LEARNING OBJECTIVES
After reading this article you will be able to:
• critically appraise using combinations of antipsychotics in the treatment of schizophrenia – specifically the use of partial agonists and dopamine blockade
• understand how receptor occupancy of antipsychotics during treatment can be calculated
• review an example of an antipsychotic combination from a recently published study using the theoretical underpinnings.
In three other articles in this issue we review the nature of partial agonism of dopamine receptors (Cookson Reference Cookson and Pimm2023a) and the qualities of three partial agonists, aripiprazole, brexpiprazole and cariprazine (Cookson Reference Cookson and Pimm2023b), and discuss the combination of a partial agonist with a full antagonist (Cookson Reference Cookson, Pimm and Reynolds2023c).
Combining antipsychotics to treat schizophrenia is discouraged for several reasons (Barnes Reference Barnes, Drake and Paton2020):
• side-effects may be exacerbated – especially those that are dose-dependent, such as hypotension, QT prolongation, weight gain and extrapyramidal symptoms
• lack of evidence of effectiveness, particularly when one drug is already used at the maximum recommended dose
• absence of proven theoretical underpinnings
• adverse interactions – although pharmacokinetic interactions between antipsychotics are few, phenothiazines may inhibit drug-metabolising enzymes (Loga et al., Reference Loga, Curry and Lader1975), notably CYP2D6.
However, combinations are frequently used for:
• mitigation of side-effects (combining a non-sedative antipsychotic with a sedative one)
• treatment failure with one antipsychotic at optimal dose
• obtaining another therapeutic effect, for example combining an antipsychotic without antidepressant effects with one with antidepressant effects
• part of cross-tapering.
In practice, the combinations used by clinicians give little consideration to their therapeutic receptor profiles. Often, they are simply combined because of an attempt to personalise treatments based on clinical intuition.
Combining a full antagonist with a partial agonist has been shown in certain circumstances to be of benefit. For example, addition of aripiprazole to risperidone has been shown able to reduce symptoms of hyperprolactinaemia without worsening psychosis. The antipsychotics most commonly combined with aripiprazole are clozapine and olanzapine, but only the combination with clozapine has shown greater effectiveness in preventing hospital admission than the antagonist (clozapine) alone in naturalistic studies (Tiihonen Reference Tiihonen, Taipale and Mehtälä2019); this may reflect better adherence to medication when side-effects such as weight gain are addressed. Such uses have – in contrast to the combination of full antagonists – a possible theoretical basis (Reynolds Reference Reynolds2021).
Dopamine hypothesis, receptor occupancy and dose
Schizophrenia arises from an interaction of genetic and environmental factors in development, resulting in specific and subtle neuronal dysfunction. According to the dopamine hypothesis, a consequence of this is a dysregulation of dopaminergic neurons, resulting in increased release of presynaptic dopamine that underlies positive psychotic symptoms; this can be relieved by antipsychotic drugs that antagonise the excess, primarily by competitive blockade of dopamine D2/D3 receptors (McCutcheon Reference McCutcheon, Reis Marques and Howes2020).
Occupancy and quantification of receptor blockade
The main measure used to estimate the strength of an antagonist is the dissociation equilibrium constant Kd, also called the dissociation constant. This is the ratio between the rate constant of association and that of dissociation. It corresponds to the concentration that will occupy 50% of receptors at equilibrium.
D2 occupancy in treating schizophrenia
Key in understanding treatment of schizophrenia are the concentrations (or doses) needed to reach sufficient levels of blockade of the D2/D3 receptors so that psychotic symptoms are controlled. The different stages of schizophrenia – first episode, chronic-relapsing and stable with maintenance treatment – require different levels of occupancy that result from different drug concentrations, which can be expressed as multiples of the dissociation constant Kd (Table 1). This might be the consequence of dopamine release increasing and decreasing in the course of the illness; other factors, such as changes in receptor numbers, would complicate the calculation.
TABLE 1 Clinical stage, required occupancy of D2/D3 dopamine receptors by antagonist antipsychotic, and concentration (or dose) expressed as multiples of the dissociation constant
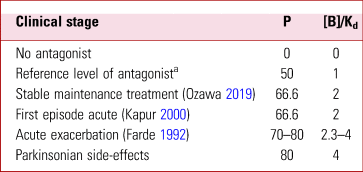
P, the proportion of receptors occupied (receptor occupancy); [B], concentration of the drug; Kd, dissociation constant.
a The reference level of 1 gives an occupancy or P of 50%. Doubling the reference level blocks about 66% of the receptors, and trebling blocks about 75%.
Receptor occupancy and dose
The relationship between dose and receptor occupancy is determined by the affinity of the drug for the receptor and can be predicted over a wide range of doses by the ‘law of mass action’, according to an equation describing a rectangular hyperbola:
where P is the proportion of receptors occupied; [B] is concentration of the drug; and Kd is the dissociation constant of the drug at that receptor (Lazareno Reference Lazareno and Birdsall1993). This signifies that when the concentration is the same as the Kd, the occupancy is 50%.
For efficacy in an acute relapse of schizophrenia in adults, occupancy of up to 80% of receptors is required (Farde Reference Farde, Nordstrom and Wiesel1992). This would correspond to a concentration up to four times the Kd (Table 1). Importantly, with older antipsychotics the onset of Parkinsonian side-effects in young adults has been shown to occur with occupancies above 80% (Table 1). Only 65% occupancy is observed in some patients in the long-term maintenance phase of schizophrenia (Ozawa Reference Ozawa, Bies and Pillai2019) and in people with a first episode (Kapur Reference Kapur, Zipursky and Jones2000).
Replacing concentrations with doses
In practice, the dose of an antipsychotic is known but the concentration is usually unknown. It is therefore helpful to know the dose of drug that will produce a concentration leading to 50% occupancy at steady state blood levels. We call that dose the DssOcc50 (the dose producing 50% occupancy at steady state blood levels), and it can be derived from positron emission tomography (PET) scans. Nyberg & Farde (Reference Nyberg and Farde2000) called this measure Ki, but to be unambiguous we use DssOcc50 here.
The formula above can use doses in place of concentrations, provided that the Kd value is substituted by DssOcc50. But there are complications in the relationship between dose and blood level (Box 1). These complications mean that the relationship between concentration and occupancy is more robust than that between dose and occupancy. The latter shows more variation between individuals due, for example, to genetic differences in drug metabolism or transport.
BOX 1 Complications concerning the relationship between dose and blood level of antipsychotic
• There are marked individual variations in absorption and metabolism of drugs (e.g. CYP2D6 status, age)
• Concentration in blood (and brain) may not be linearly related to dose
• Metabolites may play a part in clinical effects: risperidone and aripiprazole have active metabolites
• Metabolism of the drug may be induced during prolonged exposure, resulting in the need for higher doses to reach a given blood level and occupancy (e.g. chlorpromazine)
The formula tells us that the concentration or dose required to produce extrapyramidal side-effects is twice the dose needed to treat a first episode but not always higher than the dose needed for an acute relapse.
An additional complication is that a blood–brain barrier exists whereby drugs are extruded from the brain and the efficiency of this extrusion varies between drugs and with age, with corresponding differences in brain/plasma concentration ratios.
Example converting blood level from mg/ml to nM/L
The following data are from Mamo et al (Reference Mamo, Graff and Mizrahi2007).
The mean blood level on aripiprazole 10 mg/day was 126 ng/ml. Aripiprazole has a molar mass of 448.385 g/mol:
The protein binding of aripiprazole is 99.36%, giving a free aripiprazole level of 0.64% or 1.8 nM/L
Understanding occupancy with combinations of drugs
The consequence (in terms of receptor occupancy, Occ) of combining two antipsychotics depends on the concentration (or dose) and the dissociation constants (Kd values) of the two drugs. The formulae are:


where B1 is the concentration of drug 1; B2 the concentration of drug 2; Kd1 the dissociation constant of drug 1; and Kd2 the dissociation constant of drug 2 (Salahudeen Reference Salahudeen and Nishtala2017).
Using dopamine receptor affinity and occupancy to compare doses
The main laboratory measure used to estimate the strength of a receptor antagonist is the dissociation constant Kd introduced above and this is the concentration that will occupy 50% of those receptors at equilibrium.
Since clinicians seldom have access to blood levels of antipsychotics (and their interpretation would be complicated by protein binding) it would be more clinically useful to know the proportion of receptors occupied when the drug has reached a steady state level on regular dosing.
Substituting dose for concentration and assuming they are linearly related:
where DssOcc50 is the dose producing 50% occupancy at steady state.
Published data from PET scans using ligands that bind to D2/D3 receptors (particularly [11C]raclopride) provide values for DssOcc50 for several antipsychotics: amisulpride (Sparshatt Reference Sparshatt, Taylor and Patel2009), haloperidol and risperidone (Nyberg Reference Nyberg and Farde2000), aripiprazole (Sparshatt Reference Sparshatt, Taylor and Patel2010) and olanzapine (Bishara Reference Bishara, Olofinjana and Sparshatt2013). The doses shown are averaged and there is a two-fold range in the dose between individuals in the cases of risperidone, haloperidol and amisulpride (Table 2). A very high occupancy is reported for zuclopenthixol acetate (used to control severe agitation in psychosis), where the DssOcc50 is less than 12.5 mg and the maximum recommended dose is 400 mg over 3 days (Nyberg Reference Nyberg, Farde and Bartfai1995).
TABLE 2 Doses of antipsychotics producing 50% occupancy of D2/D3 receptors in positron emission tomography scans at steady state (DssOcc50) compared with lowest doses for maximum efficacy

ED95, 95% of the effective dose, defined as the lowest dose that produces the maximum effect.
Source: Leucht et al (Reference Leucht, Crippa and Siafis2020).
For efficacy in an acute relapse of schizophrenia, occupancy by D2/D3 antagonists of up to 80% of receptors is required (Farde Reference Farde, Nordstrom and Wiesel1992). This would correspond to a concentration four times the Kd or a dose four times the DssOcc50.
Leucht et al (Reference Leucht, Crippa and Siafis2020) presented graphs of dose–response relationships for a range of antipsychotics in acute schizophrenia, to compare their relative potency. Their preferred measure of potency is the ED95, 95% of the effective dose or ‘maximal effective dose’, defined as the lowest dose that produces the maximum effect.
Furthermore, examination of the ED95 values calculated by Leucht et al (Reference Leucht, Crippa and Siafis2020) shows that they are similar to four times the DssOcc50. Notably, aripiprazole is an exception (Table 2).
We can therefore substitute DssOcc50 for Kd in formulae (c) and (d) above, as shown in Box 2.
BOX 2 Formulae to determine receptor occupancy for two drugs in combination


where DssOcc50(drug1) is the dose of drug 1 producing 50% occupancy of D2/D3 receptors (seen in positron emission tomography scans) at steady state blood levels.
Other pharmacological mechanisms in antipsychotics
Many newer antipsychotics (e.g. olanzapine and risperidone) block serotonin at 5-HT2A receptors and are thought to increase presynaptic dopamine release in motor areas of the basal ganglia (Dewey Reference Dewey, Smith and Logan1995). This is thought to contribute to the avoidance of extrapyramidal side-effects (EPSE). Such an increased release would require an increase in the concentration of drug to reach the level of D2/D3 receptor occupancy that would cause EPSE.
Antipsychotic effects can arise from mechanisms other than D2/D3 blockade – clozapine being a clear example involving other mechanisms.
Example: olanzapine combined with amisulpride
The COMBINE trial, a randomised controlled trial comparing olanzapine with amisulpride or a combination of the two in acutely ill people with schizophrenia, found that the combination of olanzapine and amisulpride brought greater improvement over 8 weeks than olanzapine alone, but not significantly greater than amisulpride alone (Schmidt-Kraepelin Reference Schmidt-Kraepelin, Feyerabend and Engelke2022). However, the authors noted higher dose equivalents in the combined treatment. The mean daily doses were olanzapine 14.1 mg, amisulpride 659 mg and the combination 14.4 mg olanzapine plus 567 mg amisulpride (Table 3). The equations imply occupancy levels of 80% for olanzapine alone, 81% for amisulpride alone and 88% for the combination. One possible explanation is that for some patients occupancy levels over 80% brought about greater improvement.
TABLE 3 Striatal dopamine D2/D3 occupancy in the COMBINE study (Schmidt-Kraepelin Reference Schmidt-Kraepelin, Feyerabend and Engelke2022) of olanzapine, amisulpride and their combination

DssOcc50, the dose that will produce a concentration leading to 50% occupancy at steady state blood levels.
Exploring the exceptional partial agonism of aripiprazole
Aripiprazole monotherapy
When a partial agonist occupies receptors it competes with the transmitter – to produce some blockade – but it has an important stimulating effect, which is also known as intrinsic activity.
Aripiprazole's intrinsic activity has been calculated to be about 25% (Cosi Reference Cosi, Carilla-Durand and Assié2006; Mace Reference Mace and Taylor2009). Therefore, to achieve 75% blockade, near complete (99%) receptor occupancy is necessary with aripiprazole. It should be emphasised that although intrinsic activity is a robust measure of individual receptor responses, differences can arise in whole tissues according to the number of receptors available and which second messenger is involved.
Interestingly, the maximum recommended clinical dose of 30 mg produces about 73% antagonistic occupancy or blockade (Table 4); this would avoid Parkinsonian side-effects and could be sufficient to treat a first episode of psychosis (Robinson Reference Robinson, Gallego and John2015; Pagsberg Reference Pagsberg, Jeppesen and Klauber2017).
TABLE 4 Doses of aripiprazole, total D2/D3 receptor occupancy and antagonistic occupancy based on P = 100[dose]/(DssOcc50 + [dose]), assuming DssOcc50 is 1 mg (Sparshatt Reference Sparshatt, Taylor and Patel2010)

P, the proportion of receptors occupied (receptor occupancy); DssOcc50, the dose that will produce a concentration leading to 50% occupancy at steady state blood levels.
However, it would not necessarily be sufficient to achieve remission in an acute relapse; there, the full antagonists amisulpride, risperidone and olanzapine have greater overall efficacy than aripiprazole (Huhn Reference Huhn, Nikolakopoulou and Schneider-Thoma2019). Aripiprazole has efficacy is preventing relapse in maintenance therapy of schizophrenia compared with placebo (Kane Reference Kane, Sanchez and Perry2012), but in a long-term study of effectiveness, aripiprazole was not as effective as most other antipsychotics for continuation or preventing re-admission to hospital (Zhao Reference Zhao, Lin, Teng, Khoo, Soh and Furukawa2016).
Conclusions
We suggest that estimates of D2/D3 receptor occupancy by individual antipsychotics used in combination can be derived from formulae (c) and (d) above by substituting the dose found to produce 50% occupancy at steady state in PET scans (DssOcc50) for the dissociation constant (Kd), and using the dose of drug in place of the drug concentration (Box 2).
The formulae in Box 2 can been used to explore various combinations of antipsychotics, including combining a partial agonist with a full antagonist, in the hope of clarifying clinical findings.
Limitations
Individual antipsychotics vary in their ratios of D2 and D3 receptor binding in striatal and extra-striatal areas (Schwartz Reference Schwartz, Diaz and Pilon2000). Furthermore, the two receptor types are differentially antagonised by antipsychotic agents, with effects reflecting their different functional activity and anatomical distribution. Nor does the model presented here account for drug effects at presynaptic D2/D3 receptors.
Some antipsychotics do not show a clear hyperbolic dose–response relationship. For example, quetiapine has proved difficult to quantify in this way, with low apparent occupancy levels perhaps related to its shorter half-life and fast dissociation kinetics. Furthermore, clozapine cannot be included in this model of drug interactions because its additional efficacy in otherwise non-responsive patients likely depends on mechanisms other than D2/D3 receptor antagonism, and occupancy in acute treatment is less than 70% (Farde Reference Farde, Nordstrom and Wiesel1992). Hence, any displacement by aripiprazole will not reduce blockade of dopamine (see Cookson et al, Reference Cookson, Pimm and Reynolds2023c).
The extent of occupancy by dopamine itself is largely unknown.
The intrinsic activities of the D2 partial agonists, including aripiprazole and cariprazine, are determined from in vitro studies and may not be accurate measures of their activity in human brain. Intrinsic activity is dependent on cellular environment and second messenger systems associated with the receptor, which may vary between different tissues.
Nevertheless, we believe that the theoretical model described here provides a reasoned basis for qualitative understanding of complex clinical situations involving combinations of antipsychotics.
Acknowledgement
We are grateful to Professor Gavin Reynolds for his expert scientific advice – including the mechanism of serotonin antagonism in antipsychotics – provided during the finalisation of this article.
Author contributions
J.C. and A.B. discussed and developed the project and the concept of DssOcc50. J.P. developed the form of the manuscript. We all contributed to writing and editing the paper.
Declaration of interest
J.C. is a member of the BJPsych Advances editorial board and did not take part in the review or decision-making process for this article; he declares no other conflicts for the past 3 years. A.B. is commercial associate at Otsuka Pharmaceuticals.
MCQs
Select the single best option for each question stem:
1 Regarding the use of antipsychotic combinations in schizophrenia:
a doses of both drugs should be reduced by 50% in all cases
b side-effects produced by the single drugs are always made worse
c clinicians may be unaware of the resulting levels of occupancy at the D2 receptors
d pharmacokinetic interactions between antipsychotics are irrelevant
e extensive evidence from randomised controlled trials has been published.
2 A partial agonist with 25% intrinsic activity must occupy what proportion of receptors to achieve antagonistic occupancy of 53% of dopamine receptors?
a 99%
b 91%
c 83%
d 73%
e 71%.
3 An antipsychotic that occupies 50% of brain dopamine receptors at a dose of 5 mg daily, at steady state is likely to achieve maximum therapeutic efficacy at which daily dose?
a 5 mg
b 10 mg
c 15 mg
d 20 mg
e 25 mg.
4 In the COMBINE trial, using olanzapine together with amisulpride resulted in:
a greater improvement over 8 weeks compared with olanzapine alone
b greater improvement over 8 weeks compared with amisulpride alone
c no difference between the combination compared with olanzapine alone or amisulpride alone
d lower levels of receptor occupancy compared with the individual drugs
e greater improvement only in the extrapyramidal side-effects.
5 The following total dopamine receptor occupancy is required in patients with schizophrenia who have relapsed after responding to a combination of antipsychotics:
a 90%
b 80%
c 70%
d 60%
e 50%.
MCQ answers
1 c 2 e 3 d 4 a 5 b








eLetters
No eLetters have been published for this article.