Epidemiological studies have shown that the incidence of chronic degenerative diseases, especially coronary heart disease, osteoporosis and some cancers, is lower in the Mediterranean basin (Keys et al. Reference Keys, Aravanis, Van Buchem, Blaackburn, Buzina and Djordjevic1981; Kanis, Reference Kanis1993; Gerber, Reference Gerber1994). The beneficial effect is attributed to specific Mediterranean practices, even though other protective factors may be involved. The eating pattern highlighted refers to a diet with an increased consumption of olives, fruits and vegetables, nuts and cereals, and large quantities of olive oil (Keys, Reference Keys1995). These food items contain a variety of compounds with an antioxidant and anti-inflammatory capacity, such as polyphenols. Many in vitro and in vivo studies have been performed to elucidate mechanisms by which polyphenolic compounds may act to confer positive health effects. Investigations have shown that they are potent inhibitors of LDL oxidation in vitro (Keys, Reference Keys1995; Fito et al. Reference Fito, Covas, Lamuela-Raventos, Vila, Torrents, de la Torre and Marrugat2000). Those molecules have also been found to exhibit activity in breaking peroxidative chain reactions and preventing metal ion chelation (Manna et al. Reference Manna, Galletti, Cucciolla, Moltedo, Leone and Zappia1997). In addition to their antioxidant properties, polyphenols have been implicated in the inhibition of enzymes involved in the inflammatory process (Kohyama et al. Reference Kohyama, Nagata, Fujimoto and Sekiya1997) and the inhibition of platelet aggregation (Petroni et al. Reference Petroni, Blasevich, Salami, Papini, Montedoro and Galli1995).
Despite, however, the myriad of potential health benefits of the olive oil polyphenolics, there are few data relating to their possible preventive effect on osteoporosis, a degenerative disease. Concerning bone health, we have previously demonstrated the bone-sparing effects of the consumption of olive oil and the olive phenolic compound oleuropein on inflammation-induced bone loss in the ovariectomized (OVX) rat, an experimental model mimicking the senile osteoporosis that occurs with ageing (Puel et al. Reference Puel, Quintin, Agalias, Mathey, Obled, Mazur, Davicco, Lebecque, Skaltsounis and Coxam2004, Reference Puel, Mathey and Agalias2006). In fact, olives, which may account for 1–2 % of fresh fruit, are considered to be a better source of such polyphenols than are some virgin olive oils (Romero et al. Reference Romero, Brenes, Yousfi, Garcia, Garcia and Garrido2004). The main polyphenols in green olives are oleuropein, a glucoside ester of 3,4-dihydroxyethanol (hydroxytyrosol), elenolic acid and tyrosol (Brenes et al. Reference Brenes, Garcia, Garcia, Rios and Garrido1999). During olive-processing, these polyphenols undergo chemical transformation and fall in concentration. Oleuropein, tyrosol and hydroxytyrosol possess several biological properties attributable to their antioxidant and free radical scavenging ability (Visioli et al. Reference Visioli, Bellosta and Galli1998).
In light of this, it is important to provide further insights into the effect of such polyphenols on the skeleton. In fact, a diet providing a cocktail of different antioxidants may be more effective than supplementation with a single molecule, and it is closely a natural environment in which active molecules were found. Moreover, as polyphenols undergo chemical transformation during processing (Romero et al. Reference Romero, Brenes, Yousfi, Garcia, Garcia and Garrido2004), and because the phenolic content of virgin olive oil is influenced by the type of extraction procedure used (Montedoro & Garofolo, Reference Montedoro and Garofolo1984), we examined whether table olives (as a raw source of olive phenolic compounds) might contribute to the beneficial effect of the Mediterranean diet on bone.
The present work was carried out to evaluate the possible bone-sparing effect of the consumption of table olives – green and black Lucques olives – on bone metabolism in two experimental models of osteopenia: (1) OVX rats, to simulate hormone-deficiency-induced bone loss, and (2) OVX rats in which inflammation had been induced by subcutaneous magnesium silicate to mimic the inflammatory and oxidative status that occurs with ageing.
Materials and methods
Experimental design
The study was conducted in accordance with the regional Ethics Committee (France). One hundred and four female Wistar rats (INRA, Clermont-Ferrand/Theix, France), 6 months of age and weighing approximately 273 g, were housed in a temperature-controlled room (21°C), with a 12 h light/dark cycle. After 1 week of adaptation to the experimental conditions (control diet), the animals were either sham-operated (SH group; controls; n 26) or underwent surgical ovariectomy (OVX group; n 78) under anaesthesia using Imalgen 1000 (Merial, Lyon, France; 0·75 ml/kg body weight, intraperitoneally) and Vetranquil 1 % (Ceva santé animale, Libourne, France; 0·25 ml/kg body weight, intraperitoneally). Immediately after surgery (designated as day 0), the animals were randomly assigned to groups as follows: (1) SH given a control diet (SH); (2) OVX given a control diet (OVX); (3) OVX given green Lucques olives (L'Oulibo, Bize-Minervois, France; GO); (4) OVX given black Lucques olives (L'Oulibo, Bize-Minervois, France; BO).
The experimental diets are given in Table 1. Briefly, the control diet was a semi-purified standard diet (AIN93 modified) providing 50 g/kg of fat but deficient in vitamins E and D, and Ca (INRA, Jouy en Josas, France). In the treated group, we substituted standard fat by natural fat amount of olive. The number of olives given in each experimental diet was calculated to provide 50 g/kg of fat, according to the lipid content of the olives, i.e. 10 g/d green olives and 6 g/d black olives. Finally, NaCl (0·17 % in the control diet) was removed from both experimental diets enriched with olives. The Lucques variety of olive, mainly cultivated in the Mediterranean area of the South of France, was selected because of its high content of phenolic compounds (oleuropein; Amiot et al. Reference Amiot, Fleuriet and Macheix1986). The olives were protected from light during storage. Diets were prepared every week and stored at 4°C. During the experimental period, the daily amount of diet given to each rat was adjusted to the mean level consumed by the SH animals the previous day in order to prevent ovariectomy-induced hyperphagia.
Table 1 Composition of the experimental diet consumed by female Wistar rats
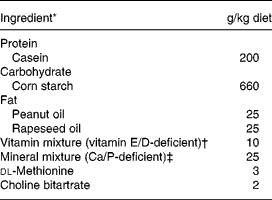
* From INRA (Jouy en Josas, France); casein (Union des caséinerie, Surgères, France), corn starch (Cerestar, Saint-Maur, France), cellulose (Durieux, Marne la Vallée, France), oil (Bailly, Aulnay sous Bois, France), vitamin mixture (Roche, Neuilly sur Seine, France), mineral mixture (Prolabo, Fontenay sous bois, France), dl-methionine and choline bitartrate (Jerafrance, Jeufosse, France).
† With dl-α-tocopherol acetate, 1·1 mg/kg; cholecalciferol, 32·25 μg/kg.
‡ With Ca 4·2 g/kg, P 4·01 g/kg, Mg 1·25 g/kg.
Three weeks before the end of the experiment (day 63), subcutaneous inflammation was provoked in thirteen animals from each of the OVX and SH groups by four injections of a suspension of sterile magnesium silicate (3·2 g total per animal; Talc; ICN Biomedicals, Illkrizch, France), as previously described (Krempien et al. Reference Krempien, Vukicevic, Vogel, Stavljenic and Buchele1988). At necropsy (day 84), urine from the previous 24 h was harvested and blood samples were collected from the abdominal aorta to measure biochemical parameters. The spleen and uterine horns were removed and weighed. The left and right femurs were cleaned of adjacent tissue and collected for mechanical testing and bone mineral density (BMD) measurement, respectively.
Tyrosol and hydroxytyrosol measurement in olives
The olives were homogenized in a blender, and 11 g of the powder obtained was extracted twice with CH3OH–H2O (1:1 v/v, 200 ml). The combined extracts were filtered and concentrated under reduced pressure before being washed three times with petroleum ether (200 ml) to remove oil, free fatty acids and other lipid contaminants. The remaining aqueous solution was partitioned twice against ethyl acetate in a water:phase ratio of 1:1. Next, the ethyl acetate extract was filtered on sodium sulphate anhydrous and evaporated to dryness at 30°C in a vacuum before being dissolved again in methanol, 100 μl of this being injected for HPLC analysis.
HPLC diode array
The chromatographic separation was conducted on a Finnigan SPECTRA HPLC system comprising of a Finnigan SPECTRA P4000 quaternary pump coupled to a Finnigan SPECTRA system UV6000LP diode array detector (Finnigan, Thermo Fischer Scientific Inc., Waltham, USA). Degassing of the mobile phase was achieved with a Finnigan SCM1000 online degasser (Finnigan). The sample was introduced into the chromatographic column through a Rheodyne 7725i injector (Rheodyne, Rohnert Park, USA) equipped with a 100 μl loop. HPLC control, data acquisition and processing were performed using ChromQuest v.4.1 software ((Thermo Fischer Scientific) connected to the HPLC unit by a Finnigan SN4000 controller (Finnigan). The extracts were chromatographed on a Lichrosorb C18 reversed phase column (250 × 4·0 mm, internal diameter 5 μm; Agilent Technologies Inc., Santa Clara, USA) equipped with a C18 Altech precolumn (Altech, Altech Associates, Deerfield, IL, USA). The column temperature was maintained at 40°C throughout the experiments with the aid of an electronically controlled oven.
HPLC with diode array detection
The chromatographic separation of the substances was performed using a gradient elution programme described in Table I. Solvent A was 0·2 % acetic acid in water (pH 3·1), and solvent B was CH3OH. The flow rate was kept constant at 1 ml/min. After each run (mobile phase composition 100 % A–0 % B), a delay time of 3 min before the next injection was necessary in order to equilibrate the column.
Detection
During the acquisition of the standard solution chromatograms for each substance, the UV spectra of all the substances were recorded with the aid of the diode array detector. The λmax was determined to be 280 nm for tyrosol and hydroxytyrosol. Spectra were also acquired for each separate substance using a Unicam UV-300 UV-Vis spectrophotometer (Unicam, Thermo Fischer Scientific) with 10 mm optical path length quartz cuvettes.
For the HPLC diode array detection procedure, peak assignment was based on the retention time (with a peak window 10 % of retention time) for every substance. On a second level, the peaks of the analytes were identified by their spectra based on diode array data.
Plasma, urine and tissue analysis
Biochemical analysis
Blood granulocyte counts were determined in blood samples using an automatic haematology counter (ABC vet; Scil Animal Care Company France, Holtzheim, France) on fresh blood.
Two plasma acute-phase proteins were measured. α1-Acid glycoprotein was determined by radial immunodiffusion using rabbit anti-rat α1-acid glycoprotein antibodies (Breuille et al. Reference Breuille, Arnal, Rambourdin, Bayle, Levieux and Obled1998). Fibrinogen was assessed by a turbidimetric assay (Biodirect, Les Ulis, France) that evaluates the precipitation of fibrinogen in the presence of (NH4)2SO4. Results were calculated from a standard scale of fibrinogen.
Plasma vitamin E was assayed by reversed-phase HPLC (Kontron series 400; Kontron, St Quentin en Yvelines, France) using a hexane extract. Briefly, tocopherol acetate (Sigma Chemical Co., St Louis, MO, USA) was added to samples as an internal standard. These were then extracted twice with hexane after ethanol precipitation of the proteins. This extract was evaporated to dryness under N gas, dissolved in methanol–dichloromethane (65:35, v/v) and injected onto a C18 column (nucleosil; 250 mm long, internal diameter 46 mm, 5 μm particles). Pure CH3OH, at a flow rate of 2 ml/min, eluted α-tocopherol in 5 min and tocopherol acetate in 6·3 min. The compounds were detected by UV light (292 nm) and quantified by internal and external calibration using daily controlled standard solutions.
Plasma triglycerides were enzymatically determined using a kit purchased from Biomerieux (Marcy-l'Etoile, France). The detection limit was 0·17 g/l.
The ferric reducing ability of plasma was determined using the method of Benzie & Strain (Reference Benzie and Strain1996), which evaluates the reduction of ferric iron to the ferrous form in presence of antioxidant components. Colorimetric measurement was performed at 593 nm, and the reaction was monitored for up to 8 min on 25 μl samples. Results were calculated from a standard scale of FeSO4.
Osteocalcin in plasma was measured by RIA using rat 125I-labelled osteocalcin, goat anti-rat osteocalcin antibody and donkey anti-goat second antibody (Biochemical Technologies, Stoughton, MA, USA).
Urinary free deoxypyridinoline (DPD) was determined in urine by competitive RIA, using rat monoclonal anti-DPD antibody coated on the inner surface of a polystyrene tube, and 125I-labelled DPD (Pyrilinks-D RIA kit; Metra Biosystems, Mountain View, CA, USA). Results are expressed as nmol DPD/mmol creatinine (Robins, Reference Robins1994). The creatinine assay kit (Biomerieux) is based on a modified Jaffe's method in which picric acid forms a coloured solution in the presence of creatinine (Cook, Reference Cook1975).
Urinary isoprostane was measured by ELISA using 15-isoprostane F2t conjugated to horseradish peroxidase and a polyclonal antibody specific for 15-isoprostane F2t (Oxford Biomedical Research, Oxford, MI, USA).
The amount of Ca in the urine was determined by atomic absorption spectrophotometry (Perkin Elmer 400; Perkin Elmer, Norwalk, CT, USA). Each sample was diluted appropriately with distilled water and lanthanum (0·1 %) for atomization. The urinary Ca excretion was calculated using the volume of urine.
Physical measurements
BMD was assessed by dual-energy X-ray absorptiometry, with a Hologic QDR-4500 A X-ray bone densitometer (Hologic, Massy, France). Total right femur BMD, as well as the BMD of two subregions, one corresponding to the distal femur metaphyseal zone, rich in cancellous bone, and the other to the diaphyseal area, rich in cortical bone, were determined (Pastoureau et al. Reference Pastoureau, Chomel and Bonnet1995).
For femoral failure load studies, the length and mean diameter of the diaphysis were measured with a precision caliper (Mitutoyo, Shropshire, UK). This parameter was then determined using a three-point bending test (Turner & Burr, Reference Turner and Burr1993) with a universal testing machine (Instron 4501; Instron, Canton, MA, USA).
Statistical methods
Results are expressed as means with their standard errors. All data were analysed using XLSTAT (Addinsoft, Paris, France). Parametric two-way ANCOVA (body weight used as a co-variate) was performed to test for any difference between bone parameters, femoral failure load and spleen weight; ANOVA was performed to test for any difference between groups. If the result was found to be significant (P < 0·05), the Student–Newman–Keuls multiple comparison test was then used to determine specific differences between means.
Results
Analysis of phenolic compounds in olives
HPLC analysis for hydroxytyrosol and tyrosol indicated that black olives contained quite a high amount of hydroxytyrosol (146 mg/100 g fresh weight), whereas this polyphenol was undetectable in the green olives (data not shown for results in this section). As far as tyrosol was concerned, black olives showed nearly nine times the concentration (143 mg/100 g fresh weight) of green olives, which provided only 16 mg/100 g fresh weight. Taking this into account and based on the amount of olives eaten, each rat received 1·62 mg/d tyrosol in the GO group and 8·56 mg tyrosol/d plus 8·76 mg hydroxytyrosol/d in the BO group.
Body weight and uterine weight
Although consuming similar amounts of food (SH, 18·5 (se 0·4) g; SH with inflammation, 18·6 (se 0·3) g; OVX, 18·6 (se 0·4) g; OVX with inflammation, 18·7 (se 0·3) g), body weight (g) was higher in the OVX than the SH rats because of pair-feeding (P < 0·001; Fig. 1, Table 2).

Fig. 1 Body weight of sham-operated rats (■), ovariectomized (OVX) rats (•), OVX rats given green Lucques olives (♦) and OVX rats given black Lucques olives ( × ) with (solid symbols, solid lines) or without (open symbols, dotted lines) inflammation. Values are expressed as means and their standard errors. *Mean values were significantly different v. OVX animals: P < 0·05.
Table 2 Bone, inflammatory state oxidative state parameters in control rats: sham-operated (SH) and ovariectomized rats (OVX) with or without inflammation at necropsy at day 84(Mean values and their standard errors for thirteen determinations in each group)

Parametric two-way ANOVA was performed to test for any difference between groups.Mean values within a row with unlike superscript letters differ.
a,b,c,d, effect of OVX and inflammation.
The success of ovariectomy was confirmed by uterine atrophy in the OVX rats (OVX 0·11 (se 0·01) g v. SH 0·72 (se 0·03) g; P < 0·0001). Neither inflammation (SH with inflammation, 0·63 (se 0·05); OVX with inflammation, 0·11 (se 0·01)) nor olive intake had any further significant effect on this parameter (GO, 0·11 (se 0·01); BO, 0·10 (se 0·01); data not shown).
Bone analysis
Bone mineral density
Inflammation elicited significant osteopenia in the total femur, the same pattern being observed for metaphyseal BMD. Concerning the diaphyseal subregion, castration was not associated with bone loss. Both experimental diets (green and black olives) elicited a different effect on those parameters. Although green olive consumption was devoid of any effect on mineralization, the black olive-enriched diet was able to restore the BMD of the diaphysis, which could explain the bone-sparing action observed for the total femur in the castrated animals given talc injections (Fig. 2).
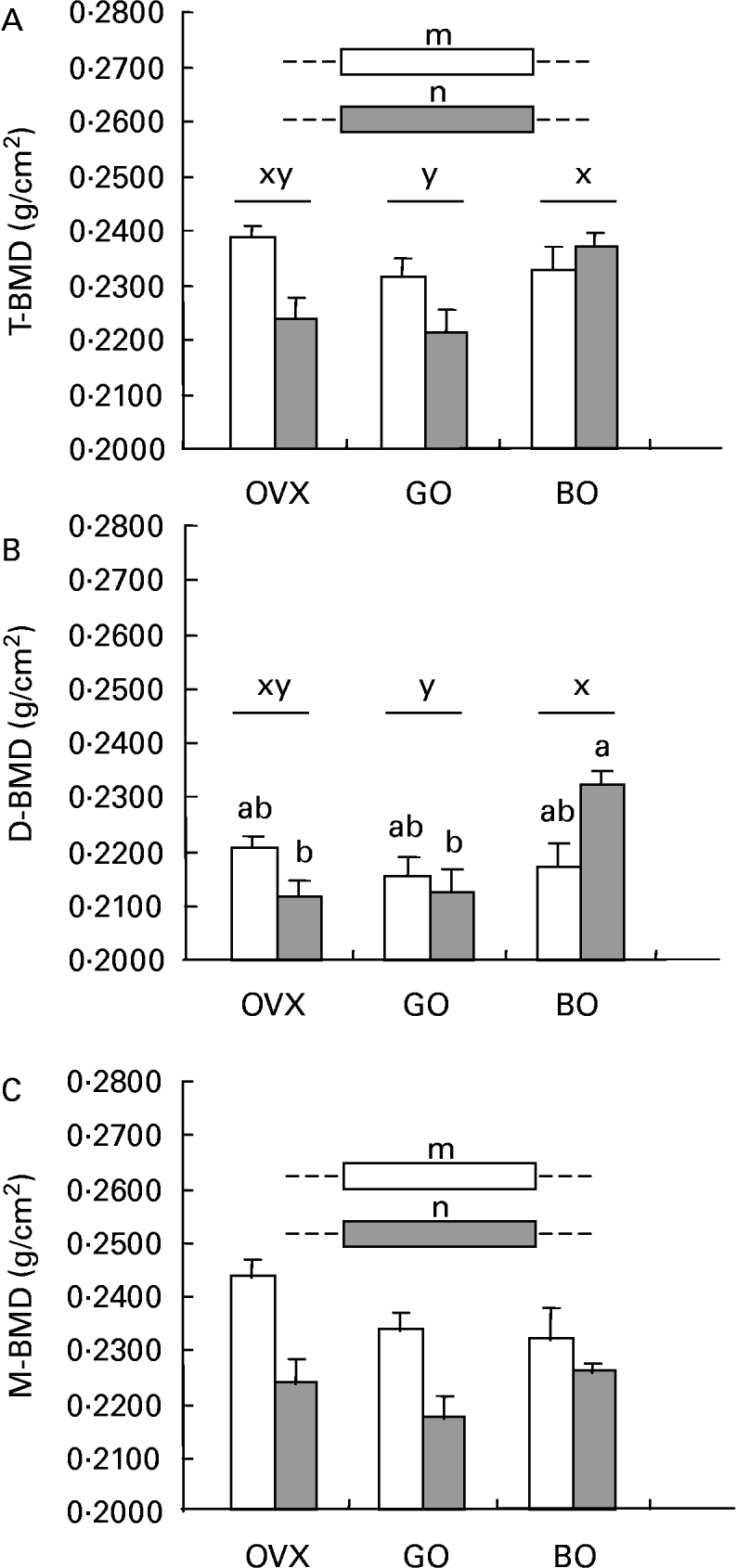
Fig. 2 (A) Total femoral (T-BMD), (B) diaphyseal (D-BMD) and (C) metaphys-eal (M-BMD) bone mineral densities measured in ovariectomized rats (OVX), OVX rats given green Lucques olives (GO), OVX rats given Black Lucques olives (BO) with (▒) or without (□) inflammation. Values are expressed as means and their standard errors, n 13. Means within a row with unlike superscript letters differ (P < 0·01): x,y, effect of diet; m, n, effect of inflammation; a,b, effect of interaction (diet × inflammation).
Bone turnover
At necropsy, no significant difference was observed in plasma osteocalcin concentration. The urinary DPD excretion (nm/mmol creatinine) measured on day 84 was significantly higher in OVX rats that had received talc than in OVX controls. Both experimental diets significantly affected this parameter (Tables 2 and 3).
Table 3 Bone parameters, inflammatory status and oxidative status in ovariectomized (OVX) rats, OVX rats given green Lucques olives (GO) and OVX rats given black Lucques olives (BO) with or without inflammation (Mean values with their standard errors for thirteen determinations)
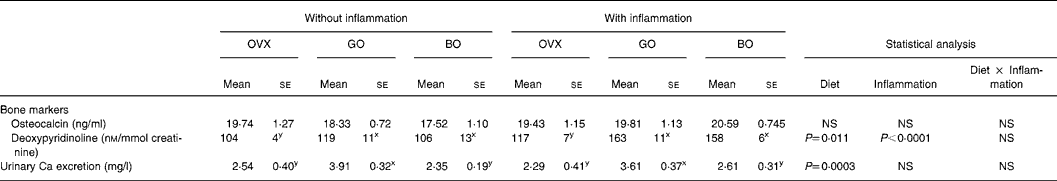
Mean values within a row with unlike superscript letters differ: x,y, effect of diet.
Urinary Ca excretion
This did not differ when ovariectomy was performed. Inflammation was devoid of any effect on this parameter as well. However, calciuria was increased by the consumption of green olives (Tables 2 and 3).
Femoral mechanical testing
No significant difference between groups was demonstrated for femoral failure load (mean value 105 (se 1) N).
Inflammatory state analysis
Plasma fibrinogen and α1-acid glycoprotein concentrations were significantly higher in OVX rats than SH groups (see Table 2, earlier). In our oestrogen-deficient rats, talc administration elicited a significant increase in every tested marker for inflammatory status (fibrinogen, α1-acid glycoprotein, granulocytes; Table 4). The rise in plasma fibrinogen was partially corrected by an intake of black Lucques olives.
Table 4 Bone parameters, inflammatory status and oxidative status in ovariectomized (OVX) rats, OVX rats given green Lucques olives (GO) and OVX rats given black Lucques olives (BO) with or without inflammation (Mean values and their standard errors for thirteen determinations)

Mean values within a row with unlike superscript letters differ: x,y, z, effect of diet.
* Co-variance analysis (body weight as co-variate).
An increase in spleen weight was observed in our animals when inflammation was provoked with talc. This trend was not prevented by either type of olive.
Oxidative stress analysis
Ferric reducing ability of plasma
This parameter decreased when a granulomatous inflammation was induced by the injection of talc into the rats. Neither black nor green Lucques olives prevented this effect.
Urinary isoprostane
In the same way, an increase was induced in OVX rats after magnesium silicate administration. An intake of black Lucques olives prevented this increase.
Plasma vitamin E level
This parameter was altered by inflammation. Both experimental diets increased the vitamin E plasma level, whether or not the animals had been given a talc injection. In the same way, plasma vitamin E:TG ratio was different in the inflammatory state. Black and green olives both significantly improved this biomarker.
Discussion
The present study demonstrates that olives, which are a commodity of great importance in Mediterranean countries (Romero et al. Reference Romero, Brenes, Yousfi, Garcia, Garcia and Garrido2004), may contribute to bone health during ageing. Indeed, the present investigation, performed on both an original experimental model of senile osteoporosis and an extensively used model of postmenopausal bone loss, showed that black Lucques olives prevented bone loss induced by ovariectomy/inflammation.
Effect of ovariectomy
Body weight is significantly increased in OVX rats compared with SH rats (Kalu, Reference Kalu1991). The weight gain, which would be prevent by pair-feeding, could be explained by a change in central leptin gene expression, responsible for promoting weight gain and adiposity after ovariectomy (Torto et al. Reference Torto, Boghossian, Dube, Kalra and Kalra2006).
As expected, femoral bone loss in OVX rats, the most classically used model of postmenopausal osteopenia (Kalu, Reference Kalu1991), was induced in the distal metaphysis of the femur (rich in cancellous bone that is mainly involved in metabolic functions; see Table 2, earlier). The femoral diaphysis (rich in cortical bone that fulfils essentially mechanical and protective functions) was not affected, as femoral failure load, an indicator of mechanical properties. This osteopenia probably resulted from an increase in bone resorption, as shown by the higher urinary DPD excretion in OVX than SH rats.
Although the OVX rats is suitable for studying problems that are relevant to hormone-deficiency-induced bone loss, it does not totally fulfil the conditions for senile osteoporosis because of a further dysregulation of the immune response, a chronic low-grade inflammation and an impaired oxidative status occurring with ageing (Finkel & Holbrook, Reference Finkel and Holbrook2000; Bruunsgaard et al. Reference Bruunsgaard, Pedersen and Pedersen2001; Pawelec et al. Reference Pawelec, Barnett and Forsey2002). This is why we set up an animal model in which we attempted to imitate those perturbations by both performing an ovariectomy and eliciting a chronic inflammation. An acute-phase response was induced in the OVX rat by the subcutaneous injection of particles poorly degraded by phagocytic cells, such as magnesium silicate (Minne et al. Reference Minne, Pfeilshifter, Scharla, Mutschelknauss, Schwarz, Krempien and Ziegler1984).
Effect of inflammation
In agreement with Dinarello (Reference Dinarello1984), subcutaneous talc granulomatosis in OVX rats developed many aspects of the acute-phase response, such as an increase in two positive acute-phase proteins – fibrinogen and α1-acid glycoprotein – the levels of which remained high after 3 weeks (see Table 4, earlier), hypertrophy of the spleen and a raised level of granulocytes. In this experimental model, we fulfilled the effect of ageing, i.e. an alteration in secondary lymphoid organ and plasma protein synthesis (Papet et al. Reference Papet, Dardevet, Sornet, Bechereau, Prugnaud, Pouyet and Obled2003). Moreover, in these conditions, we observed modifications in the pro-oxidant/antioxidant balance. The urinary excretion of isoprostane, a marker of in vivo oxidative stress, increased, whereas there was a decline in the total antioxidant capacity of the plasma (as indicated by ferric reducing ability of plasma values).
Regarding bone health, consistent with previous data (Minne et al. Reference Minne, Pfeilshifter, Scharla, Mutschelknauss, Schwarz, Krempien and Ziegler1984; Pfeilschifter et al. Reference Pfeilschifter, Wuster, Vogel, Enderes, Ziegler and Minne1987), we found that OVX animals that had been subcutaneously injected with magnesium silicate showed a significant trabecular bone loss. In our experimental conditions, an impairment of BMD was even observed in the cortical area, but this was not sufficient to alter its mechanical properties. Targetting bone metabolism, talc injection was associated with increased bone resorption, osteoblastic activity staying unchanged.
Effect of black olives
Black Lucques olives exhibited a bone-sparing effect, as shown by a higher BMD of the total femur than was seen in control OVX rats with inflammation (see Fig. 2, earlier). This could be accounted for by a potent effect on the diaphyseal subregion, whereas there was no change in the metaphyses. The impact on bone metabolism was not, however, consistent, as an increase in the urinary excretion of DPD was measured. Nevertheless, DPD and osteocalcin reflected the rats' status when measurement was performed, but not an accumulated effect.
The prevention of demineralization might result from a reduced inflammatory state, as shown by the lower plasma fibrinogen and α1-acid glycoprotein levels in OVX rats given black olive varieties, even though the splenomegaly and rise in granulocyte level were not prevented. The liver is in fact involved in the synthesis of acute-phase proteins, whereas granulocytes are produced by bone marrow; they may thus not be correlated with time. Conversely, the spleen contributes to the macrophage system, as does the bone marrow. In addition, an improvement in oxidative stress, measured by isoprostane excretion, was observed under our conditions. This protection against oxidative stress was related to the optimal level of plasma antioxidants. Rats that consumed black olives exhibited a better antioxidant status: higher levels of vitamin E and a higher vitamin E:TG ratio than were seen in OVX rats with inflammation. This better vitamin E status was mainly due to the richness of vitamin E in black olives. In addition, because of the high fatty acid content of the black olives, the intestinal absorption of vitamin E may have been stimulated.
Finally, enough antioxidant polyphenols, such as tyrosol and hydroxytyrosol, may have been present to save the endogenous vitamin E. In fact, dietary vitamin E appears to have a protective role since it prevents the deleterious effect of skeletal unloading on bone mass (Smith et al. Reference Smith, Lucas, Turner, Evans, Lerner, Brackett, Stoecker and Arjmandi2005). A direct effect of tyrosol and hydroxytyrosol could not be ruled out to explain the anti-inflammatory and antioxidative effects of black olives, these properties having been established in the literature (Petroni et al. Reference Petroni, Blasevich, Salami, Papini, Montedoro and Galli1995; Wiseman et al. Reference Wiseman, Mathot, de Fouw and Tijburg1996; de la Puerta et al. Reference de la Puerta, Ruiz Gutierrez and Hoult1999, Reference de la Puerta, Martinez-Dominguez and Ruiz-Gutierrez2000; Visioli et al. Reference Visioli, Caruso, Galli, Viappiani, Galli and Sala2000a, Reference Visioli, Galli, Plasmati, Viappiani, Hernandez, Colombo and Salab; Bertelli et al. Reference Bertelli, Migliori, Panichi, Longoni, Origlia, Ferretti, Cuttano and Giovannini2002). The levels of hydroxytyrosol and its derivatives in fresh ripe olives suitable for table olive production have been found to vary from 100 to 5610 mg/kg (Blekas et al. Reference Blekas, Vassilakis, Harizanis, Tsimidou and Boskou2002). In the olives used in the present study, the hydroxytyrosol concentration was 1460 mg/kg (in the black olives, the green olives being devoid of hyroxytyrosol), and that of tyrosol 1430 mg/kg, compared with 162 mg/kg in green olives.
Some studies have suggested anti-inflammatory effects for tyrosol: this phenolic has been shown to be able to modulate the increase in cytokine release by lipopolysaccharide-stimulated peripheral blood mononuclear cells (Bertelli et al. Reference Bertelli, Migliori, Panichi, Longoni, Origlia, Ferretti, Cuttano and Giovannini2002). Tyrosol is also effective in inhibiting the oxidation of cholesterol in LDL (Caruso et al. Reference Caruso, Berra, Giavarini, Cortesi, Fedeli and Galli1999). Nevertheless, in olive fruit, many other compounds, such as fatty acids and other micronutrients, that could act singly and/or synergistically to protect bone loss in these experimental conditions could also be involved.
Effect of green olives
Regarding the effect of the green Lucques variety of olive, no bone-sparing effect was observed. In the same way, no anti-inflammatory activity was exhibited. Despite an improvement in antioxidant status, as shown by a rise in vitamin E and vitamin E:TG ratio, a lack of effect on oxidative stress, as indicated by isoprostane excretion, should also be noted. The lower effectiveness compared with black olives could be due to the lack of hydroxytyrosol and to the smaller amount of tyrosol, which is actually less antixoxidant than hydroxytyrosol (owing to the lack of cathechol function; de la Puerta et al. Reference de la Puerta, Martinez-Dominguez, Ruiz-Gutierrez, Flavill and Hoult2001).
Moreover, there are differences between the two varieties in the degree of ripeness and in processing methods, which could explain the variation in composition of the olives and thus the difference in effect on bone. In particular, green olives are characterized by a higher (0·832 g) NaCl content, compared with the amount provided by black olives (0·480 g; v. 0·0357 g in the control diet). It is thus possible that such an intake of NaCl was involved in the depletion of bone mineral. Indeed, the skeleton serves as a substantial reservoir of labile base in the form of alkaline Ca salts, which can be mobilized to defend blood pH (Barzel, Reference Barzel1995). This is confirmed by the increased urinary Ca loss in the green olive diet shown in the present study (see Table 3, earlier). In fact, osteoclasts and osteoblasts are known to respond to small changes in pH, a slight drop in pH causing a burst of bone resorption (Krieger et al. Reference Krieger, Sessler and Bushinsky1992).
In conclusion, black Lucques olives prevented bone loss in an experimental model of senile osteoporosis in which ovariectomy was coupled with low-grade inflammation and impaired oxidative status. This bone-sparing effect seemed to result from an improvement in both inflammatory and oxidative status. The association between an adequate diet, as a whole, and bone mass needs to be emphasized because many consumers mistakenly think that Ca alone is important for bone development and maintenance. The traditional Mediterranean diet may thus appear useful in the prevention of osteoporosis.
Acknowledgements
We thank Bernard Lyan and Fabienne Bécherau for technical assistance, and Jean-François Martin for statistical analysis.








