During the last decades, decreasing trends in sperm counts have been reported in multiple studies( Reference Swan, Elkin and Fenster 1 – Reference Virtanen, Jørgensen and Toppari 4 ). The reasons for this downward trend have not been fully elucidated, but exposures adversely affecting prenatal testicular development as well as exposures and lifestyle factors during childhood and young adulthood have been suggested( Reference Virtanen, Jørgensen and Toppari 4 , Reference Nordkap, Joensen and Blomberg Jensen 5 ). However, relatively little work has been devoted to understanding the modifiable determinants of semen quality, as a marker of men’s reproductive potential( Reference Povey, Clyma and McNamee 6 ), which could lead to the design of clinical and public health interventions( 7 ).
Diet is a modifiable risk factor that can be assessed in clinical practice and inform actions for disease prevention( Reference Mahe, Carsin and Zeeny 8 ). Previous epidemiological studies suggest that diet may be associated with semen parameters( Reference Jensen, Swan and Skakkebaek 9 – Reference Salas-Huetos, Bulló and Salas-Salvadó 14 ). Animal food intake, and in particular intake of red meats and fish, has received particular attention since their nutritional profile and their contamination with persistent organic pollutants and hormonal residues may affect testicular function( Reference Jensen, Heitmann and Blomberg Jensen 11 , Reference Swan, Liu and Overstreet 15 – Reference Afeiche, Williams and Gaskins 19 ). Although literature is scarce, red meats have consistently been associated with worse semen quality, while fish intake appears to have the opposite relation( Reference Afeiche, Williams and Gaskins 19 – Reference Afeiche, Gaskins and Williams 22 ). However, these studies have been conducted in different populations and settings, with diverse age and health status, and only one previous work was performed in healthy young men( Reference Afeiche, Williams and Gaskins 19 ). Therefore, the aim of this study is to investigate the association of intakes of meats, including red, white, processed and fish meats, with semen quality parameters and reproductive hormones among healthy young men from Spain.
Methods
Study population
The Murcia Young Men’s Study (MYMS) was a cross-sectional study conducted in 2010–2011 that enrolled healthy men between 18 and 23 years of age at the University of Murcia (Region of Murcia, Spain). MYMS is part of a multicentre international study (Finland, Denmark, Spain and USA) aimed at evaluating the role of environmental contaminants on semen quality. The same study population has been used in previously published works( Reference Mínguez-Alarcón, Mendiola and López-Espín 10 , Reference Chavarro, Mínguez-Alarcón and Mendiola 12 , Reference Cutillas-Tolín, Mínguez-Alarcón and Mendiola 23 , Reference Mínguez-Alarcón, Chavarro and Mendiola 24 ). Participants were recruited through flyers posted at university campuses. Men were eligible to participate if they were born in Spain after 31 December 1987, and were able to contact their mother to complete a questionnaire.
A total of 240 men contacted the study staff. Of these, 223 (92·9 %) met the eligibility criteria and 215 completed a study visit and agreed to participate in the study. During the visit, men underwent a physical examination, completed questionnaires concerning diet, lifestyle, medical and reproductive history and provided semen and blood samples. Six men who reported implausible total energy intake (>20920 kJ/d) and three who had a hydrocele were excluded from the analysis. The final analytical sample comprised 206 men (85·8 %). Participants received a €50 gift card for their participation in the study. Written informed consent was obtained from all subjects. The Research Ethics Committee of the University of Murcia approved this study (no. 495/2010, approved 14 May 2010).
Physical examination
Men’s height and weight were measured using a wall stadiometre and digital scale (Tanita SC 330-S) to determine their BMI. The presence of varicocele, hydrocele or other scrotal abnormalities was recorded. Testicular volume was assessed with a Prader orchidometre (Andrology Australia). All physical examinations were performed by the same investigator (J. M.) to minimise variability in study measures.
Dietary assessment
Participants completed a 101-item semi-quantitative FFQ previously validated in several adult populations in Spain( Reference Vioque and Gonzalez 25 , Reference Vioque, Navarrete-Muñoz and Gimenez-Monzó 26 ). Men reported their usual intake of foods and supplements over the past year. The FFQ had nine categories for frequency of consumption, ranging from never to ≥6 times/d. Total meat intake was defined as the sum of red meat, white meat and fish. We grouped specific meat types in seven groups (online Supplementary Table S1): three types of red meat, one type white meat and three types of fish.
Semen analysis
Men were instructed to abstain from ejaculation for 48 h before sample collection but were not excluded if this was not the case. At the time of sample collection, they also provided information on time of the previous ejaculation. Semen samples were obtained by masturbation at the clinic, and lubricants were not used. Ejaculate volume was estimated by sample weight, assuming a semen density of 1·0 g/ml. Sperm concentration was evaluated by haemocytometer (Improved Neubauer; Hauser Scientific Inc.). Sperm motility was evaluated as either motile or immotile according to the WHO criteria( 27 ), and the percentage of total motile sperm and progressive motility were calculated. Smears for morphology were made, air-dried, fixed, Papanicolaou stained and evaluated using strict criteria( Reference Menkveld, Stander and Kotze 28 ). Total sperm count (volume × sperm concentration) was also calculated. The same specialist biologist carried out all the semen analyses. An external quality control on semen samples throughout the study period was carried out in collaboration with the University of Copenhagen’s Department of Growth and Reproduction. To assess inter-laboratory variation in sperm concentration analysis, five sets of duplicate semen samples (600 µl each) were sent by mail during the study period from the University of Copenhagen’s Department of Growth and Reproduction to the Murcia Andrology Laboratory. The samples were blinded, non-diluted fresh sperm specimens from regular semen donors that were preserved by adding 10 µl of a 3 m sodium azide solution per 1 ml of the ejaculate after liquefaction. Results showed no systematic differences. The mean inter-examiner CV was 4·0 %, ranging between 1·7 and 7·1 %.
Reproductive hormone measurement
Blood samples were drawn from men’s cubital veins and centrifuged; the serum was separated, stored and frozen at –80°C. Serum samples were shipped to Rigshospitalet (Copenhagen, Denmark) and stored at –20°C until hormone analysis was performed. The methods have been outlined previously( Reference Asklund, Jørgensen and Skakkebaek 29 ). Briefly, hormone assessments were performed simultaneously to reduce intra-laboratory variations. Serum levels of follicle-stimulating hormone (FSH), luteinising hormone (LH) and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer). Intra- and inter-assay variations were <5 % in each of the three assays. Serum total testosterone (TT) levels were determined using a time-resolved fluoroimmunoassay (dissociation enhanced lanthanide fluoroimmunoassay; DELFIA) with intra- and inter-assay variations of <8 %. Oestradiol (E2) was measured by RIA (Pantex) with an intra-assay variation of <8 % and an inter-assay variation of <13 %. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd) with intra- and inter-assay variations of 13 and 18 %, respectively. When inhibin B was above approximately 100 pg/ml, the intra-assay variation was <7 % and the inter-assay variation was <6 %. The majority of the men had levels above 100 pg/ml. Calculated free testosterone (FT) levels were determined using the equation of Vermeulen et al. ( Reference Vermeulen, Verdonck and Kaufman 30 ), assuming a fixed albumin of 43·8 g/l.
Statistical analysis
Sample size was calculated assuming anticipating an effect size of 0·15, for a prespecified α and power level of 5 and 80 %, respectively. With a non-response rate of 20 %, a minimum sample size of 157 subjects was needed( Reference Soper 31 ). Participants’ characteristics were summarised using the median and interquartile range (IQR) and absolute frequency and percentage across quartiles of total meat intake. Statistical differences across quartiles of meat intake, red meat intake and fish meat intake were assessed using Kruskal–Wallis tests for continuous variables and χ 2 tests for categorical variables. Semen volume, sperm concentration, total sperm count, morphologically normal sperm, FSH levels and E2 levels were log(ln) transformed before analysis to improve normality. After analysis, we back-transformed them to show values in original scale. Multivariate linear regression models were used to investigate the association of each group of meat intake (in categories) with semen quality parameters and reproductive hormones with raw data and after adjusting for confounders. We used ANCOVA to calculate adjusted reproductive outcomes and 95 % CI as dependent continuous variables for each category of meat intake and covariates as independent variables. An association was considered when we found a statistically significant linear trend among categories of meat intake as ordinal variable. Explicit substitution analyses were performed for the foods for which the unspecified substitution was statistically significant.
Confounding was assessed based on participants’ characteristics associated with meat intake and semen parameters or reproductive hormones. Based on this criteria, models were adjusted for age (years), BMI (kg/m2), smoking (current smoker v. not current smoker), physical activity (h/week), television (TV) watching (h/week) and total energy intake (kJ/d). The remaining types of meat (continuous) and dietary patterns (continuous) were also included into the models to address the possibility that observed associations would be explained by the overall food choices rather than intake of specific meats. Specifically, as described elsewhere in this population( Reference Cutillas-Tolín, Mínguez-Alarcón and Mendiola 23 ), we used principal components analysis with orthogonal transformation based on predefined food groups( Reference Hu, Rimm and Stampfer 32 ) to obtain uncorrelated factors (dietary patterns) with simpler structures. Terms for intake of meats were excluded from this analysis. We retained the first two factors (online Supplementary Table S2) based on the amount of variance explained by the factor, the scree plot and the interpretability of the factors. Every subject was given a score for the two identified patterns according to their food consumption, and each participant appears in results for both dietary patterns. All analyses of semen parameters were also adjusted for abstinence time. Analyses of sperm motility were additionally adjusted for time to start semen analysis, and analyses of reproductive hormones were adjusted for time of blood draw. Analyses were performed with the statistical software IBM SPSS 21.0 (IBM Corporation).
Results
Participants were predominantly Caucasian (97·6 %) and non-smokers (68·1 %). They had a median age of 20·5 (IQR 19·6–21·5) years and BMI of 23·7 (IQR 21·8–25·4) kg/m2. The median physical activity and TV watching was 9 and 20 h/week, respectively (Table 1, online Supplementary Table S3). A total of seventy-one subjects had a surgical scar in the genital area or lower abdomen mostly due to frenectomies or circumcision (53·5 %), appendectomy (16·9 %) and inguinal hernia repair (9·9 %) (data not shown). The most consumed meat product was processed red meat (29 %), followed by dark meat fish (22 %), white meat (18 %), unprocessed red meat (12 %), white meat fish (11 %), shellfish (5 %) and organ meats (3 %). Men in highest quartile of total meat intake had a higher total energy intake. Meat intake was positively related to physical activity and to the Mediterranean and Western dietary patterns, and inversely related to TV watching. Red meat intake was positively related to energy intake and the Western dietary pattern, and negatively related to TV watching. Fish intake was positively associated with energy intake, the Mediterranean dietary pattern and physical activity. No other subject characteristics were significantly different across quartiles of total meat intake, red meat or fish intake (Table 1, online Supplementary Table S3). Semen parameters and reproductive hormone levels were within normal limits for healthy adult men (Table 1, online Supplementary Table S3).
Table 1 Participant characteristics according to intakes of red meat or fish * (Murcia Young Men’s Study (n 206)) (Medians and interquartile ranges (IQR) for continuous variables; numbers of participants and percentages for categorical variables)
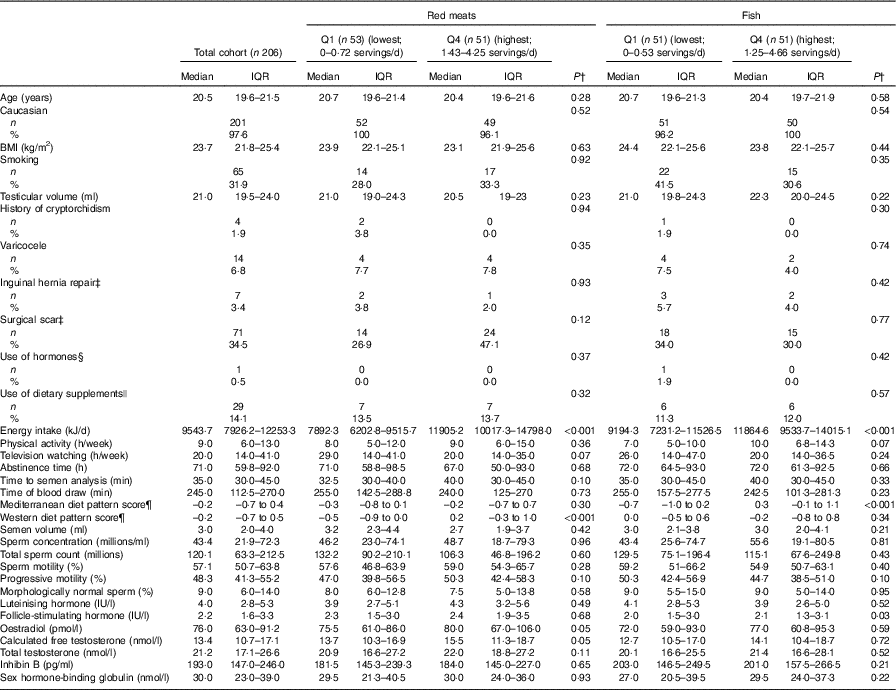
Q, quartile.
* Total red meat includes processed and unprocessed red meats and organ meat and total fish meat includes white fish meat, dark fish meat and shellfish.
† Kruskal–Wallis test for continuous variables and χ² test for categorical variables.
‡ Physical examination in the genital area (including lower abdomen).
§ Self-report of any use of dehydroepiandrostendione, androstenedione, creatinine, steroids or other muscle buildings.
|| Vitamins and minerals.
¶ Dietary pattern scores without total meat intake.
Total meat intake was unrelated to semen quality parameters (Table 2, online Supplementary Table S4) or reproductive hormone levels (Table 3, online Supplementary Table S5). Similarly, when the major categories of meat were examined, we found no association of intakes of red meats, white meats or fish with semen quality parameters (Table 2) or reproductive hormone levels (Table 3).
Table 2 Multivariable adjusted * semen parameters in relation to meat intake (Murcia Young Men’s Study (n206))
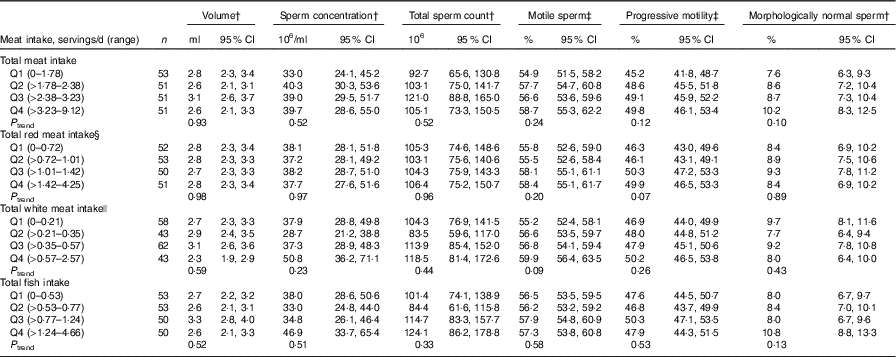
Q, quartile.
* Adjusted for energy content intake, intakes of the remaining meats, dietary patterns, age, BMI, smoking, physical activity, television watching and abstinence time.
† Back-transformed to original scale.
‡ Additionally adjusted for time to start semen analysis (min).
§ Includes processed and unprocessed red meat and organ meat.
|| Includes chicken with and without skin, rabbit, quail and duck.
Table 3 Multivariable adjusted * reproductive hormone levels in relation to meat intake (Murcia Young Men’s Study (n 206))
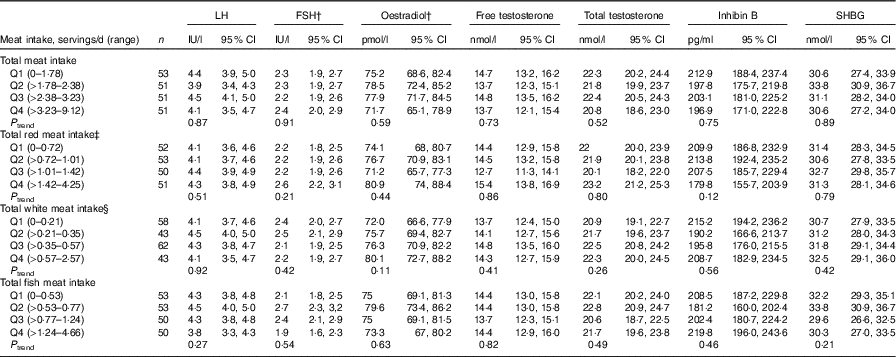
LH, luteinising hormone; FSH, follicle-stimulating hormone; SHBG, sex hormone-binding globulin; Q, quartile.
* Adjusted for energy content intake, intakes of the remaining meats, dietary patterns, age, BMI, smoking, physical activity, television watching and time of blood draw.
† Back-transformed to original scale.
‡ Includes processed and unprocessed red meat and organ meat.
§ Includes chicken with and without skin, rabbit, quail and duck.
We then evaluated intake of sub-categories of meat in relation to semen quality and reproductive hormones (online Supplementary Tables S6–S9). In these analyses, shellfish intake was positively related to progressive sperm motility (online Supplementary Tables S6 and S8). The multivariate adjusted progressive sperm motility for men in increasing quartiles of shellfish intake was 45·2 (95 % CI 42·1, 48·3), 42·0 (95 % CI 38·2, 45·7), 49·4 (95 % CI 46·9, 51·8) and 53·2 (95 % CI 50·0, 56·4) % (P trend<0·001) (Fig. 1, online Supplementary Table S8). This association was similar when we used men who reported no intake of shellfish as the reference category and divided the remaining men in quartiles of intake. In this analysis, the multivariable adjusted progressive sperm motility for men who did not consume shellfish and men in increasing quartiles of intake was 44·8, 44·7, 42·2, 49·4 and 53·2 %, respectively (P trend<0·001), and similar pattern was found for total sperm motility. Furthermore, there was an inverse relation between shellfish intake and E2 levels (online Supplementary Table S4). The multivariable-adjusted serum E2 concentrations for men in increasing quartiles of shellfish intake were 77·2, 86·6, 76·1 and 67·8 pmol/l (P trend = 0·02) (online Supplementary Table S4). Last, we examined the difference in semen quality parameters associated with the substitution of other types of meat with shellfish. Consuming shellfish instead of organ meats was associated with higher total and progressive sperm motility (P trend<0·001). A similar association was observed when shellfish was eaten instead of white meat fishs (motile sperm, P trend = 0·03; progressive motility, P trend = 0·004) or dark meat fishs (motile sperm, P trend = 0·03; progressive motility, P trend = 0·006) (data not shown).
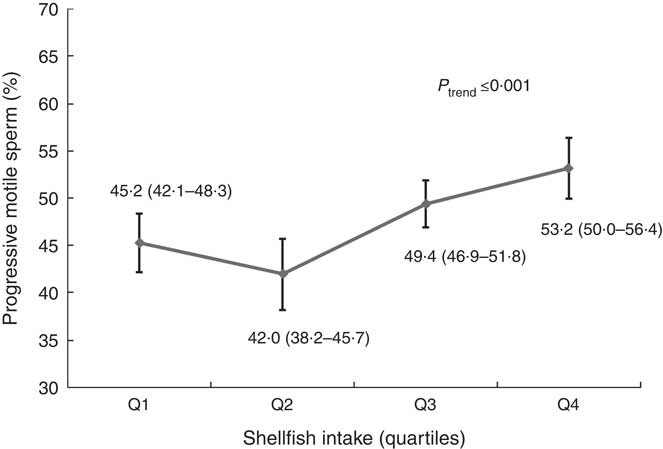
Fig. 1 Shellfish intake in relation to progressive sperm motility among healthy young men. Estimates are adjusted for total energy intake, intakes of other meats, dietary patterns, age, BMI, smoking, physical activity, television watching, abstinence time and time to start semen analysis. Q, quartile.
We also found an inverse association between organ meat intake and sperm motility (online Supplementary Tables S6 and S8). The multivariate percentage of progressively motile sperm for men who did not consume organ meats was 50·2 (48·3–52·1) % compared with 44·8 (95 % CI 42·4, 47·2) % for men who consumed organ meats (P = 0·001) (online Supplementary Table S8). LH levels for organ meat consumers were higher than those of non-consumers (4·6 v. 4·0 IU/l) after multivariable adjustment (P = 0·03). Levels of TT, FT and E2, as well as the TT:LH and FT:LH ratios were unrelated to organ meat intake (data not shown). In addition, unprocessed red meat intake was positively associated with LH (P trend = 0·02) and SHBG (P trend = 0·001) in adjusted models. No other associations were identified with semen quality parameters (online Supplementary Table S3) or reproductive hormone levels (online Supplementary Table S4).
As shellfish intake was related to higher progressive motility and lower E2 levels, and organ meat to lower progressive motility and higher LH levels, we examined whether LH or E2 were related to progressive motility in this population, and whether further adjustment for these hormones affected the association of shellfish and organ meat intake with semen quality. LH and E2 were unrelated to semen quality in this population, and additional adjustment did not change the previously observed associations (data not shown).
Discussion
In this cross-sectional study of healthy young men, we found that total meat intake was unrelated to semen quality or reproductive hormone levels. When subgroups of meat were separately considered, however, shellfish intake was associated with higher progressive motility and organ meat intake was inversely related to this outcome. We also found association with reproductive hormones that did not explain the association with semen quality. The remaining types of meat were unrelated to semen quality parameters or reproductive hormone levels. The relevance of the observed association of shellfish and organ meat intakes with semen quality and reproductive hormones should be further evaluated, given that these specific relations have only been evaluated in a small number of previous studies( Reference Afeiche, Williams and Gaskins 19 ).
Our findings are consistent with those of previous studies that explored the association between fish and seafood intake with semen quality. Two case–control studies among men in fertility clinics have reported significantly higher shellfish intake among normospermic men than among oligoasthenoteratospermic patients( Reference Afeiche, Williams and Gaskins 19 ) and greater seafood intakes as a protective factor for asthenozoospermia( Reference Eslamian, Amirjannati and Rashidkhani 21 ). Greater sperm motility has also been previously related to higher consumption of fish and other seafood( Reference Vujkovic, de Vries and Dohle 33 ), while in other study no associations were found with any semen indicators( Reference Braga, Halpern and Figueira Rde 34 ). Afeiche et al. (22) reported a positive association between intake of dark meat fish intake and total sperm count as well as a positive relation between white meat fish intake and morphologically normal sperm among men presenting to a fertility clinic but not among healthy young men( Reference Afeiche, Williams and Gaskins 19 ).
Shellfish is an important source of PUFA( Reference Dong 35 ), and previous work has related higher consumption of n-3 PUFA to better sperm motility( Reference Safarinejad 36 ). However, because we did not observe an association between dark meat fish intake and semen quality in this study, and previous work in this cohort has not found associations between intakes of n-3 and semen quality parameters or reproductive hormones( Reference Chavarro, Mínguez-Alarcón and Mendiola 12 , Reference Mínguez-Alarcón, Chavarro and Mendiola 24 ), intake of n-3 PUFA is unlikely to explain our findings. Instead, the relation between shellfish intake with sperm motility may be due to the intake of micronutrients highly concentrated in shellfish such as Zn( Reference Dong 35 ). Zn is involved in male reproductive function including spermatogenesis, sperm maturation and sperm activation( Reference Zhao, Dong and Hu 37 ). A recent meta-analysis showed that Zn supplementation in infertile men was associated with better semen quality parameters including sperm motility( Reference Zhao, Dong and Hu 37 ).
To our knowledge, only three previous studies have addressed the association between intake of organ meat and semen parameters. Organ meat intake has been previously hypothesised to lower semen quality( Reference Afeiche, Williams and Gaskins 19 ). Contrary to our findings, a similar study among young men in the USA found organ consumption to be positively related to sperm motility( Reference Afeiche, Williams and Gaskins 19 ). Previous studies among infertile men have not found a relationship between organ consumption and sperm motility( Reference Afeiche, Williams and Gaskins 19 , Reference Afeiche, Gaskins and Williams 22 ). As literature is scarce and inconsistent, this association may represent a chance finding and more studies are needed to clarify this discrepancy.
Several limitations should be discussed. The cross-sectional design is not strong to distinguish causal from non-causal relationships and the small sample size could result in missing true associations. However, results of the semen analysis were unknown to participants, essentially blinding them to the study outcome, and thus decreasing the likelihood of reverse causation. Diet was assessed using an FFQ and like all other diet assessment tools, FFQ are subject to measurement error. However, this questionnaire has been validated previously in Spanish populations( 27 ). In addition, FFQ are known to be better at ranking than at estimating exact intakes. Our analytic strategy of making comparisons between quantiles of intake rather than relying on continuous measures of intake is not only better aligned with this characteristic of FFQ but also protects against the influence of individuals with very high intakes, thus resulting in conservative estimates of the associations examined. Moreover, as the consumption of shellfish and organ meats was very low (3–5 %) and the multiple comparisons developed in the statistical analysis, we cannot rule out the possibility of residual confounding or chance findings that could explain the associations reported. However, we adjusted for a large number of potential confounders, including known predictors of semen quality as well as lifestyle factors associated with meat intake, including other dietary behaviours as captured by data-derived dietary patterns. Finally, although the homogeneity of study participants may have increased the internal validity of the study, it may limit the generalisability of our results to men facing difficulties with fertility.
In conclusion, we found no relation of total meat intake nor intake of major types of meat (red meats, white meats and fish) with markers of testicular function. Nevertheless, analyses of subgroups of meat suggest that intake of shellfish may be beneficial for sperm motility, whereas the intake of organ meats may have the opposite relation. Due to the scarcity of literature in this population and in specific types of meats, additional research is needed to confirm or refute these findings.
Acknowledgements
This work was supported by the Seneca Foundation, Regional Agency of Science and Technology, grant no. 08808/PI/08; Ministerio de Ciencia e Innovación, Instituto Carlos III (FIS), grant no. PI10/00985; National Institutes of Health, grant no. P30DK046200.
A. M. T.-C., J. M. and N. J. were involved in study conception and study design. J. M. was involved in study execution and acquisition of data. A. B. M.-C. performed the statistical analysis. A. B. M.-C., L. M.-A., J. E. C. and J. J. A.-G. contributed to data analysis and interpretation. A. B. M.-C., L. M.-A. and J. E. C. drafted the manuscript. J. V. and N. J. were involved in critical revision for important intellectual content. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003458







