Normal pregnancy is associated with insulin resistance and altered glucose tolerance that has been well characterised in human subjects and animal models(Reference Catalano, Tyzbir and Wolfe1–Reference Gilbert, Pere and Baudelin3). While the peripheral tissues (especially muscle) exhibit a ≥ 50 % decrease in insulin sensitivity by late pregnancy(Reference Butte4–Reference Lain and Catalano6), hepatic glucose production (HGP) continues to be suppressed by hyperinsulinaemia(Reference Connolly, Papa and Smith5, Reference Lain and Catalano6). Oral glucose tolerance tests are widely used during pregnancy for the diagnosis of impaired glucose tolerance and gestational diabetes. Mixed meal tolerance tests have been suggested as a more informative and physiological alternative to glucose tolerance tests, since they provide data about metabolism of all macronutrients, not just carbohydrate, and they stimulate the release of glucagon, in addition to insulin and incretin hormones(Reference Maki, Rains and Dicklin7–Reference Wolever, Chiasson and Csima9). The metabolic response to a mixed meal, and particularly the role of the liver in the disposition of a mixed meal, has been less well studied than disposition of a glucose load during pregnancy, however.
The conscious dog model is near-unique in its ability to allow assessment of hepatic glucose uptake (HGU), because its size allows cannulation of the vessels supplying and draining the liver. These cannulations are too invasive to be employed in humans, and no reports of measurement of HGU in rodents have been published, underscoring the difficulty of transferring the technique to small animal models. Therefore, we used the conscious dog to assess the roles of the liver and peripheral tissues in the disposition of a standardised mixed meal, particularly in regard to the uptake of glucose and synthesis of glycogen. Quantifying the disposition of a mixed meal provides a better understanding of the way the body meets both the fetal and maternal nutrient requirements during late pregnancy.
Methods
Animals and surgical procedures
Studies were conducted on normal conscious female non-pregnant (NP) and pregnant (P) mongrel dogs (n 16; eight animals per group). Vanderbilt University (Nashville, TN, USA) has a comprehensive animal care and use programme that is registered with the United States Department of Agriculture and fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC-International). The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee before initiation of the studies, and all institutional, federal and AAALAC guidelines were adhered to in conducting these studies. The P dogs were studied in the 7th–8th week of gestation (total duration of pregnancy approximately 9 weeks). The NP dogs had basal oestrogen and progesterone concentrations. Body weights at the time of experimentation were 23·7 (sem 1·2) and 26·3 (sem 1·0) kg for NP and P dogs, respectively (P = 0·10). Before the day of the study, all dogs were fed once daily a diet of meat (Pedigree; Mars PetCare, Franklin, TN, USA) and chow (Purina Lab Canine Diet no. 5006; PMI Nutrition, Henderson, CO, USA) with a combined composition of 34 % protein, 14·5 % fat, 46 % carbohydrate and 5·5 % fibre based on dry weight. The diet was nutritionally adequate for P dogs, and the animals in both groups consumed approximately 6908–7745 kJ/d (1650–1850 kcal/d). Approximately 16 d before the experiment, each dog underwent a laparotomy under general anaesthesia (isoflurane, 0·8 %). Sampling catheters were placed in the femoral artery, portal vein, left common hepatic vein and right common iliac vein; ultrasonic flow cuffs (Transonic Systems, Ithaca, NY, USA) were positioned about the hepatic artery, portal vein and external iliac artery; and a gastrostomy tube was inserted as described previously(Reference Moore, Pagliassotti and Swift10, Reference Wasserman, Lacy and Bracy11). The distal ends of the catheters and flow probes were placed in subcutaneous pockets. Criteria for use of an animal for experimentation were as described previously(Reference Connolly, Holste and Aglione12).
Experimental procedures
All dogs were fasted for 18 h before the experiment. On the morning of the experiment, a primed (36 μCi), continuous (0·3 μCi/min) infusion of [3-3H]glucose was begun via a cephalic vein. The experiment consisted of a 90 min equilibration period ( − 120 to − 30 min), a 30 min basal period ( − 30 to 0 min), a 30 min period (0–30 min) of continuous intragastric infusion of a liquid-defined formula diet and then a 360 min postprandial period (30–390 min). The meal consisted of 38 % protein, 17 % fat and 45 % carbohydrate, in the form of whey protein (ProMod; Abbott Nutrition, Columbus, OH, USA), lipid emulsion (Microlipid; Nestlé, Minneapolis, MN, USA) and d-glucose (Sigma, St Louis, MO, USA); the macronutrient contributions to total energy intake were designed to be as close as possible to the animals' usual diet. Each animal received 2·8 g protein/kg, 0·6 g lipid/kg and 3·2 g glucose/kg, and 250 μCi [U-14C]glucose was added to the formula infused.
Blood was drawn every 15 min during the basal and early postprandial periods, with the sampling interval being increased later in the postprandial period. At the end of the study, each animal was killed with an overdose of pentobarbital. A biopsy from each of the seven maternal liver lobes and three skeletal muscles (sartorius, gracilis and gastrocnemius) was immediately freeze-clamped in liquid N2 and stored at − 80 °C for future analysis of glycogen content.
Sample analysis
Samples were analysed for haematocrit, plasma glucose, NEFA, insulin, glucagon, [3H]glucose and [14C]glucose-specific activities, and blood glucose, lactate, alanine, glycerol, β-hydroxybutyrate, acetoacetate and gluconeogenic amino acids as described previously(Reference Connolly, Holste and Aglione12, Reference Pagliassotti, Holste and Moore13). 14CO2 measurements were performed in triplicate on arterial, portal vein and hepatic vein blood(Reference Moore, Pagliassotti and Swift10). 14C-labelled glucose, lactate and alanine were separated by ion-exchange chromatography(Reference Moore, Pagliassotti and Swift10, Reference Shiota, Golden and Katz14). Glycogen content of liver and muscle was analysed enzymatically following acid precipitation(Reference Keppler, Decker and Bergmeyer15).
Calculations
Arterial, portal and hepatic vein substrate concentrations were used for calculation of organ balance by the arteriovenous difference technique. The rate of substrate delivery to the liver (hepatic load; HL) was calculated as follows: HL = A × Q A+P × Q P, where A and P are the blood or plasma concentrations of the substrate in the femoral artery and portal vein, respectively, and Q A and Q P are the rates of blood or plasma (as appropriate) flow through the hepatic artery and portal vein, respectively. Plasma flow was derived from blood flow; plasma flow = blood flow × (1 − haematocrit). Net hepatic balances (NHB) of substrates were calculated with the following formula: NHB = H × Q H − HL, where H is the hepatic vein substrate concentration (blood or plasma) and Q H is the total hepatic blood or plasma flow (i.e. Q A+Q P). Net fractional substrate extraction by the liver was the ratio of NHB:HL. Net gut balance was calculated as follows: net gut balance = (P − A) × Q P. Plasma glucose values were converted to blood values as described previously(Reference Pagliassotti, Holste and Moore13). Net gut and hepatic balances of [14C]glucose and 14CO2 (in disintegrations per min (dpm)/kg per min) were calculated as for other substrates, but using plasma flows. The results were divided by the inflowing glucose specific radioactivity (dpm/mg glucose) to convert them to glucose; the specific activity of glucose in the meal was used for net gut uptake and oxidation, and the weighted specific activity of glucose in the artery and portal vein was used for net hepatic uptake and oxidation. The HL and balance data for substrates are expressed per 100 g liver weight, to correct for differences in body size between the groups. However, the data were also calculated per kg body weight, and the conclusions did not differ. Maternal liver weights at the end of the study were 715 (sem 43) and 836 (sem 72) g in the NP and P groups, respectively (P = 0·14).
Hepatic glycogen synthesis (cold) was calculated as the difference between the final glycogen concentrations in the mixed meal-fed animals and the concentrations in liver biopsies taken from 18 h-fasted NP (n 5) and P (third-trimester; n 4) dogs. Net deposition of glycogen in liver was also calculated using both [3H]glucose and [14C]glucose by dividing hepatic tracer glycogen accumulation (dpm/g liver) by the average inflowing tracer glucose specific activities. Indirect glycogen synthesis was determined by the difference between 14C- and 3H-determined glycogen synthesis.
The hindlimb of the dog is approximately 66 % muscle, with most of the remainder of the mass being bone, and therefore it served as an index of muscle glucose uptake(Reference Wasserman, Lacy and Bracy11). Hindlimb balance = (A − I) × Q I, where I and Q I indicate the iliac vein substrate concentration and iliac artery blood flow, respectively. Hindlimb fractional extraction was calculated as hindlimb balance divided by load to the limb (A × Q I).
The trapezoidal rule was used to calculate the area under the curve (AUC) for the 390 min prandial and postprandial periods. Only positive excursions from zero (i.e. net gut production of substrates) were used in the calculation of AUC for gut disposition of glucose. Both positive and negative excursions (i.e. net balance) were included in the calculations of hepatic and hindlimb disposition of substrates.
Repeated-measures ANOVA was used for statistical comparisons of time-course data, with the Tukey test for post hoc analysis (Systat, Chicago, IL, USA). For comparison of single items of data (e.g. AUC or glycogen concentrations between the groups), t tests were used unless the data failed the test of normality, in which case Mann–Whitney rank sum tests were used (as indicated later).
Results
Hormone concentrations
Arterial plasma insulin concentrations tended to be higher in NP v. P dogs both in the basal period and following the meal, but this did not reach statistical significance (P = 0·21 for the time-course data; Fig. 1). The peak postprandial concentrations in NP dogs (438 (sem 78) pmol/l) were obtained 15 min after the end of the meal, but the peak in the P animals (336 (sem 84) pmol/l) was not evident until 60 min postprandially (P = 0·34 for peak concentrations). The timing of the peak was highly variable within both groups. The AUC of the insulin response (change from basal concentrations) was 88 230 (sem 16 314) and 69 750 (sem 19 512) pmol/l per 390 min in NP and P dogs, respectively (P = 0·23). The glucagon and cortisol concentrations did not differ between the groups at any time (Fig. 1).
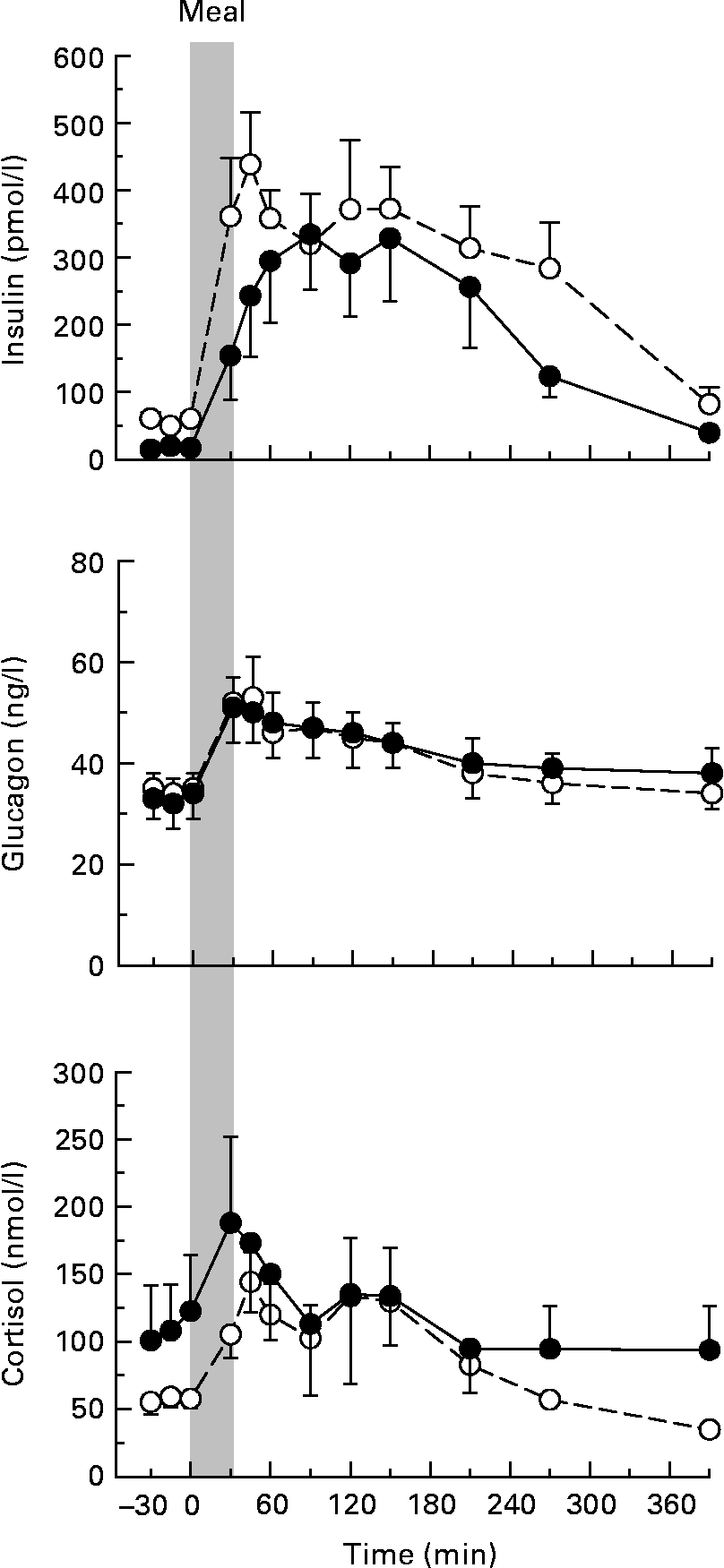
Fig. 1 Arterial plasma concentrations of insulin, glucagon and cortisol in non-pregnant (NP; –○–) and pregnant (P; –●–) dogs. Values are means, with their standard errors represented by vertical bars (n 8 per group). The grey bar indicates the period of continuous intragastric meal infusion. Mean values were not significantly different between the groups.
Glucose concentrations and balance data
Arterial plasma glucose concentrations did not differ between the groups in the basal period, but the postprandial concentrations between 60 and 210 min were significantly higher in the P group (Fig. 2), and the AUC of the change from basal concentrations was 2-fold higher in the P group (187 990 (sem 33 590) v. 86 680 (sem 12 140) mg/l per 390 min, P < 0·05). Portal vein plasma glucose was significantly higher in the P v. NP group from 90 to 390 min (AUC 198 910 (sem 14 510) v. 302 850 (sem 45 400) mg/l per 390 min, P < 0·05). Hepatic blood flow (Table 1) tended to be lower in the P group, although neither hepatic artery (P = 0·31) nor portal vein (P = 0·27) blood flow differed significantly between the groups. Both groups demonstrated a significant postprandial rise in portal vein flow. The hepatic glucose load did not differ significantly between the groups (Fig. 2).

Fig. 2 Arterial and portal vein plasma glucose concentrations, net gut glucose output, hepatic glucose load, net hepatic glucose uptake and net hepatic fractional glucose extraction in non-pregnant (NP; –○–) and pregnant (P; –●–) dogs. Values are means, with their standard errors represented by vertical bars (n 8 per group). The grey bar indicates the period of continuous intragastric meal infusion. * Mean values were significantly different between the groups (P < 0·05; post hoc analysis).
Table 1 Blood flow (ml/min)†
(Mean values with their standard errors, n 8 per group)

NP, non-pregnant; P, pregnant.
* Mean values were significantly different from those of basal rate in the same group (P < 0·05).
† Total hepatic blood flow is the sum of the hepatic artery and portal vein flows.
‡ Mean values were not significantly different between the groups.
During the basal period, both groups exhibited net gut glucose uptake, which shifted to net gut glucose output (NGGO) concomitantly with feeding. Overall, NGGO was not significantly different between the groups (Fig. 2), and the AUC totalled 68·2 (sem 3·2) and 61·9 (sem 7·4) % of the administered glucose in the NP and P groups, respectively (P = 0·52). The peak rate of NGGO occurred at 45 min in the NP group compared with 90 min in the P group. By 390 min, the NP group had returned to basal rates of net gut glucose uptake, but the P group continued to display NGGO. If net gut glucose balance in the two groups was compared only at the 390 time point, using the Mann–Whitney test, the rates would have been significantly different ( − 0·7 (sem 0·5) v. 0·9 (sem 0·5) mg/kg per min in NP v. P dogs, respectively; P < 0·05). The AUC of gut glucose oxidation was similar in the two groups (11 (sem 5) and 12 (sem 11) mg/kg per 390 min (P = 0·99), or a total of 220 (sem 97) and 306 (sem 295) mg/390 min (P = 0·78), in NP and P dogs, respectively; Fig. 3).

Fig. 3 Postprandial glucose oxidation by the gastrointestinal tract, liver and hindlimb in non-pregnant (NP; –○–) and pregnant (P; –●–) dogs. Values are means, with their standard errors represented by vertical bars (n 8 per group). * Mean values were significantly different between the groups (P < 0·05).
The rates of net hepatic glucose output during the basal period were not significantly different between the groups, and both groups shifted to net hepatic glucose uptake (NHGU) during the postprandial period, although the shift appeared to be slightly slower in the P group (Fig. 2). The AUC of NHGU (calculated as change from basal rates) were 3691 (sem 475) and 5304 (sem 1105) mg/100 g liver 390 min in NP and P dogs, respectively (P = 0·38). If expressed per kg body weight, NHGU would have totalled 1108 (sem 135) and 1562 (sem 296) mg/kg per 390 min in NP and P dogs, respectively (P = 0·16). HGU calculated with [14C]glucose totalled 2723 (sem 1230) and 4471 (sem 1450) mg/100 g liver per 390 min in NP and P dogs, respectively (P = 0·34). Hepatic glucose oxidation was higher in the P group than in the NP group during the early postprandial period (P < 0·05; Fig. 3), although oxidation was a minor fate in the liver and the other organs and tissues examined. The AUC of hepatic fractional glucose extraction was approximately 40 % greater in the P group (P = 0·13; Fig. 2).
Hindlimb blood flow was not significantly different between the groups (P = 0·39; Table 1). In addition, hindlimb glucose uptake (Fig. 4) and glucose oxidation (Fig. 3) did not differ significantly between the groups. The AUC of hindlimb fractional glucose extraction was nearly 10-fold higher in the NP group than in the P group, however (Fig. 4; P < 0·05).

Fig. 4 Hindlimb glucose uptake and fractional extraction in non-pregnant (NP; ![]() ) and pregnant (P;
) and pregnant (P; ![]() ) dogs. Values are means, with their standard errors represented by vertical bars (n 8 per group). The grey bar indicates the period of continuous intragastric meal infusion. The inset histogram shows the area under the curve (AUC) of change from basal in fractional extraction (NP, □; P, ■). * Mean values were significantly different from those of the NP group (P < 0·05).
) dogs. Values are means, with their standard errors represented by vertical bars (n 8 per group). The grey bar indicates the period of continuous intragastric meal infusion. The inset histogram shows the area under the curve (AUC) of change from basal in fractional extraction (NP, □; P, ■). * Mean values were significantly different from those of the NP group (P < 0·05).
Non-glucose substrates
Lactate concentrations and balance data did not differ significantly between the groups at any time (Table 2). In the early postprandial period, there was a surge of net hepatic lactate output in both groups, with a consequent rise in arterial lactate concentrations. The AUC of net hepatic lactate output was 50 % greater in the NP group than in the P group (P = 0·07). Hindlimb lactate uptake increased in parallel with the increase in circulating concentrations.
Table 2 Concentrations and net hepatic and hindlimb balances of lactate, glycerol, NEFA and Ala
(Mean values with their standard errors, n 8 per group)

NP, non-pregnant; P, pregnant.
* Mean values were significantly different between the groups (P < 0·05; post hoc analysis).
† Negative rates indicate net uptake.
Basal arterial blood glycerol concentrations tended to be higher (P = 0·15) in the P v. NP group. The concentrations and net hepatic glycerol uptakes in both groups fell during the early postprandial period and then returned to basal by the end of the study (Table 2). Hindlimb glycerol uptake was significantly lower in the P v. NP group during the basal period, but the rate declined similarly in both groups in the postprandial period. Arterial NEFA concentrations were significantly higher in the P v. NP group during the basal and early postprandial period. The NEFA concentrations and net hepatic uptake rates fell in both groups in the postprandial period (Table 2), and the concentrations and net hepatic output of β-hydroxybutyrate declined as well. β-Hydroxybutyrate and acetoacetate concentrations and balance data did not differ between the groups (see Table S1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn).
Arterial concentrations of alanine and net hepatic uptake of alanine were significantly lower in the P v. NP group during the basal period (Table 2). With the exception of glycine, the arterial concentrations and net hepatic and hindlimb uptakes of the other gluconeogenic amino acids increased similarly in the postprandial period (see Table S2 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). In the P group, arterial glycine concentrations increased postprandially while there was no increase in the NP group, resulting in a significant difference in concentrations during 30–210 min.
Muscle and hepatic glycogen
The glycogen concentrations of the three skeletal muscles were very homogeneous, and therefore the values were averaged for each dog. The muscle glycogen concentrations at the end of the study were 37 % higher in NP v. P dogs, although the rates of muscle glycogen synthesis did not differ significantly (Table 3).
Table 3 Maternal glycogen‡
(Mean values with their standard errors, n 8 per group)
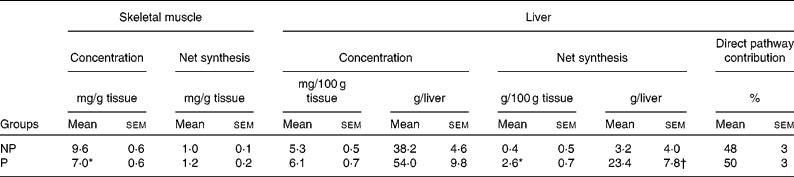
NP, non-pregnant; P, pregnant.
Mean values were significantly different from those of the NP group: *P < 0·01; †P < 0·05.
‡ Glycogen concentrations are from biopsies taken at the end of the study. Skeletal muscle concentrations are the mean of concentrations in three muscles (sartorius, gracilis and gastrocnemius), and hepatic concentrations are the weighted (for the percentage contribution of each lobe to total liver mass) mean of seven liver lobes.
Hepatic glycogen concentrations in the dogs from which biopsies were taken after an 18 h fast averaged 4·9 (sem 0·5) and 3·6 (sem 0·9) mg/100 g liver in NP and P dogs, respectively (P = 0·07). Because the livers of P dogs were larger (P < 0·01) than those of NP dogs, the total hepatic glycogen content was virtually identical in NP and P dogs after the 18 h fast (24·8 (sem 3·0) and 23·9 (sem 7·6) g, respectively). In the meal-fed animals, hepatic glycogen concentrations at the end of the study tended to be greater in the P v. NP group (P = 0·14 or 0·07, depending on whether the data are expressed in mg/g liver or g/liver; Table 3). Postprandial net hepatic glycogen synthesis was 6- to 7-fold greater in the P v. NP group, but the percentage contribution of the direct pathway did not differ between the groups (P = 0·80).
Discussion
The P dogs exhibited marked postprandial hyperglycaemia. We previously observed an increased AUC of plasma glucose concentrations in normal P v. NP female dogs following an oral glucose load(Reference Moore, Menon and Coate16), which is consistent with the present results. Glucose absorption appeared to be prolonged in P v. NP dogs, which might have resulted from reduced gut motility as a consequence of elevated progesterone in the P animals(Reference Liu, Chen and Liu17) or a delay in gastric emptying related to the hyperglycaemia in the P group(Reference Jones, Berry and Kong18, Reference Kuo, Gentilcore and Nair19). The hepatic glucose loads (a determinant of the rate of NHGU(Reference Cherrington20)) in the two groups were virtually identical throughout the postprandial period, despite the postprandial hyperglycaemia in P dogs. This occurred because hepatic blood flow tended to be lower in P v. NP dogs, which is consistent with our previous findings(Reference Connolly, Papa and Smith5). The rate of NHGU (the sum of two processes, HGP and HGU) under postprandial conditions has not been assessed previously in human subjects during pregnancy because it is not possible to cannulate the portal vein. Likewise, to our knowledge, there is only one previous report quantifying NHGU during pregnancy in a non-ruminant animal model, an assessment of the response to meal feeding in P rabbits(Reference Pere, Baudelin and Briggs21). However, in rabbits, unlike human subjects and dogs(Reference Catalano, Tyzbir and Wolfe1, Reference Connolly, Papa and Smith5), the liver exhibits insulin resistance during normal pregnancy(Reference Hauguel, Gilbert and Girard22). This might be related to the fact that NEFA concentrations are markedly elevated in P v. NP rabbits after an overnight fast(Reference Pere, Baudelin and Briggs21). While NEFA concentrations tend to be moderately elevated in normal overnight-fasted P v. NP women and dogs, the magnitude of the increase is much less than in P rabbits (approximately 1·0- to 1·3-fold in women(Reference Catalano, Nizielski and Shao23–Reference Sivan, Homko and Chen25) and dogs(Reference Connolly, Holste and Aglione12, Reference Connolly, Aglione and Smith26) v. 1·8-fold in rabbits(Reference Pere, Baudelin and Briggs21)). Although it has been suggested that circulating NEFA levels are poorly correlated with the suppressibility of endogenous glucose production in pregnancy(Reference Sivan, Homko and Whittaker27), NEFA concentrations are a key determinant of endogenous glucose production in the NP state(Reference Cherrington, Edgerton and Sindelar28). HGP in normal P women and dogs is known to be suppressible by insulin under euglycaemic conditions to a similar extent as in NP controls(Reference Catalano, Tyzbir and Wolfe1, Reference Connolly, Papa and Smith5), and the current data indicate that postprandial HGU is at least as great during late pregnancy as in NP females, in the presence of similar changes in insulin concentrations. In our previous findings in NP and P dogs studied under clamp conditions with matched levels of hyperinsulinaemia and hyperglycaemia in the presence of portal vein glucose infusion, the AUC of NHGU was significantly reduced in the P group(Reference Moore, Smith and Lacy29). This suggests that hyperglycaemia following the mixed meal feeding allowed the P group to achieve normal or even enhanced rates of hepatic glucose disposal.
Cardiac output increases in pregnancy in the dog(Reference Blanco, Tórtora and Rodríguez30), as in other species, and thus it might seem surprising that hepatic and hindlimb blood flow tended to be lower in P dogs than in NP dogs. In previous studies, we have observed that total hepatic blood flow has been both higher(Reference Connolly, Holste and Aglione12, Reference Connolly, Aglione and Smith26) and lower(Reference Connolly, Papa and Smith5, Reference Connolly, Aglione and Smith31) in P dogs than in NP controls, when total flow per animal is considered, but it has generally tended to be lower in P v. NP animals when expressed per 100 g of maternal liver(Reference Connolly, Holste and Aglione12). Hindlimb flow has also shown a tendency to be lower during pregnancy(Reference Connolly, Papa and Smith5). In numerous investigations, ultrasonic flow probes have yielded results comparable with those with an independent technique, indocyanine green extraction, both in P and NP dogs(Reference Connolly, Papa and Smith5, Reference Connolly, Aglione and Smith31, Reference Canniff, Smith and Lacy32). Indocyanine green was not infused in the current studies because the postprandial lipaemia renders its measurement virtually impossible. However, we are confident, based on our previous comparisons, that the tendency towards lower blood flows in the P group did not arise from systematic underestimation by the flow probes. Near term, the uterus and products of conception account for a greater percentage of total body weight in dogs than in humans, and canine uteroplacental flow increases progressively from early to late pregnancy(Reference Nautrup33). Thus, the most likely explanation for the tendency towards lower hindlimb and hepatic blood flow in the P group, in comparison with the N group, is that a large percentage of maternal cardiac output is required for perfusion of the uteroplacental vessels with a concomitant relative decrease in flow to some maternal tissues. A decrease in the proportion of total blood flow directed to the liver is supported by studies in normal P women(Reference Munnell and Taylor34). Data regarding changes in hepatic blood flow in human pregnancy are conflicting, however. Studies with dye extraction indicate no change in the rate of hepatic blood flow in women during normal pregnancy(Reference Munnell and Taylor34). In contrast, ultrasonography indicates that portal vein blood flow is increased in women during the late second and third trimesters(Reference Clapp, Stepanchak and Tomaselli35, Reference Nakai, Sekiya and Oya36). The maximal velocity of portal vein flow actually decreases progressively during human pregnancy(Reference Bozgeyik, Ozdemir and Kocakoc37), but an increase in vessel diameter has been reported by one group(Reference Clapp, Stepanchak and Tomaselli35), explaining how portal vein flow could increase. Both portal vein blood flow and diameter have been found to be highly dependent on body position in P women(Reference Clapp, Stepanchak and Tomaselli35), which may explain some of the disparity in results.
Post-study hepatic glycogen concentrations did not differ between NP and P dogs, which are consistent with the data from rats in the fed state(Reference Herrera, Knopp and Freinkel38). Total net hepatic glycogen synthesis in the P group was 6- to 7-fold higher than in the NP group, however. The calculated net hepatic glycogen synthesis is an underestimate of the actual value in both groups, because the biopsies in the fasted groups were taken after an 18 h fast, the time at which mixed meal feeding was administered in the study groups. If the fasting biopsies had been taken at the same time as those in the meal-fed animals, the fasting period would have been 24·5 h, and the hepatic glycogen content would have been further reduced since canine hepatic glycogen levels are on a steep portion of the depletion curve during 18–24 h of fasting(Reference Moore, Pagliassotti and Swift10, Reference Hendrick, Frizzell and Williams39). Moreover, it is probable that the hepatic glycogen levels in P dogs would have declined more rapidly than those in NP animals, since basal HGP in both dogs and human subjects is greater during late pregnancy than in the NP state(Reference Catalano, Tyzbir and Wolfe1, Reference Connolly, Holste and Aglione12), with glycogenolysis accounting for a substantial portion of basal HGP(Reference Connolly, Holste and Aglione12, Reference Connolly, Aglione and Smith26). The relatively depleted state of basal hepatic glycogen levels in P dogs might have contributed to the rapid rate of hepatic glycogen synthesis in the P group following meal feeding, since greater hepatic glycogen depletion achieved by longer fasting (i.e. 42 v. 18 h) is known to be associated with a more rapid rate of hepatic glycogen synthesis during duodenal glucose infusion in normal dogs(Reference Galassetti, Hamilton and Gibbons40). Additionally, both oestradiol and progesterone, hormones that are elevated more than 2-fold during late pregnancy compared with anoestrus(Reference Concannon, McCann and Temple41), stimulate hepatic glycogen synthesis in rats and mice(Reference Carrington and Bailey42–Reference Kalkhoff44). The percentage contribution of the direct and indirect pathways did not differ between the NP and P groups, indicating that the rate of synthesis via both pathways was stimulated in P dogs. The enhancement of glycogen synthase activity stimulated by the increase in postprandial hepatic glucose 6-phosphate probably resulted in both glucose and gluconeogenic substrates being directed towards glycogen synthesis. Consistent with this, postprandial net hepatic lactate output tended to be reduced in the P group, indicating that glycolytic carbon was directed towards glycogen synthesis rather than hepatic release. The marked enhancement of postprandial hepatic glycogen synthesis is likely to be a significant contributor to the maintenance of glucose homeostasis in pregnancy, replenishing the stores needed to maintain a constant source of glucose for fetal and maternal metabolism.
Under hyperinsulinaemic euglycaemic clamp conditions, hindlimb glucose uptake is reduced approximately 33 % in dogs during late pregnancy(Reference Connolly, Papa and Smith5), providing clear evidence of peripheral insulin resistance. Nevertheless, the insulin response to the mixed meal was not enhanced in the P group, in keeping with our previous finding that the canine β-cell exhibits no apparent adaptation (hypertrophy, hyperplasia or neogenesis) during pregnancy(Reference Moore, Menon and Coate16). In spite of the insulin resistance of pregnancy and the lack of compensatory insulin secretion, the P group's postprandial hindlimb glucose uptake was unimpaired. Postprandial hyperglycaemia apparently allowed P dogs to overcome the pregnancy-associated peripheral insulin resistance in order to maintain rates of hindlimb glucose uptake indistinguishable from those in the NP group, because adjustment of the glucose uptake rates for the load of inflowing glucose demonstrated that the AUC of fractional glucose extraction by the hindlimb was markedly lower in P v. NP dogs. Muscle glycogen synthetic rates were at least as rapid in P dogs as in NP dogs, but the muscle glycogen concentrations were significantly lower in the P v. NP group at the end of the study. Hindlimb glucose oxidation was similar in the two groups, consistent with lower basal muscle glycogen concentrations and/or greater rates of peripheral lipid accumulation in pregnancy.
The glucagon response to hypoglycaemia is markedly blunted during late pregnancy in the normal human subjects, rats and dogs(Reference Connolly, Aglione and Smith26, Reference Canniff, Smith and Lacy32, Reference Rossi, Lapaczewski and Diamond45, Reference Rosenn, Miodovnik and Khoury46). The increase in the hormone in response to mixed meal feeding was indistinguishable in the NP and P groups; however, demonstrating that there is no inherent defect in glucagon secretion during pregnancy. Thus, the impairment of the counter regulatory glucagon response in late pregnancy is likely to be related to a specific defect in the sensing or response to hypoglycaemia, as we have suggested previously(Reference Canniff, Smith and Lacy32).
In the basal period, P dogs exhibited no net hepatic lactate output, and their arterial NEFA concentrations were higher and glycerol concentrations tended to be higher than those in NP dogs. Similarly, hepatic ketone release tended to be elevated in the P animals. All of these findings reflect a longer fasted condition(Reference Moore, Pagliassotti and Wasserman47), in keeping with the description of pregnancy as a state of accelerated starvation(Reference Metzger, Ravnikar and Vileisis24). Circulating glycerol concentrations (a sensitive indicator of lipolysis) declined to similar levels in both groups after the meal, demonstrating that suppression of lipolysis under postprandial conditions remained intact in P dogs.
The classic hyperinsulinaemic euglycaemic clamp technique has been described as the gold standard for examining insulin sensitivity(Reference Defronzo48), but it has a limited ability to assess the full dynamic range of liver glucose disposal, which includes both suppression of HGP and enhancement of HGU. Glucose disposal in response to enteral intake of glucose or a mixed meal is a function of the interaction of numerous factors, including insulin and glucagon secretion, peripheral insulin sensitivity, the degree of suppression of HGP, and the rate of HGU(Reference Defronzo48). The portal signal generated by enteral glucose delivery serves both to enhance liver glucose uptake and suppress muscle glucose uptake(Reference Cherrington20). Indices derived from the oral glucose tolerance test can serve as estimates of both muscle and liver insulin sensitivity and have been determined to be highly predictive of the development of type 2 diabetes(Reference Abdul-Ghani, Matsuda and Balas49). Thus, glucose(Reference Abdul-Ghani, Matsuda and Balas49, Reference Abdul-Ghani, Williams and DeFronzo50) or mixed meal(Reference Maki, Rains and Dicklin7) ingestion provides a useful physiological tool for assessing glucose disposal.
In conclusion, pregnancy was associated with postprandial hyperglycaemia following mixed meal feeding, but hepatic and hindlimb glucose uptake was unimpaired compared with the NP state. The liver in P dogs played a very active role in glucose metabolism, with the hepatic glycogen synthetic rate (net mass of glycogen deposited over the postprandial time period) and glucose oxidation being significantly greater in the P v. NP group. The avid nature of postprandial hepatic glycogen synthesis in the P animals probably ensures the maintenance of adequate maternal glucose concentrations to allow an uninterrupted supply of glucose to the products of conception. The potential for use of a mixed meal rather than an oral glucose load to assess glucose metabolism in pregnancy deserves further examination, since it can elicit information not only about glucose tolerance but also about other aspects of postprandial metabolism.
Acknowledgements
The present study was supported by NIH DK 058134, Juvenile Diabetes Foundation International Research grant 193113 (C. C. C.), and American Diabetes Association Research Award 7-06-RA-96 (M. C. M.). The Hormone Assay & Analytical Services Core and the Metabolic Physiology Shared Resource Core of the Vanderbilt Diabetes Research and Training Center, supported by NIDDK grant DK-20593, made substantial contributions to the study. The authors have no conflicts of interest. M. C. M. participated in designing the experiments and was responsible for the analysis and interpretation of the data and preparation of the manuscript. M. S. S. collected and analysed the data. C. C. C. participated in designing these experiments, obtained funding for them, and participated in the collection, analysis and interpretation of the data.









