In many countries the 7-valent pneumococcal conjugate vaccine (PCV7) (and more recently PCV10 and PCV13) has been incorporated into national infant immunization schedules [Reference van Deursen1, Reference Miller2]. Vaccination of children with this vaccine leads to a decrease in the incidence of invasive pneumococcal disease (IPD) caused by the vaccine serotypes, both in vaccinated children and in unvaccinated adults [Reference van Deursen1, Reference Miller2]. However, the incidence of non-vaccine serotype IPD increases to some extent in all age groups after implementation of the vaccine. In The Netherlands, PCV7 was introduced in June 2006 for all newborns. This led to a net decrease in IPD incidence in children and in adults aged ⩾65 years. On the other hand, in younger adults (18–49 years), incidence of IPD shows an increasing trend in The Netherlands [Reference van Deursen1]. In other countries both increases and decreases in IPD incidence have been reported for this age group [Reference Miller2–Reference Pilishvili6].
Recently, a study was published investigating the risk of pneumococcal pneumonia in mothers of young infants in the UK [Reference Hak, Shea and Jick7]. The annual incidence rate of pneumococcal pneumonia in mothers appeared to have increased since the introduction of PCV7. Moreover, an association was found between the risk of pneumococcal pneumonia in mothers and the PCV7 immunization status of their first-born children [Reference Hak, Shea and Jick7]. Two different studies also reported an increased carriage rate of (non-vaccine serotype) pneumococci in parents of children vaccinated with PCV7 compared to parents of unvaccinated children [Reference van Gils8, Reference Flasche9]. While in these publications fathers and mothers have not been analysed separately, a Dutch study on pertussis showed that the transmission rate between infants and their mothers is higher than between infants and other household members [Reference de Greeff10].
In this study we aimed to investigate the changes in the incidence of IPD in young female adults in The Netherlands since the introduction of PCV7. We analysed the data of the Dutch national IPD surveillance study by sex, focusing on the age group of young parents, i.e. aged 20–45 years [Reference van Deursen1].
The number of IPD cases in adults aged 20–45 years in the period between 1 June 2008 and 31 May 2010 (post-PCV7 implementation period) was compared to the number of cases in the period between 1 June 2004 and 31 May 2006 (pre-PCV7 implementation period). The Dutch national IPD surveillance is based on the identification of all IPD cases by nine sentinel laboratories throughout the country, covering ∼25% of the Dutch population [Reference van Deursen1]. Annual IPD incidence rates were estimated by dividing the total number of IPD cases in a specific year by 25% of the total Dutch population. Incidences were compared by providing incidence rate ratios (IRR), 95% confidence intervals (CI) and P values, calculated by Episheet (http://www.drugepi.org/dope-downloads/). We considered P values <0·05 to be significant.
The incidence of non-vaccine serotype IPD increased significantly by 60% (P = 0·03) in women aged between 20 and 45 years (Table 1), leading to a non-significant increase in overall IPD in women of this age group from 3·80 to 5·26/100 000 persons. The incidence of non-vaccine serotype IPD in men of the same age remained stable. Further analysis showed that the increase in non-vaccine-type IPD in women was almost entirely caused by serotype 1:14 of the increment of 19 non-vaccine serotype IPD cases were serotype 1 (Table 1). The second largest serotype increase in young female adults was serotype 7F, with three cases. The serotype-1 cases were equally distributed over the nine sentinel laboratories, which makes a regional outbreak unlikely. Six (25%) of the 24 serotype-1 female IPD patients had an underlying comorbidity compared to 15 (31%) of 48 female IPD patients infected with one of the other serotypes. In patients aged <20 years the number of serotype-1 IPD cases was small for both sexes (Table 2). In patients aged >45 years the incidence of serotype-1 disease increased both in men and in women (Table 2). In this age group the overall incidence of non-vaccine-type IPD increased significantly with 39% (P = 0·00) in men and 19% (P = 0·04) in women (not shown in the Table).
Table 1. Invasive pneumococcal disease incidence in The Netherlands in patients aged between 20 and 45 years
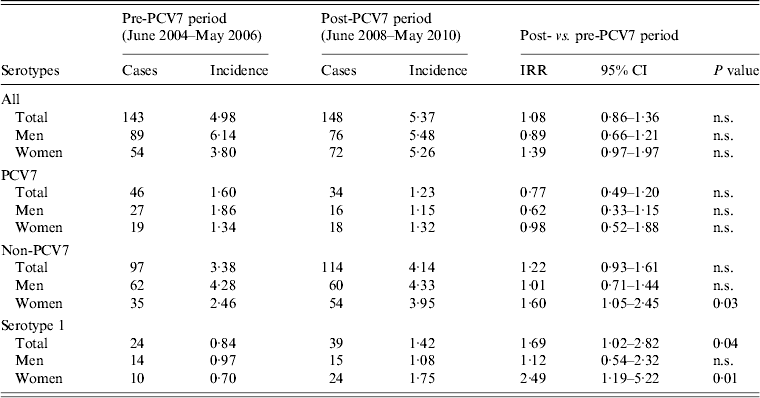
PCV7, Seven-valent pneumococcal conjugate vaccine; cases, number of cases included in the surveillance study (covering ±25% of the Dutch population); incidence, cases/100 000 persons; IRR, incidence rate ratio; CI, confidence interval; n.s., non-significant (P ⩾ 0·05).
Table 2. Invasive pneumococcal disease serotype-1 incidence in The Netherlands
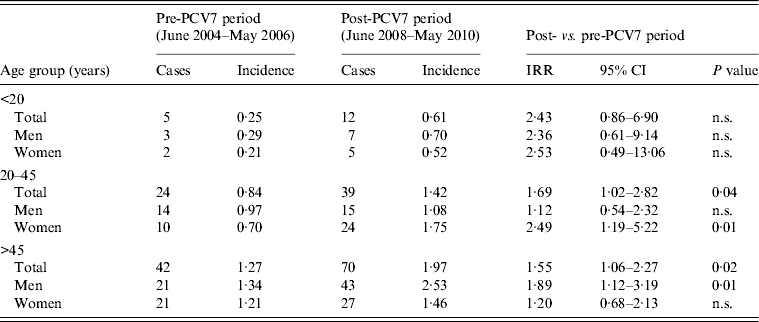
PCV7, Seven-valent pneumococcal conjugate vaccine; cases, number of cases included in the surveillance study (covering ±25% of the Dutch population); incidence, cases/100 000 persons; IRR, incidence rate ratio; CI, confidence interval; n.s., non-significant (P ⩾ 0·05).
Timing of the increase in non-vaccine serotype IPD in young women could suggest an association with the implementation of PCV7. Mothers, possibly having closer contact with their vaccinated babies and toddlers than fathers, may become more exposed to non-vaccine-type pneumococci. It is, however, striking that this increase in IPD incidence is mainly caused by a single serotype.
Colonization with serotype 1 did not increase after vaccine implementation, neither in children, nor adults [Reference van Gils8, Reference Flasche9]. However, serotype 1 has a very high invasive disease potential and is rarely found as nasopharyngeal colonizer [Reference Brueggemann and Spratt11–Reference Brueggemann13]. Several studies suggest that the incidence of serotype-1 disease in Europe has been naturally fluctuating, with regular peaks every couple of years in the absence of pneumococcal vaccines [Reference Miller2, Reference Harboe14]. Therefore, it is not possible to determine whether the observed increase in serotype-1 disease in young women after introduction of PCV7 has occurred by a change in acquired immunity or by chance [Reference Cobey and Lipsitch15]. If the increasing incidence of serotype-1 disease in young female adults could be attributed to the implementation of PCV7, it would be expected that women would also be more susceptible than men in this age group to other post-vaccine implementation serotype shifts. The incidence of vaccine-type IPD did not decrease more in young women than in young men (Table 1), nor were there differences between the sexes with respect to non-vaccine serotypes other than serotype 1.
Serotype-1 IPD is less commonly seen in patients with comorbidities than other serotypes and the prognosis of serotype-1 disease is relatively good [Reference Jansen16, Reference Weinberger17]. In a study by Grau et al. it was shown that in young healthy adults with IPD the proportion of women as well as the proportion of serotype-1 disease was higher than in adults with comorbidities [Reference Grau18]. Serotype 1 could have a predilection for young female adults, but it is not known what the biological principle behind this would be.
This study is clearly limited by small case numbers and by the unknown parenthood status of the patients. However, our data show a marked increase in the incidence of serotype-1 IPD in young women after introduction of PCV7 in The Netherlands, which was not seen in men in the same age group. Now that PCV10 or PCV13, both including serotype 1, have been introduced in most countries, the observed increased incidence of IPD caused by this serotype may not have great clinical relevance. However, identification of the underlying mechanisms behind this increase by carefully designed epidemiological studies on the spread of pneumococci within families could be applicable to other serotypes as well. We invite other investigators to analyse their IPD surveillance data by sex in order to determine whether our observation can be confirmed in other populations.
ACKNOWLEDGEMENTS
This study was supported in part by an unrestricted research grant from Pfizer Pharmaceuticals. The sponsor played no role in the study design, data analyses, and preparation, review or approval of the manuscript. The authors thank all medical students for making data collection possible, and all participating hospitals and sentinel laboratories for their cooperation.
DECLARATION OF INTEREST
E. Sanders reports receiving grants from Pfizer and GlaxoSmithKline for research on pneumococcal infections; from Baxter for research on immunodeficiency disease, consulting fees from Pfizer and GlaxoSmithKline, lecturing fees from Pfizer and GlaxoSmithKline, and grant support from Pfizer and GlaxoSmithKline for pneumococcal vaccine studies. She is involved with IDM committees for vaccine studies of Pfizer and GlaxoSmithKline. A. van der Ende reports receiving grants from Pfizer for research on pneumococcal infections. G. Rijkers reports receiving consulting and lecturing fees from Pfizer.




