critical interventions such as those associated with complex trauma care, stroke thrombolysis and sepsis therapies have further underlined the importance of a clinically broad but temporally focused specialty. The provision of emergency care is therefore a key function of most major hospitals.
Irrespective of most other health system features, patients with acute severe illness or injury present to or are taken to an emergency department (ED). In the United Kingdom these departments have been officially termed accident and emergency (A&E) departments since the Platt report of 1961 (Anon, 1961). However, accidents and associated injuries have diminished in frequency across western Europe, with a particularly large reduction in injuries associated with road traffic accidents, interpersonal violence and occupational activities (Eurostat, 2017). Consequently, the mix of patients presenting to the ED or A&E has changed significantly over the last 50 years. Case volumes have, however, continued to rise in excess of population growth, especially since 2000; the reasons for this vary but most countries have seen an increased demand for the treatment of acute exacerbations of chronic disease and conditions associated with frailty and ageing (see Chapter 4). Ageing and population growth do not fully explain this growth in demand.
It is increasingly recognized that the presentation of illness is not related solely to aetiology or pathology and it is apparent that we are witnessing a changing utilization of emergency medical systems by patients of all age cohorts.
This chapter looks at the branch of medicine responsible for the initial treatment of many of these emergencies and the emergency departments within the acute general hospitals in which they are usually based.
The development of contemporary emergency medicine
Emergency medicine is a relatively new specialty. First formally recognized in the United Kingdom and the USA in the mid-1960s, it has undergone major changes in many countries since then (Reference Totten and BellouTotten & Bellou, 2013). Often starting as a service that provided triage and emergency treatment for victims of injury, modern emergency medicine covers the early management and investigation of a broad range of conditions in addition to trauma, from infectious diseases to psychiatric illness (Box 7.1). Most recently the specialty has evolved in response to pressures to specialize and standardize with demonstrable improvement in outcomes. For conditions such as sepsis, major trauma and stroke it has become increasingly clear that early expert intervention makes a significant difference (Reference CameronCameron, 2014).
Box 7.1 Defining emergency medicine
Emergency medicine has been defined as “a field of practice based on the knowledge and skills required for the prevention, diagnosis and management of acute and urgent aspects of illness and injury affecting patients of all age groups with a full spectrum of episodic undifferentiated physical and behavioural disorders; it further encompasses an understanding of the development of pre-hospital and in-hospital emergency medical systems and the skills necessary for this development” (International Federation for Emergency Medicine, 2018).
“It is a specialty in which time is critical. The practice of Emergency Medicine encompasses the pre-hospital and in-hospital triage, resuscitation, initial assessment and management of undifferentiated urgent and emergency cases until discharge or transfer to the care of another physician or health care professional.” (European Society for Emergency Medicine, 2007)
In some countries it became common for patients admitted as acute emergencies to be dealt with in a single specialist emergency department rather than in different wards, clinics and departments within the hospital. This provided a rationale for the development of a separate specialty. There were also historic factors, including the development of major trauma care first developed in North America, in part in response to experience gained in the Vietnam War, spreading first to the British Isles in the 1980s and adopted more widely in Europe after 1994.
The provision of emergency medical care varies widely between and, at times, within countries. Internationally, two principal models have evolved, the continental European model (sometimes referred to as the “Franco-German” model) and the Anglo-Australasian-American model (Reference ConeCone et al., 2015). In continental Europe emergency care tends to focus on the critical care end of the spectrum and the management and investigation of emergencies requiring hospital care is undertaken by inpatient specialties. In this model the most seriously injured and ill are typically attended to by anaesthetist-led teams at the scene and often receive extensive treatment before transfer to the operating theatre, intensive treatment unit, or medical or surgical ward. In the Anglo-Australasian-American model treatment at the scene is usually more limited and predominantly paramedic led; the emphasis is on rapid transport to an appropriate hospital where emergency department clinicians are responsible for the investigation and management of the patients and the consequent decision to discharge or admit them.
Proponents of the European model assert that better outcomes are achieved when organ-specific specialists lead the investigation and treatment of patients. The Anglo-Australasian-American model contends that most patients do not arrive with an organ or even a specialty-based diagnosis but have multiple and often interacting conditions. However, the models have in general arisen not as a reflection of deliberate policy choices based on these views but because of circumstance, resource availability and history.
There are no high quality comparative studies of different systems. Moreover, most countries operate some components of each model. Thus, trauma care has evolved to reinforce pre-hospital interventions and direct transfer to major trauma units and centres, whereas patients who meet criteria to consider sepsis are often insufficiently “labelled” to allow triage to particular hospitals or even inpatient teams.
The emergency medicine care pathway
Ideally the emergency care system should commence at the first point of contact between the patient and a clinician or emergency call system. Current systems, though far from mature, aim to coordinate services to provide the most appropriate response to the medical needs of the patient. Where this is achieved, such a system should reduce the inappropriate use of hospitals, ensure that patients who require urgent care receive it promptly, and make best use of limited resources.
Entering the emergency department
In most countries significant numbers of patients self-present to the emergency department. The remainder attend following advice or referral from a general practitioner (GP) or other clinician, by way of a telephone triage service, or following an attendance and subsequent conveyance by an ambulance. In Denmark and Norway patients are required to have sought advice from the GP or ambulance service prior to coming to the emergency department. In the Netherlands this approach is strongly encouraged by the use of insurance deductibles and by providing very accessible general practice services.
Most countries now have a telephone service dedicated to providing urgent health care advice and signposting. The United Kingdom uses lay advisers assisted by computer algorithms; these do not attempt to provide a diagnosis but instead lead to a recommended course of action (disposition). The limitations of this approach and the consequent over-triage to both GP and emergency department care has led to a recent commitment to increase the proportion of calls handled by a clinician, as happens routinely in the systems operated in Sweden and Denmark. All these systems aim to ensure that patients are directed to the right point of care without unreasonable delay or duplication of effort, although the extent to which they achieve this can be unclear (see below).
The Franco-German model has traditionally put doctors in ambulances or cars as a key part of the immediate response. There are some signs of convergence between the United Kingdom model and Franco-German models of pre-hospital care, with initiatives to “front-load” more highly trained clinicians (doctors, paramedics and advanced nurse practitioners), either as staff on an ambulance or dispatched by car from a hospital. The evidence from the United Kingdom for better patient outcomes from pre-hospital deployment of doctors is very limited. The use of medically staffed helicopters for this role has grown significantly but there are major concerns about their cost-effectiveness (Reference BledsoeBledsoe et al., 2006; Reference Butler, Anwar and WillettButler, Anwar & Willett, 2010; Reference DelgadoDelgado et al., 2013).
The ambition of all systems is to bring triage and immediate treatment to the earliest safe and effective point in the pathway. In consequence it is expected that the development of ambulances staffed with personnel and equipment that allow greater assessment and evaluation skills will enable these same services to either treat and discharge more patients at the scene or convey them to non-hospital providers of health care.
Paramedics can also transport patients to specialist units directly, bypassing non-specialist hospitals and in some cases the emergency department. Hyperacute stroke centres (Reference RamsayRamsay et al., 2015), trauma units and specialist ST elevation myocardial infarction units have evolved across Europe over the last 10 years. Accessing these facilities without intermediate delays has seen an improvement in outcomes that has more than offset the consequential increased journey time, although in many countries only limited progress has been made in achieving change in structures and processes (Reference AlbrechtAlbrecht et al., 2017).
These “condition-specific” diversion protocols require either autonomous paramedic practice or make use of telephone/online support from a specialist clinician based at the hospital. In the United Kingdom enhanced paramedic training and autonomy has enabled up to 40% of emergency ambulance calls to be managed without transport to an emergency department (National Audit Reference OfficeOffice, 2017).
In a number of countries (including the Netherlands, Spain, England, and Norway) primary care centres staffed by GPs or nurses can offer a range of treatment for minor injuries and minor ailments.
Minor injury units were associated with many community hospitals in the United Kingdom even before the creation of the National Health Service (NHS). These units have been variously staffed by GPs and nurse practitioners. The effectiveness and utility of such units, especially in more rural settings, has become increasingly recognized.
In urban areas of the United Kingdom the recognition that at least 20% of patients attending an emergency department can be better dealt with by primary care staff has incentivized the development of co-located primary/urgent care services. Such facilities can decongest the emergency department. These are also an increasing feature of the Dutch system.
The hospital emergency department
The emergency department provides care in the first phase of almost all acute medical episodes that are of a severity sufficient to require hospital resources. Traditionally this was not the case. For most of the 20th century, the majority of acute admissions were seen and assessed by a GP/family doctor who arranged both transfer to hospital and direct admission to an appropriate ward under an inpatient specialty team. In 2017 fewer than 25% of admissions were managed in this way in the United Kingdom; 75% of acute admissions now enter the hospital via the emergency department.
By necessity, emergency departments usually offer care to all types of acute illness and injury, physical and mental health problems, and to all ages. However, the way services are organized varies considerably within and between health systems. For example, obstetric emergencies are usually seen in maternity units, while isolated mental health problems may be seen in a geographically separate mental health facility. Paediatric attendances represent one in four emergency department visits (Reference TangTang et al., 2010) and although few in number in the United Kingdom, specialist paediatric emergency departments are common in other parts of Europe, especially in France.
Triage is widely used in emergency departments to prioritize cases so that those with time-critical conditions and greatest symptom severity are treated first. Although formal systems have been evaluated and shown to be reliable they do have significant over and under triage consequences with both resource and risk implications (Reference ParentiParenti et al., 2014).
Ideally triage would not be required because patients could be treated immediately. “See and treat” approaches (Reference ParkerParker, 2004) aim to use triage resources to treat patients rather than risk managing the queue. Such approaches may be difficult to maintain during periods of peak demand.
Senior physician involvement in the initial assessment of all attenders has benefits for ED performance (Reference AbdulwahidAbdulwahid et al., 2016) and quality of care (Reference OredssonOredsson et al., 2011). This, however, creates a paradox in resource-constrained services with the least ill or injured being assessed by the most senior clinician.
There is only limited published evidence that streaming of patients into different tracks, performing laboratory analysis in the emergency department, or shifting responsibility for ordering certain radiological investigations to nurses results in shorter waiting time and length of stay (Reference OredssonOredsson et al., 2011), although this is likely to reflect the absence of rigorous research rather than the lack of any effect, as their advantages are intuitive.
The performance of some of these models may also depend on local circumstances – for example, streaming of primary care type patients may be of limited value where most patients see a GP prior to attending, but may be very useful in circumstances where access to local primary care is poor. Data from the United Kingdom indicate that there is considerable variation in the proportion of patients whose needs can be better addressed by primary care clinicians (15% to 40%) (Reference Moulton, Mann and TempestMoulton, Mann & Tempest, 2014). This is an area in which further research and evaluation is required.
Resuscitation is a core component of all emergency medicine systems. In most cases resuscitation of the most seriously ill and injured will be led by an emergency medicine team. They will usually be supported by other specialties – in particular, anaesthesia, intensive care, surgery, and orthopaedics. In some European systems the intensive care team is responsible for resuscitation. Nevertheless, irrespective of the lead clinicians the process of care of seriously ill and injured patients is becoming more standardized with evidence-based guidelines for the management of cardiac arrest, major trauma, paediatric resuscitation and other severe illness such as septic shock.
Improvements in road safety measures, falling levels of interpersonal violence, improving workplace safety, and reductions in suicide (European Association for Injury Prevention and Safety Promotion (EuroSafe), 2013) mean that while major trauma is an important component of emergency medicine, its share of the work of the emergency department is decreasing. In the United Kingdom major trauma accounts for less than 1% of emergency department attendances. It was therefore apparent by the 1990s that it was neither appropriate nor feasible for every emergency department/acute hospital to maintain and deliver high quality trauma care. In consequence trauma services were reorganized into a tiered response with a network of trauma units acting as spokes to the major trauma centre hubs with a demonstrable improvement in outcomes (Reference CelsoCelso et al., 2006). In 2013 results from the Trauma Audit and Research Network (TARN) national audit show that one in five patients who would have died before the networks are now surviving severe injuries (Reference McCulloughMcCullough et al., 2014).
The formal designation of trauma centres in Europe has been a slower process than in the USA, which may reflect a lower incidence of severe trauma – in particular the much lower incidence of penetrating trauma associated with gun and knife crime. There are also significant historical, logistical and political difficulties in ensuring that the wide range of specialist services required for an integrated trauma centre are located on the same site. An issue in eastern Europe and the countries of the former Soviet Union is the siting of specialist institutes on different sites that would need to be relocated to create an integrated trauma service.
A significant proportion of patients attending an emergency department will require hospital admission. In the United Kingdom this varies from 15% to 35% depending on case-mix. The proportion is higher in the Netherlands and higher again in Norway. Of those not requiring admission a proportion will require a short period of observation, further investigation or time to establish the efficacy of initial treatment. Historically such patients were admitted to a co-located observation ward. Such units continue to provide appropriate care for many patients attending emergency departments, including those recovering from procedures requiring sedation or anaesthesia, or awaiting specialist interpretation of radiological investigations, such as computerized tomographic pulmonary angiograms. Other patient groups that benefit from such a facility include elderly patients who have fallen and are being assessed by therapists and frailty teams (see Chapter 4).
In many countries people with mental health problems requiring an emergency response are taken directly to mental health units. Patients who have self-harmed and need medical treatment for poisoning or injuries may need to attend the emergency department for assessment and treatment of their physical injury or toxicological emergency. For this reason many health systems have established specialized mental health teams based in the emergency departments.
In the United Kingdom recent systems of “street triage” for mental illness have shown a reduction in ED attendances and the number of compulsory detentions (Reference Dyer, Steer and BiddleDyer, Steer & Biddle, 2015). This system combines police, ambulance and a mental health clinician in a single response vehicle. These initiatives have been shown to lead to improvements in the quality of care of mental health patients, reduced delays and have delivered significant resource savings (Reference TadrosTadros et al., 2018).
Emergency departments have an increasing role in secondary prevention. This may be at an individual patient level, e.g. detection and intervention for high risk alcohol intake, or at system level, e.g. injury surveillance to detect trauma hotspots or emerging causes of trauma.
Admission and post-acute care
In some countries, such as the United Kingdom and the Netherlands, many patients who require further investigation and care are moved to acute medical or surgical assessment units. These units may be run either by internal general medicine specialists or by the emerging specialty of acute medicine. These units have a very active approach to treatment with the aim of further front-loading senior review to optimize care and reduce length of stay.
Patient flow is a major issue for many emergency departments. In particular, inability to move patients who require admission into the right hospital bed or promptly arrange a safe discharge home can substantially delay care and impede efficiency. When patients require admission it is highly desirable that they are admitted to a bed managed by the specialty appropriate to their condition. It is clear that admission to a bed that is available but inappropriate is associated with higher mortality (Reference George and WilkinsonGeorge & Wilkinson, 2016) and increased lengths of stay.
There are also problems in the emergency department if admission is delayed because of lack of bed capacity; staff become stretched as they have to assess and care for new patients as well as those awaiting admission. There is evidence from Canada, Australia and the United Kingdom that high levels of emergency department crowding are associated with treatment delays (Reference GaieskiGaieski et al., 2017) and increased mortality (Reference SunSun et al., 2013; Reference Filippatos and KarasiFilippatos & Karasi, 2015).
Recognition of the iatrogenic harm caused by delays has encouraged greater scrutiny of patient flows and attendant obstacles to prevent or minimize such risks. While there are many internal hospital processes that can facilitate patient flow, effective discharge is particularly important. Shortages of nursing homes, intermediate care, home care and other support have a significant impact on the effectiveness of the emergency care system.
Discharged patients may be referred for follow-up by other specialties, such as follow-up of patients with fractures by the orthopaedic service. Others will be referred back to their GP and many will be discharged with no requirement for further follow-up. It is increasingly recognized that the ability to discharge a patient with a referral to a specialist clinic within 48 hours is both expedient and usually preferable to admission for many patients.
Workforce
The model and levels of staffing of emergency departments vary considerably depending on a combination of history, primary care provision, and the availability of emergency medicine specialists to provide dedicated staffing.
The Franco-German model has generally used junior medical staff, GPs and nurses with early senior specialist review of most cases before further investigation and treatment. By contrast the Anglo-American-Australian model has supported the development of emergency medicine specialists, with referral of about 25% of patients to specialty teams after assessment, stabilization, investigation and treatment has commenced.
The rising workload has outstripped the ability of the United Kingdom, Ireland and New Zealand to provide sufficient fully trained emergency medicine doctors to manage the service safely and within various process targets. Consequently, various strategies to make better use of other staff groups have been introduced. Such staff include advanced nurse/clinical practitioners, physician associates, paramedics and frailty practitioners. However, the changing nature of the case-mix related to an ageing demographic and the attendant problem of multimorbidity has created real problems for the Franco-German model. Patients presenting with single illness issues represent a minority of patients requiring admission; for this reason reliance upon traditional specialist inpatient teams is increasingly malaligned to patient need.
The role of the senior emergency medicine doctor varies among countries. The main determinant seems to be the number of senior doctors within a department at any one time. Many studies have shown the advantages of early senior intervention in a wide range of conditions improving outcomes and increasing admission avoidance (Reference PurdyPurdy, 2010). Senior staff leading and supervising cases in the resuscitation room is almost universal but there are varying approaches to the deployment of senior staff within the remainder of the emergency department. The most common models of this role are:
Delivery – where the senior staff see and treat patients throughout their care.
Instigation – where the senior clinician undertakes a rapid assessment and defines a plan which is then implemented by junior staff.
Attending – where junior staff see the patients initially and then check with senior staff before referral or discharge.
Consulting – where junior staff see the patient and ask for help when they perceive the need.
Emergency nurse practitioners are now well established in the United Kingdom but their acceptance in other countries is limited. They can provide a safe and effective minor injury service, although in some departments they may be more expensive than a junior doctor model as they are relatively well paid and may take longer to complete their work (Reference SakrSakr et al., 2003; Reference WilsonWilson et al., 2009). The barriers to implementation are related to emergency department culture, physician reimbursement systems and case-mix. In the United Kingdom advanced clinical practitioner programmes have been established to develop nurses, paramedics and pharmacists to become autonomous practitioners seeing a wide range of cases in the emergency department (Reference SwannSwann et al., 2013). Other somewhat niche roles have also been developed, such as emergency department practitioners (with a background as anaesthetic assistants/operating department practitioners) who can undertake investigations and invasive procedures in the resuscitation room.
The use of geriatricians in the emergency department has been shown to be effective at reducing admissions and is not associated with a high readmission rate (Reference Jones and WallisJones & Wallis, 2013). This may be better in a dedicated unit rather than in the main emergency department (Reference Sophia and BashirSophia & Bashir, 2014) and if supported by a wider MDT to facilitate assessment and discharge.
Every emergency department also needs support from imaging and pathology services, although there is an increasing use of near-patient testing (see Chapter 10). Turnaround times can be reduced but quality control may be more difficult and near-patient testing is often relatively expensive (Reference AshaAsha et al., 2014; Reference Larsson, Greig-Pylypczuk and HuismanLarsson, Greig-Pylypczuk & Huisman, 2015). Certain other specialties are often considered mandatory to support any emergency department that receives undifferentiated emergency cases; these include anaesthetics, intensive care, general medicine, general surgery, orthopaedic trauma, and paediatrics.
It is increasingly recognized that emergency medicine can be highly stressful (Reference BergerBerger, 2013) with consequent challenges for both recruitment and retention. High demand and low job control were found to be common in a systematic review but other factors included insufficient support at work, an imbalance between effort and reward, and organizational injustice (Reference Basu, Qayyum and MasonBasu, Qayyum & Mason, 2016).
Barriers to delivering optimal care
The key barriers to delivering optimal care are: rising levels of demand; inefficient use of resources; and downstream delays in the system which lead to queues and consequential overcrowding. These are compounded by problems arising from the physical design of some emergency departments and the workforce challenges discussed above.
Managing demand
It has been clearly demonstrated that difficulty in accessing primary care is related to higher emergency department attendances. Conversely, systems that have easy timely access to primary care have less emergency department usage. Similarly many emergency department attendances are preceded by unsuccessful attempts to obtain a primary care appointment. In some studies interventions to improve primary care access have resulted in reduced emergency department attendances (Reference WhittakerWhittaker et al., 2016), although extending the hours primary care is available has a limited effect and may not be cost-effective. For those systems where the emergency department receives substantial numbers of primary presentations the provision of co-located primary care facilities has been effective in decongesting the emergency department itself. In England alternative sites, such as urgent treatment centres, have been developed so people can be assessed and treated in a lower acuity setting than an emergency department.
Telephone advice lines that aim to direct the user to the most appropriate location in an appropriate timescale do not seem to reduce the workload to emergency departments and there is some evidence they increase overall demand (Reference TurnerTurner et al., 2013; Reference CollinsCollins, 2015). This may be improved by involving doctors and other clinicians in the call decision-making (Reference Anderson and RolandAnderson & Roland, 2015).
Good chronic disease management may be able to reduce the number of acute episodes if better control of the patient’s condition can result in fewer episodes of acute need, even if the general trajectory is one of deterioration, although this is rarely easy. Equally important is the early detection of, and intervention for, deterioration. A key part of this is patient education and a good understanding of their disease, together with an agreed plan ranging from self-care (e.g. home-held antibiotics for COPD), to seeking urgent advice before deteriorating further. Support for nursing and residential homes can also avoid attendances at the emergency department, by ensuring optimal ongoing care, good end of life planning, and the use of telehealth advice before calling an ambulance (Reference NickNick et al., 2015).
There is no evidence to support the idea that public information campaigns can reduce attendance at emergency departments. For most people it is a rare event for a different condition each time, and the appropriateness of attendance depends not only on medical considerations but also factors such as availability of alternatives and social support. Some disease-specific education campaigns have increased the early recognition of acute episodes but invariably have a high cost of false positives with consequent increase in emergency department attendances. Diverting people away is unlikely to be effective as most people believe the emergency department is the correct location for their care (Reference Anderson and RolandAtenstaedt et al., 2015) and there are some significant risks.
Although often advocated as a means to reduce demand, there is no evidence that co-payment schemes reduce inappropriate attendances (Reference ReedReed et al., 2005; Reference Selby, Fireman and SwainSelby, Fireman & Swain, 1996; Reference Siddiqui, Roberts and PollackSiddiqui, Roberts & Pollack, 2015) and they bring many other problems, often costing more to operate than they raise, while deterring necessary care. In Ireland attendances at emergency departments have increased significantly despite a co-payment system designed to encourage primary care use.
Finally social changes are also impacting on emergency departments. In the United Kingdom the “liberalization” of the alcohol licensing laws in 2005 has produced a wholly predictable increase in alcohol-related presentations. The public, media and politicians increasingly expect care seven days a week and 24 hours per day; this in turn has produced a disproportionate increase in demand during “anti-social” hours with major consequences for staffing.
Queues and overcrowding
Demand for emergency care is subject to high levels of variability – both seasonal and at different times of day and days of the week. Although there is some predictability to this, average hourly attendances are subject to wide variation (greater than 50%) (Reference BluntBlunt, 2014). Consequently, systems need to be able to deal with surges in demand and hence there needs to be some redundancy built into staffing and the physical environment. The situation is made worse by the fact that activity in planned care is often even more variable. These variations in demand, both predictable and random, combined with capacity constraints associated with historically high bed occupancy rates, ensure that emergency departments are prone to significant overcrowding during peak periods. This phenomenon is endemic to health systems in many countries, including Ireland, the Netherlands, South Africa, the United Kingdom and the USA – indeed, it is the one almost invariable feature of all current health care systems! Avoiding overcrowding requires better alignment of resources to demands. Within the emergency department this means speedy responses for requests for specialist consultation, access to diagnostics, enhanced bed availability, and prompt discharge when hospital care has been completed.
Within the wider hospital, delays in decision-making, investigation, discharge planning and discharge will mean that beds are not available to admit patients. This can lead to extensive overcrowding. Emergency departments are seldom staffed or designed to deal with a large group of patients awaiting admission. As a result standards of care deteriorate, key interventions are delayed or omitted, and both morbidity and mortality rise (Reference ForsterForster et al., 2003; Reference GuttmannGuttmann et al., 2011; Reference BodenBoden et al., 2016).
Effective design
The design of emergency departments needs to support effective and efficient function and many national guidelines exist (Department of Health and Social Care, 2013). Good design can also improve user experience and reduce aggression (Design Reference CouncilCouncil, 2011). But because buildings persist longer than any models of care, it is important to include flexibility in design to allow for increases in attendances but also to support new and future models of care. This design also needs to address the specific requirements of a range of groups including children, the elderly, those with cognitive impairment, those with mental health problems, and those with infectious diseases.
The future
Prevention measures have had sustained and large effects. Road safety initiatives – including safety belts, crash helmets, speed control, car design, and alcohol limits – have all contributed to the reduction in deaths and injury from vehicle incidents, especially in developed countries. Other health and safety interventions, especially in building design and the workplace, have also reduced the trauma demand in many emergency departments. Trauma is therefore a decreasing component of the workload in many emergency departments and its nature is also changing with the growth of an older population increasing the importance of injuries from falls (Reference KehoeKehoe et al., 2015; Reference SivarajasingamSivarajasingam et al., 2016).
The increase in population size, the current and projected disproportionate increases in the numbers of patients in the ninth decade of life and the consequent importance of managing frailty, co-morbidities, and cognitive impairment are pressing challenges.
Specialization of health care and the growth of new technologies over the past 40 years have delivered extraordinary improvements in patient care and outcomes but this trend now means that seldom can a single inpatient specialist or team deal with the majority of medical or surgical emergencies. Consequently there is a need for more precise diagnosis by the emergency medicine clinician before referral. This has substantial resource implications both for the emergency medicine workforce and the support services of pathology and diagnostic imaging. The development of time-critical interventions similarly requires sufficient resources to deliver such treatments on a 24/7 basis. New staff roles can be developed to help support this – not just as extensions of the roles of nurses and other professionals. Where emergency medicine grows, it will be important to ensure that other specialisms withdraw from offering more general support for emergency care.
Crucially, the need to maintain minimum caseloads to sustain expertise means that often subspecialty services will be reorganized to fewer centres. In the future it is likely that common non-complex conditions will be treated in most hospitals but for less common/more complex conditions and treatments patients will need to be transferred to larger centres. Inevitably such patients will continue to present to any emergency department. Properly configured and resourced networks (akin to those established for trauma) will need to be established and supported to ensure optimal care and appropriate transfer for all patients.
Approximately 15% of all emergency hospital admissions in England involve the 1% of people in their final year of life. There is more to do to ensure that patients who are at the end of life do not spend their last hours in an emergency department through appropriate advanced planning and ensuring that ambulance and other services have information available about these plans immediately available.
Finally Denmark, the Netherlands and the United Kingdom have all seen significant reductions in the number of emergency departments, with more expected in other countries. In addition, some departments may shut to ambulance attendances overnight. The drivers for these changes include the general trend to hospital regionalization, optimal utilization of the scarce emergency medicine workforce, and the need to ensure on-site provision of many other services to support the delivery of 21st-century care that can deliver optimal outcomes.





















