Soils are the dominant terrestrial sink for carbon, containing three times as much C as above-ground plant biomass, and acting as a host for both organic and inorganic C, as soil organic matter and pedogenic carbonates, respectively. This article reviews evidence for the generation within the soil solution of dissolved C derived from plants and recognition of its precipitation as carbonates. It then considers the potential value of this process for artificially-mediated CO2 sequestration within soils. The ability of crops such as wheat to produce organic acid anions as root exudates is substantial, generating 70 mol/(y kg) of exuded C, equivalent to the plant's own ‘body weight’. This is still an order of magnitude less than measured C production from Icelandic woodlands (Moulton et al., 2000), which have no other possible source of C. Thus, there is apparently no shortage of available dissolved C, as bicarbonate in solution, and so the formation of pedogenic carbonates will be controlled by the availability of Ca. This is derived from mineral weathering, primarily of silicate minerals (natural plagioclase feldspars and pyroxenes; artificial cement and slag minerals). Within the UK, existing industrial arisings of calcium silicate minerals from quarrying, demolition and steel manufacture that are fine-grained and suitable for incorporation into soils are sufficient to account for 3 MT CO2 per year, compensating for half of the emissions from UK cement manufacture. Pursuing these arguments, it is shown that soils have a role to play as passive agents in the removal of atmospheric CO2, analogous to the use of reed beds to clean contaminated waters.

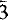 ,
, 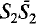 double sheets, with composition (Si
double sheets, with composition (Si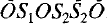 …; the corresponding composition is [Ca
…; the corresponding composition is [Ca