Clozapine remains the treatment of choice for refractory schizophrenia despite its association with a wide range of adverse effects, both trivial and life-threatening. Clozapine is well known to cause blood dyscrasias Reference Atkin, Kendall, Gould, Freeman, Lieberman and O'Sullivan1 but it is also associated with other serious adverse effects such as seizures, Reference Devinsky, Honigfeld and Patin2 intestinal obstruction, Reference Townsend and Curtis3 myocarditis, Reference Killian, Kerr, Lawrence and Celermajer4 diabetes, Reference Lund, Perry, Brooks and Arndt5 thromboembolism and cardiomyopathy. Reference Hagg, Spigset and Soderstrom6
The frequency and variety of these effects might be expected to afford a relatively higher mortality in those receiving clozapine compared with other antipsychotics. However, studies suggest that clozapine reduces overall mortality Reference Sernyak, Desai, Stolar and Rosenheck7 (at least when compared with periods when not taking clozapine), Reference Walker, Lanza, Arellano and Rothman8 probably because it lowers suicide risk. Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi9
In a recent study, Reference Atkinson, Douglas-Hall, Fischetti, Sparshatt and Taylor10 we found that death was a common cause of clozapine treatment cessation. We wanted to compare reasons for discontinuation with another second-generation antipsychotic but were aware that covert non-adherence might confound our results (non-adherence with clozapine in responsive patients results in relapse and so is readily confirmed). In our study we compared reasons for stopping clozapine with a matched cohort of patients stopping risperidone long-acting injection.
Method
This study was approved by the South London and Maudsley Drug and Therapeutics Committee as part of its on-going audit programme. We used pharmacy computer records to determine all patients registered in to receive clozapine in the South London and Maudsley NHS Foundation Trust between March 2002 and October 2006 and identified all patients ceasing treatment during this period. Each person who discontinued clozapine (clozapine group) was matched by age and gender at discontinuation with a person who discontinued risperidone long-acting injection without knowledge of reason for discontinuation. Details of those who discontinued risperidone were obtained from our database of 277 patients developed for a previous study. Reference Young and Taylor11 Risperidone patients began treatment in or after August 2002 and ceased treatment before October 2004.
We did not perform a power calculation but used all reliable data available to us at the time. Patients in both cohorts received standard care (i.e. there was no extraneous intervention), were largely drawn from the local population and all were prescribed their antipsychotics by secondary-care psychiatrists. A small proportion in each group (<10%) were tertiary referrals.
We obtained from case notes data on ethnicity, diagnosis, duration of treatment and reason for discontinuation. We categorised reason for discontinuation as: patient decision (partial or non-adherence, or patient request or refusal); adverse effects (clinician decision to withdraw because of unacceptable side-effects); ineffective (clinician assessment of inadequate effect); death; or other. Where death was the cause of discontinuation but the exact cause of death unclear, we obtained death certificates from the public record office.
We also established mortality rates for all those known to have been treated with either drug during the observation periods stated. Study cohorts' expected mortality rates were calculated using age-specific population mortality rates for 2002 supplied by the UK Office for National Statistics. 12 Standardised mortality ratios (SMRs) were calculated using the indirect standardisation method.
Results
During the study period, 592 patients were registered to receive clozapine, 368 continued and 224 were deregistered (63 did not start clozapine or left our services during the study period). Thus, 161 patients received clozapine for at least 1 week and later discontinued. In total, 277 patients received at least one injection of risperidone, of whom 27 were lost to follow-up. Of the remaining 250, 184 discontinued and 161 were matched to patients who had discontinued clozapine (Table 1). Reasons for drug discontinuation for each group are shown in Table 2.
Table 1 Patient characteristics
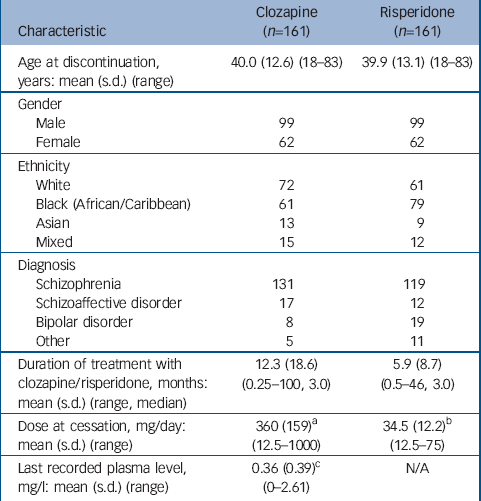
| Characteristic | Clozapine (n=161) | Risperidone (n=161) |
|---|---|---|
| Age at discontinuation, years: mean (s.d.) (range) | 40.0 (12.6) (18-83) | 39.9 (13.1) (18-83) |
| Gender | ||
| Male | 99 | 99 |
| Female | 62 | 62 |
| Ethnicity | ||
| White | 72 | 61 |
| Black (African/Caribbean) | 61 | 79 |
| Asian | 13 | 9 |
| Mixed | 15 | 12 |
| Diagnosis | ||
| Schizophrenia | 131 | 119 |
| Schizoaffective disorder | 17 | 12 |
| Bipolar disorder | 8 | 19 |
| Other | 5 | 11 |
| Duration of treatment with clozapine/risperidone, months: mean (s.d.) (range, median) | 12.3 (18.6) (0.25-100, 3.0) | 5.9 (8.7) (0.5-46, 3.0) |
| Dose at cessation, mg/day: mean (s.d.) (range) | 360 (159) a (12.5-1000) | 34.5 (12.2) b (12.5-75) |
| Last recorded plasma level, mg/l: mean (s.d.) (range) | 0.36 (0.39) c (0-2.61) | N/A |
N/A, not applicable
Table 2 Reasons for discontinuation
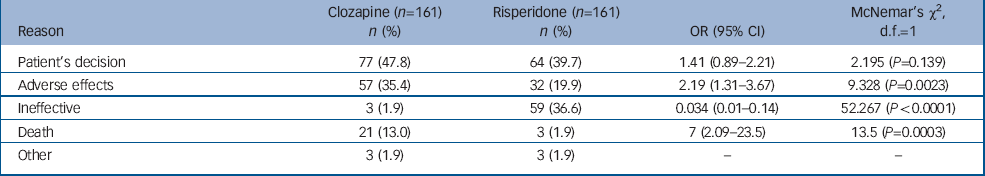
| Reason | Clozapine (n=161) n (%) | Risperidone (n=161) n (%) | OR (95% CI) | McNemar's χ2, d.f.=1 |
|---|---|---|---|---|
| Patient's decision | 77 (47.8) | 64 (39.7) | 1.41 (0.89-2.21) | 2.195 (P=0.139) |
| Adverse effects | 57 (35.4) | 32 (19.9) | 2.19 (1.31-3.67) | 9.328 (P=0.0023) |
| Ineffective | 3 (1.9) | 59 (36.6) | 0.034 (0.01-0.14) | 52.267 (P<0.0001) |
| Death | 21 (13.0) | 3 (1.9) | 7 (2.09-23.5) | 13.5 (P=0.0003) |
| Other | 3 (1.9) | 3 (1.9) | - | - |
a. Dose known for 85 of 161 patients
b. Every 2 weeks
c. Plasma level recorded for 132 of 161 patients; of these, 27 (20.5%) had plasma levels of 0.0-0.05 mg/l, indicating probable non-adherence
Mean age of those who died on clozapine was 49.2 years (s.d.=14.5, range 30–83), mean duration of treatment was 38.2 months (s.d.=29.5, range 3–100). Mean last recorded dose was 412 mg/day (s.d.=141, range 100–650) and mean last recorded clozapine level (known for 18 of 21 patients) was 0.48 mg/l (s.d.=0.31, range 0.02–1.12). Mean plasma level in those surviving (known for 114 of 140 patients) was 0.34 mg/l (s.d.=0.41, range 0.0–2.61); 25 of these had levels (0.0–0.05 mg/l) suggesting non-adherence. The deaths occurring in people receiving risperidone were in patients aged 48, 65 and 81 years. Duration of treatment with risperidone injection was 40 months, 2 weeks and 38 months respectively. We did not record duration of illness for study participants but mean duration of illness in the risperidone cohort was about 11 years. Reference Young and Taylor11
Cause of death in clozapine patients was: pneumonia (n=5), lung carcinoma (n=3), other carcinoma (n=2), myocardial infarction (n=2), cerebrovascular accident (n=2), clozapine overdose (n=2), gastrointestinal haemorrhage (n=1), cardiac arrest (n=1), left ventricular failure (n=1), asphyxia during restraint (n=1) and sepsis (n=1). There was no evidence of neutropenia or agranulocytosis in any patients at the time of death. Cause of death was established from case notes in 8 patients and death certificate in the remaining 13. Cause of death in those taking risperidone was: myocardial infarction (n=1), left ventricular failure (n=1) and sudden unexplained death (n=1). There were no deaths in the unmatched patients discontinuing risperidone.
Overall, we established outcome for 529 patients (mean age in March 2002, 36.4 years (s.d.=11.6)) receiving clozapine for at least a week during a period of 4.67 years and 250 patients (mean age 38.6 years, (s.d.=13.8)) receiving at least one risperidone injection during a period of 2.25 years. Mortality rate was 8.5 (95% CI 5.53–13.07) per 1000 patient-years for clozapine patients and 5.3 per 1000 patient-years (95% CI 1.7–16.61) for those receiving risperidone injection. Expected mortality rates were 2.04 per 1000 patient-years and 3.51 per 1000 patient-years respectively. Standardised mortality ratios were 4.17 (95% CI 2.78–6.26) for clozapine and 1.51 (95% CI 0.49–4.65) for risperidone patients.
Discussion
Reasons for discontinuation differed between clozapine and risperidone injection: adverse effects and death were more commonly recorded as reasons of discontinuation with clozapine and ineffectiveness was more often reported with risperidone. These findings have important implications for practice.
Mortality rates and cause of death
We also found that clozapine use was associated with an increased risk of death. Age at death was very low and mortality rate was higher for clozapine patients than that expected for an age-matched UK general population. Our observed mortality rate for clozapine (8.5 per 1000 patient-years) is similar to that seen in other studies. Reference Walker, Lanza, Arellano and Rothman8,Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi9 The contribution of schizophrenia to the observed increased mortality in this study cannot be discounted, Reference Osby, Correia, Brandt, Ekbom and Sparen13 although the risperidone group did not show an increased SMR (although the small number of deaths did not allow accurate determination of SMR).
It is probable that both schizophrenia and the use of antipsychotics each contribute to the previously observed increased mortality, although individual contributions are difficult to discern. Reference Joukamaa, Heliovaara, Knekt, Aromaa, Raitasalo and Lehtinen14 It has been suggested that the mortality gap between the normal population and those with schizophrenia is growing, possibly because of increased use of atypical drugs. Reference Saha, Chant and McGrath15
Cause of death suggests some contributory role for clozapine: six cardiovascular deaths may have been associated with clozapine's metabolic effects, Reference Henderson, Cagliero, Gray, Nasrallah, Hayden and Schoenfeld16 although, again, the influence of schizophrenia itself on cardiovascular mortality is clearly important. Reference Curkendall, Mo, Glasser, Rose Stang and Jones17 In addition, five patients died of a primary pneumonia, a condition previously described as a cause of death in clozapine patients Reference Walker, Lanza, Arellano and Rothman8 and one known to be more common in elderly people receiving atypical antipsychotics. Reference Knol, van Marum, Jansen, Souverein, Schobben and Egberts18 However, pneumonia is not a widely accepted adverse effect of clozapine, Reference Sernyak, Desai, Stolar and Rosenheck7 except perhaps as a result of aspiration of saliva and even then only in rare cases. Reference Hinkes, Quesada, Currier and Gonzalez-Blanco19 It is possible that smoking plays a part. Smoking may increase the incidence of bronchial infection, leading to an acute reduction in smoking frequency which then provokes clozapine toxicity Reference Derenne and Baldessarini20,Reference Zullino, Delessert, Eap, Preisig and Baumann21 and which may subsequently allow the development of pneumonia (patients are more sedated and less mobile). We were unable to establish smoking status for any of our participants because case notes are unreliable sources of this information. Clozapine is known to reduce smoking behaviour Reference Procyshyn, Tse, Sin and Flynn22 so it seems unlikely that smoking was more prevalent in the clozapine group than the risperidone group. None the less, the observation that three patients died of lung cancer suggests a significant prevalence of smoking in the clozapine group.
Study limitations
Limitations of our method include the potentially unreliable nature of data derived from case notes and the failure to collect information on other factors likely to affect outcomes (smoking status, concurrent physical illness, illness duration). Indeed, as clozapine is often reserved for treatment of longstanding, refractory illness, duration of illness and exposure to antipsychotics are likely to be relatively greater.
Clinical implications
The number and causes of death in the clozapine group suggest an important role for prevention and management of physical illness. Testing for obesity, dyslipidaemia, hypertension and diabetes are clearly essential but studies show that physical monitoring is rarely undertaken in people taking antipsychotics, Reference Paton, Esop, Young and Taylor23,Reference Taylor, Young, Esop, Paton and Walwyn24 although interventions to improve monitoring can be effective. Reference Barnes, Paton, Hancock, Cavanagh, Taylor and Lelliott25 Similarly, smoking cessation should be encouraged and assistance offered. Moreover, given the findings of this study, any bronchial infection occurring in a patient on clozapine should provoke immediate and aggressive treatment. Consideration might also be given to the addition of other drugs (e.g. aripiprazole) to clozapine, which might mitigate its adverse metabolic effects. Reference Henderson, Kunkel, Nguyen, Borba, Daley and Louie26
Our study emphasises the need for prudence in the choice of antipsychotic and the necessity for close physical monitoring of patients receiving antipsychotics, particularly clozapine. It also highlights the problems faced by clinicians who feel obliged to use clozapine because of its effectiveness, while being aware of the likelihood of severe adverse effects.





eLetters
No eLetters have been published for this article.