Ancient practices of Buddhism and Asian traditions frequently emphasize attending to experiences with equanimity to liberate one from suffering. Attending to experiences with equanimity can be referred as mindful attention (Grossman, Reference Grossman2010), often undertaken to experience reduced “sufferings” such as the pain of anxiety (Kabat-Zinn et al., Reference Kabat-Zinn, Massion, Kristeller, Peterson, Fletcher, Pbert and Santorelli1992). Mindfulness has been defined as directing one’s attention in the present moment while adopting a nonjudgmental perspective toward experiences (Kabat-Zinn, Reference Kabat-Zinn2005). Trait or dispositional mindfulness represents the tendency to behave like this as an innate individual characteristic (Baer, Smith, Hopkins, Krietemeyer, & Toney, Reference Baer, Smith, Hopkins, Krietemeyer and Toney2006), while state mindfulness refers to changes in the state resulting from, for example, meditation interventions (Lau et al., Reference Lau, Bishop, Segal, Buis, Anderson, Carlson and Devins2006). It has been frequently suggested that mindfulness either as trait or practice might show beneficial effects through emotion regulation (see review Chambers, Gullone, & Allen, Reference Chambers, Gullone and Allen2009). Anxiety, on the other hand, can be regarded as an affective state, disposition, or trait (Scherer, Reference Scherer2009) and impairs top-down processing/executive control, with an example being enhanced susceptibility toward irrelevant salient stimuli (Eysenck & Calvo, Reference Eysenck and Calvo1992; Eysenck, Derakshan, Santos, & Calvo, Reference Eysenck, Derakshan, Santos and Calvo2007; Moser, Becker, & Moran, Reference Moser, Becker and Moran2012). Anxiety may be elicited transiently in response to a threatening situation (state anxiety) or it may also be sustained as a stable property of an individual reflecting their anxiety trait (Spielberger, Gorsuch, & Lushene, Reference Spielberger, Gorsuch and Lushene1970). Furthermore, it has been suggested that stressful events occurring in early or recent life may contribute to psychopathology underlying some anxiety disorder (Faravelli et al., Reference Faravelli, Lo Sauro, Lelli, Pietrini, Lazzeretti, Godini and Ricca2012). Stress may be regarded as an emergent process that interacts with the environment, past or recent events, homeostatic states and brings about psychophysiological reactions (Epel et al., Reference Epel, Crosswell, Mayer, Prather, Slavich, Puterman and Mendes2018). The physical or psychological stimulus or event that may trigger physiological responses in the form of a stressful or anxious state typically described as stressors. These stressors are often attributed as a potential and sometimes an unpredictable threat to the individual (Hannibal & Bishop, Reference Hannibal and Bishop2014). The most widely studied mindfulness intervention program, mindfulness-based stress reduction, was developed initially to relieve the stress occurring in patients suffering from chronic pain (Kabat-Zinn, Lipworth, & Burney, Reference Kabat-Zinn, Lipworth and Burney1985).
Several empirical studies consistently suggest that mindfulness and anxiety are inversely related to each other either at state or trait levels. Here, we present a summary of the lines of evidence which may support this inverse relationship between mindfulness and anxiety, possibly through an emotion regulation mechanism. In this review article, state and trait form of mindfulness and anxiety will be used interchangeably as one, as both forms are intimately linked to each other. The current review provides the accounts of a range of measurement indices that have been used in investigations in the mindfulness and anxiety literature. These indices comprise subjective well-being (Chang, Huang, & Lin, Reference Chang, Huang and Lin2015; Coffey & Hartman, Reference Coffey and Hartman2008; Walsh, Balint, Smolira, Fredericksen, & Madsen, Reference Walsh, Balint, Smolira, Fredericksen and Madsen2009), cognitive processes (Di Francesco et al., Reference Di Francesco, Simione, López-Ramón, Belardinelli, Lupiáñez and Raffone2017; Moore & Malinowski, Reference Moore and Malinowski2009; Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, Reference Pacheco-Unguetti, Acosta, Callejas and Lupiáñez2010; Tang et al., Reference Tang, Ma, Wang, Fan, Feng, Lu and Posner2007), electrophysiology neuroanatomy (Bishop, Reference Bishop2009; Etkin et al., Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Mocaiber et al., Reference Mocaiber, Pereira, Erthal, Figueira, Machado-Pinheiro, Cagy and de Oliveira2009; Tang et al., Reference Tang, Lu, Geng, Stein, Yang and Posner2010; Way, Creswell, Eisenberger, & Lieberman, Reference Way, Creswell, Eisenberger and Lieberman2010), neuroendocrine markers (Brown, Weinstein, & Creswell, Reference Brown, Weinstein and Creswell2012; Rosenkranz et al., Reference Rosenkranz, Davidson, Maccoon, Sheridan, Kalin and Lutz2013), immunological (Black & Slavich, Reference Black and Slavich2016; Davidson et al., Reference Davidson, Kabat-Zinn, Schumacher, Rosenkranz, Muller, Santorelli and Sheridan2003; Rosenkranz et al., Reference Rosenkranz, Lutz, Perlman, Bachhuber, Schuyler, MacCoon and Davidson2016), and genetic research (Epel, Daubenmier, Moskowitz, Folkman, & Blackburn, Reference Epel, Daubenmier, Moskowitz, Folkman and Blackburn2009; Jacobs et al., Reference Jacobs, Epel, Lin, Blackburn, Wolkowitz, Bridwell and Saron2011; Schutte & Malouff, Reference Schutte and Malouff2014). The current review article is an attempt to aggregate this set of evidence to offer a broad spectrum of indices for future mindfulness-based intervention (MBI) research. This may facilitate the investigation of the efficacy of such programs by reducing the cost of the intervention and proving sensitive indices that may reveal the individual differences in responsiveness to the program under consideration.
1. Evidence suggesting an inverse relationship between mindfulness and anxiety
1.1 Self-report measures
Self-report measures are subjective measures estimating subjective feelings such as traits or states measured by questionnaires or interviews on a measurable scale and estimate these features as mathematical scores (Howard, Reference Howard1994; Razavi, Reference Razavi2001). Due to the lack of consensus on the operational definition of mindfulness, there are an array of mindfulness questionnaires which measure different aspects of mindfulness (see Table 1). Although the current article lists a range of questionnaires mentioned in mindfulness literature, it does not provide any specific recommendation on just one questionnaire (for details, see review by Chiesa, Reference Chiesa2013) with the appropriateness of the use of each depending on how mindfulness is described in a study. In contrast, anxiety, for which there is a better consensus on its definition, is most widely measured by the State-Trait Anxiety Inventory, developed by Spielberger et al. (Reference Spielberger, Gorsuch and Lushene1970), while stress is often quantified by the Perceived Stress Scale developed by Cohen, Kamarck, and Mermelstein (Reference Cohen, Kamarck and Mermelstein1994).
Table 1. Summary of questionnaires available to measure various aspects of mindfulness

Source: Information from (Chiesa, Reference Chiesa2013).
Two studies on self-report measures, one in a nonclinical group (Coffey & Hartman, Reference Coffey and Hartman2008) and another in a clinical population (with mood and anxiety disorder) (Desrosiers, Vine, Klemanski, & Nolen-Hoeksema, Reference Desrosiers, Vine, Klemanski and Nolen-Hoeksema2013), showed that both mindfulness and anxiety could be mediated through emotion regulation strategies. These strategies could be worry, reappraisal, nonacceptance, or rumination, which might be attenuated by “buffering actions” of mindfulness, which might consequently mitigate the symptoms associated with anxiety. In addition, a study showed that trait mindfulness could predict lower anxiety and negative affect in response to anxiogenic stressors (Arch & Craske, Reference Arch and Craske2010). A mindfulness-based longitudinal survey also showed a change in the trajectory of state mindfulness could predict post-intervention enhancement in trait mindfulness and decreased psychological distress levels after 7 weeks of training (Kiken, Garland, Bluth, Palsson, & Gaylord, Reference Kiken, Garland, Bluth, Palsson and Gaylord2015). Furthermore, it was suggested that mindfulness-based therapy could alleviate the symptoms of anxiety via emotion regulation (Hofmann, Sawyer, Witt, & Oh, Reference Hofmann, Sawyer, Witt and Oh2010; Tang, Holzel, & Posner, Reference Tang, Holzel and Posner2015). Therefore, future mindfulness studies investigating anxiety should consider the role of emotion regulation in mediation between mindfulness and anxiety.
1.2 Behavioral measures
There are some independent studies which have demonstrated that higher trait mindfulness, irrespective of whether one practices meditation or not, is associated with better executive functions. Executive functions can be defined as a set of regulatory mechanisms or higher order cognitive functions necessary for general-purpose control of behavior (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Miyake and Friedman, Reference Miyake and Friedman2012). For instance, conflict control is an executive function that was shown to be predicted by trait mindfulness scores in a study employing the color Stroop task (Moore & Malinowski, Reference Moore and Malinowski2009) and another studying using the Attentional Network Test (Di Francesco et al., Reference Di Francesco, Simione, López-Ramón, Belardinelli, Lupiáñez and Raffone2017). Furthermore, a recent meta-analytic review on anxiety suggested that it, in addition to being related to impairment of working memory, is correlated to measures on a wide range of cognitive process (see a review by Moran, Reference Moran2016). For example, it was shown that under a low cognitive load state, individuals with higher trait anxiety levels showed impaired conflict control (Bishop, Reference Bishop2009). Additionally, a recent study (Jaiswal, Tsai, Juan, Liang, & Muggleton, Reference Jaiswal, Tsai, Juan, Liang and Muggleton2018) showed that when participants were grouped based on their mindfulness and anxiety scores, the group with high mindfulness and low anxiety had higher efficiency of cognitive control, reflected in higher accuracy on the Stroop task as well as in its associated electrophysiology (Jaiswal, Tsai, Juan, Muggleton, & Liang, Reference Jaiswal, Tsai, Juan, Muggleton and Liang2019). They also showed higher visual working memory capacity on a change-detection task. It seems reasonable to observe impaired conflict control and working memory in high-anxiety or low mindfulness individuals, as both executive functions have been reported to be correlated with each other (Kane & Engle, Reference Kane and Engle2003). These reports indicate that conflict control and working memory capacity are two cognitive functions which are sensitive to both mindfulness as well as anxiety. However, the above-mentioned studies did not account for the role of emotion regulation in these cognitive functions. Therefore, future studies should integrate emotional manipulation to observe the effect of mindfulness and anxiety over these behavioral measures.
1.3 Electrophysiological measures
1.3.1 Event-related potential (ERP)
Electroencephalography (EEG) is an objective approach that may allow assessment of fundamental brain properties (temporally as well as oscillatory) of both mindfulness and anxiety. Event-related potential (ERP) is a temporally sensitive measure of EEG that provides measures of electrophysiological dynamics associated with behavioral and emotional phenomena (Luck & Kappenman, Reference Luck and Kappenman2012; Schupp et al., Reference Schupp, Cuthbert, Bradley, Cacioppo, Ito and Lang2000). A study that employed passive viewing of emotional and neutral images showed that the late positive potential (LPP) component of ERPs was higher in amplitude in a low mindfulness group than in a high mindfulness group in response to high arousal unpleasant images (Brown, Goodman, & Inzlicht, Reference Brown, Goodman and Inzlicht2013). This component is a positive deflection in the ERP that appears after the presentation of emotional stimuli and is regarded as an index of emotional responses in the visual modality (Schupp et al., Reference Schupp, Cuthbert, Bradley, Cacioppo, Ito and Lang2000). A higher LPP amplitude observed in a low mindfulness group was suggested to reflect a higher emotional reactivity in this group in response to unpleasant stimuli (Brown et al., Reference Brown, Goodman and Inzlicht2013). In another study, it was shown that high-trait anxiety people showed a higher LPP in response to unpleasant stimuli than for neutral stimuli, but this was not the case in low-trait anxiety people (Mocaiber et al., Reference Mocaiber, Pereira, Erthal, Figueira, Machado-Pinheiro, Cagy and de Oliveira2009). However, this observation was contextual and was modulated by the instructions the participants were given. When they were told the stimuli presented were fictitious, the difference between unpleasant and neutral condition LPP amplitudes was not significant in either group. When the instructions stated that images were obtained from real-life conditions, only the high-trait anxiety group showed a higher LPP amplitude in the unpleasant condition than in the neutral condition. The lack of any difference in LPP amplitude between the unpleasant and neutral stimuli in the fictitious condition suggests that even high-anxiety individuals can attenuate emotional reactivity, presumably by employing a reappraisal strategy for emotion regulation. Future psychotherapeutic programs should incorporate reappraisal to understand the role of emotion regulation in attenuation of emotional reactivity and the LPP component can be a potential index to measure the degree of emotional reactivity among different sets of individuals and in different conditions. Although both studies (Brown et al., Reference Brown, Goodman and Inzlicht2013; Mocaiber et al., Reference Mocaiber, Pereira, Erthal, Figueira, Machado-Pinheiro, Cagy and de Oliveira2009) were carried out independently, the pattern of LPP modulation as a function of trait mindfulness and trait anxiety is consistent with an inverse relationship between these two traits. Therefore, it will be beneficial to measure the LPP component by employing an emotional manipulation while assessing behavioral or electrophysiological characteristics of populations varying in levels of mindfulness and anxiety.
1.3.2 Resting-state EEG
Resting-state EEG, a measure of spontaneous brain activity, can be quantified by a power spectrum over the range of EEG oscillations (Putman, Reference Putman2011). The EEG oscillations reflect the complexity of activity in neural populations in the brain and can be used to estimate the degree of integration among different brain areas. For instance, global mode oscillations in delta, theta, and alpha frequencies reflect global activity between different cortical regions, while local mode oscillations in beta and gamma frequencies denote events in local cortical areas (Knyazev, Reference Knyazev2007). Global mode oscillations have been thought of as reflecting the properties of three critical mental faculties: motivation, emotion, and attentional inhibition (Knyazev, Reference Knyazev2007). Mindfulness is suggested to affect the regulation of motivation and emotion, as well as attentional control (Greeson & Brantley, Reference Greeson, Brantley and Didonna2009; Tang et al., Reference Tang, Holzel and Posner2015). These processes are reflected by physiological brain oscillations, where these oscillations, in general, have been linked to motivation (delta frequencies), to emotion (theta frequencies) and attentional inhibition processes (alpha frequencies) (Knyazev, Reference Knyazev2007). A recent meta-analysis showed that mindfulness, in general, is associated with elevated theta and alpha activity, argued to reflect a relaxed yet alert state. No systematic patterns in the delta, beta, or gamma oscillations were reported (Lomas, Ivtzan, & Fu, Reference Lomas, Ivtzan and Fu2015). There appears to be a need for more comprehensive research on oscillatory neural characteristics relating to mindfulness and how it may be reconciled with EEG oscillation research on anxiety (see review Schutter & Knyazev, Reference Schutter and Knyazev2012).
Resting-state EEG is often used as a biomarker for the psychophysiological pathology associated with anxiety and other mental disorders (Fitzgerald & Watson, Reference Fitzgerald and Watson2018; Oathes, Bruce, & Nitschke, Reference Oathes, Ray, Yamasaki, Borkovec, Castonguay, Newman and Nitschke2008; Schutter & Knyazev, Reference Schutter and Knyazev2012). Several reports have suggested that gamma activity may be a suitable marker of fear and anxiety, with an elevated level of gamma power observed in anxious people (Gemignani et al., Reference Gemignani, Santarcangelo, Sebastiani, Marchese, Mammoliti, Simoni and Ghelarducci2000; Oathes et al., Reference Oathes, Ray, Yamasaki, Borkovec, Castonguay, Newman and Nitschke2008; Oya, Kawasaki, Howard, & Adolphs, Reference Oya, Kawasaki, Howard and Adolphs2002; Sebastiani, Simoni, Gemignani, Ghelarducci, & Santarcangelo, Reference Sebastiani, Simoni, Gemignani, Ghelarducci and Santarcangelo2003). In addition to investigation of EEG oscillations in anxiety, cross-frequency coupling is another critical index that might predict levels of anxiety and cognitive abilities. Cumulative evidence demonstrates that coupling between delta and beta frequencies becomes stronger in anxious situations or among anxious people (see Schutter & Knyazev, Reference Schutter and Knyazev2012). The stronger delta–beta coherence in anxious people may be indicative of a functional relationship between the cortex and limbic system (Poppelaars, Harrewijn, Westenberg, & van der Molen, Reference Poppelaars, Harrewijn, Westenberg and van der Molen2018; Putman, Reference Putman2011) that may be a factor in impairment of emotion regulation. Currently, there is a lack of resting-state EEG studies looking at how different global mode oscillations might modulate the amplitude of local mode oscillations. Consequently, there is potential insight to be gained from investigating the modulations of fundamental brain oscillations during a resting state. While it appears that there is plenty of literature available on resting state in the context of anxiety, very few studies offer a comprehensive insight into the dynamics of brain oscillations associated with mindfulness in a resting state. Since the resting state which, unlike a meditation state involved in mindfulness practice, does not require any active mental effort, it might be associated with brain oscillations features which can better represent the characteristics of individuals and potentially relate to their levels of trait mindfulness and/or trait anxiety. Future studies could beneficially attempt to investigate the dynamics of brain oscillations and could aid the reconciliation of the relationship between anxiety and mindfulness.
1.3.3 Electrocardiograms/heart rate variability (HRV)
Electrocardiogram (ECG) is a measure of the electrical activity of the heart and is another electrophysiological measure which is affected by anxiogenic or stressful stimulus. The beat-to-beat variation in ECG is measured by an index called heart rate variability (HRV) (Akselrod et al., Reference Akselrod, Gordon, Ubel, Shannon, Berger and Cohen1981). HRV can be a biomarker of flexibility and it is affected by continuous interaction between the sympathetic and parasympathetic nervous systems to make adjustments in response to the environmental changes (Thayer & Lane, Reference Thayer and Lane2007; Thayer & Sternberg, Reference Thayer and Sternberg2006). Increased HRV is an indicator of better well-being and cardiovascular health and is often found to be reduced in patients suffering from anxiety (Kemp & Quintana, Reference Kemp and Quintana2013). There are submeasures of HRV, such as high-frequency HRV (HF-HRV) (0.15–0.40 Hz) which corresponds to respiratory sinus arrhythmia (RSA) and low-frequency HRV (LF-HRV) (0.04–0.15 Hz) corresponding to the Traube–Hering–Mayer wave(Krygier et al., Reference Krygier, Heathers, Shahrestani, Abbott, Gross and Kemp2013; Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017). Examples of changes in these measures can be seen in vagus nerve regulation of parasympathetic activity which modulates changes in RSA and HF-HRV. The LF-HRV can be produced by both parasympathetic and sympathetic activity or baroreflex activity, but it is principally produced by the parasympathetic system (Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017). The neuroendocrine system mainly regulates sympathetic activity through secretion of norepinephrine and catecholamine hormones, and this brings about transient fluctuations in HRV. HF-HRV and LF-HRV are the most widely studied HRV measures in the context of mindfulness effects (Krygier et al., Reference Krygier, Heathers, Shahrestani, Abbott, Gross and Kemp2013; Libby, Worhunsky, Pilver, & Brewer, Reference Libby, Worhunsky, Pilver and Brewer2012; Sun et al., Reference Sun, Hu, Pan, Liu and Huang2019; Takahashi et al., Reference Takahashi, Murata, Hamada, Omori, Kosaka, Kikuchi and Wada2005).
A line of studies has shown that mindfulness meditation is associated with higher HRV (Krygier et al., Reference Krygier, Heathers, Shahrestani, Abbott, Gross and Kemp2013; Libby et al., Reference Libby, Worhunsky, Pilver and Brewer2012; Takahashi et al., Reference Takahashi, Murata, Hamada, Omori, Kosaka, Kikuchi and Wada2005). This increase in HRV is often illustrated by an increase in normalized units of HF (HFn.u.), which is estimated by the formula (HF/(HF-LF)) (Takahashi et al., Reference Takahashi, Murata, Hamada, Omori, Kosaka, Kikuchi and Wada2005). Therefore, it should be kept in mind while interpreting a higher HRV that it not necessarily always contributed by increases in the HF band, it may also be due to a decrease in the LF band. For example, Krygier et al. (Reference Krygier, Heathers, Shahrestani, Abbott, Gross and Kemp2013) observed that after 10 days of Vipassana training, a form of mindfulness training, an increase in HFn.u. was seen, which was further reportedly contributed to by a decrease in LF power rather than an increase in HF power. An increase in HFn.u. is interpreted as enhanced parasympathetic activity, which is a characteristic feature associated with mindfulness training that involves slowing down of breath and bringing “calmness” to the body and relief from stressful reactions (Krygier et al., Reference Krygier, Heathers, Shahrestani, Abbott, Gross and Kemp2013). Therefore, HRV may serve as a potential index for measuring several features associated with mindfulness and anxiety that involve dynamic fluctuations in the body in response to a changing internal and external environment.
1.4 Hemodynamic neuroimaging
Hemodynamic neuroimaging involves blood oxygen level-dependent measurements made using functional magnetic resonance imaging (fMRI). fMRI that offers a very high spatial resolution, but a relatively low temporal resolution and provides an indirect measure of the activity of a brain region either related to a task or due to spontaneous modulation. Due to its high spatial resolution, fMRI has become a broadly used technique, including in numerous clinical studies of cognition (e.g., see the review by Glover, Reference Glover2011). Some fMRI studies of emotional responses have shown that individual differences in mindfulness (Creswell, Way, Eisenberger, & Lieberman, Reference Creswell, Way, Eisenberger and Lieberman2007; Way et al., Reference Way, Creswell, Eisenberger and Lieberman2010) or in anxiety (Bishop, Duncan, Brett, & Lawrence, Reference Bishop, Duncan, Brett and Lawrence2004; Etkin et al., Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Gold et al., Reference Gold, Shechner, Farber, Spiro, Leibenluft, Pine and Britton2016; Killgore & Yurgelun-Todd, Reference Killgore and Yurgelun-Todd2005) can predict the level of activation in amygdala and prefrontal cortex (PFC). For example, Creswell et al. (Reference Creswell, Way, Eisenberger and Lieberman2007) observed that people with higher trait mindfulness showed enhanced PFC activity and attenuated amygdala activity while performing an affect-labeling task compared to effects in a gender-labeling (control) task. High-trait anxiety individuals have shown an opposite pattern, with Etkin et al. (Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004) observing that people with high-trait anxiety showed higher activity in basolateral activity in the amygdala when they processed masked fearful faces unconsciously in comparison to conscious processing of non-masked faces. In addition to the amygdala, PFC is actively involved in high-anxiety individuals, with these individuals showing reduced recruitment of PFC in response to fearful distractors compared to neutral face distractor conditions while performing an identification task (Bishop et al., Reference Bishop, Duncan, Brett and Lawrence2004).
The above pattern of findings are further strengthened by converging evidence suggesting functional connectivity between PFC and amygdala that aids emotion regulation processes modulated by either mindfulness or anxiety (Creswell et al., Reference Creswell, Way, Eisenberger and Lieberman2007; Davidson, Reference Davidson2002; Gold et al., Reference Gold, Shechner, Farber, Spiro, Leibenluft, Pine and Britton2016; Holzel et al., Reference Holzel, Hoge, Greve, Gard, Creswell, Brown and Lazar2013; Shin & Liberzon, Reference Shin and Liberzon2010). It was demonstrated that in generalized anxiety disorder patients who showed negative coupling between amygdala and PFC functional connectivity altered into positive coupling after 8 weeks of mindfulness training, but this was not seen in a stress management education (control) group (Holzel et al., Reference Holzel, Hoge, Greve, Gard, Creswell, Brown and Lazar2013). However, functional connectivity in most studies has been examined for specific tasks with very few studies investigating this network in a spontaneous resting state context which can better reflect individual differences due to traits. (Lim, Teng, Patanaik, Tandi, & Massar, Reference Lim, Teng, Patanaik, Tandi and Massar2018). For instance, Bishop (Reference Bishop2009) observed reduced PFC activity due to high-trait anxiety while performing a conflict task in a low attentionally demanding state, while Creswell et al. (Reference Creswell, Way, Eisenberger and Lieberman2007) found high PFC during an affect-labeling task in high mindful people, in addition to reduced bilateral amygdala activity.
Although these neuroimaging reports show evidence in line with an inverse relationship between mindfulness and anxiety, the nature of amygdala–PFC activity appears to be generally task-dependent. A recent study on task-independent resting state (Lim et al., Reference Lim, Teng, Patanaik, Tandi and Massar2018) showed that high-trait mindfulness individuals showed greater connectivity and anticorrelations between default mode network and salience network than did low mindfulness people. Lim et al. (Reference Lim, Teng, Patanaik, Tandi and Massar2018) further explained about the possible roles of these two networks, with the default mode associated with mind-wandering which is decreased by an increase in the level of trait mindfulness (Mrazek, Smallwood, & Schooler, Reference Mrazek, Smallwood and Schooler2012). The salience network was suggested to play a role in coordinating between externally and internally directed attention supported by executive control and default mode networks, respectively (Menon & Uddin, Reference Menon and Uddin2010), thus facilitate switching between mind-wandering and task-readiness states (Sridharan, Levitin, & Menon, Reference Sridharan, Levitin and Menon2008). Therefore, stronger connectivity between the default mode and salience networks might indicate greater ability to regulate mind-wandering and task-readiness states in high mindfulness people. These observations (Lim et al., Reference Lim, Teng, Patanaik, Tandi and Massar2018) highlight the importance of exploring brain regions beyond the amygdala and PFC as are frequently reported in mindfulness and anxiety literature. For example, Frewen et al. (Reference Frewen, Dozois, Neufeld, Lane, Densmore, Stevens and Lanius2010) observed a positive correlation between left amygdala activity and a trait mindfulness “observing” component, unlike many previous studies reporting negative correlation between trait mindfulness and amygdala (Creswell et al., Reference Creswell, Way, Eisenberger and Lieberman2007; Way et al., Reference Way, Creswell, Eisenberger and Lieberman2010). This difference in Frewen et al. (Reference Frewen, Dozois, Neufeld, Lane, Densmore, Stevens and Lanius2010) study from previous studies could be due to the fact that they used a paradigm of auditory modality in which the participants had to listen to a writtenscript and further asked to imagine as if those incidents were happening at that moment. Unlike Frewen et al. (Reference Frewen, Dozois, Neufeld, Lane, Densmore, Stevens and Lanius2010), the previous studies reported here had shown a negative correlation with amygdala and had used paradigms in visual modality wherein the participants would either label the affect (Creswell et al., Reference Creswell, Way, Eisenberger and Lieberman2007) or match the affect (Way et al., Reference Way, Creswell, Eisenberger and Lieberman2010) from the faces shown on a screen. Therefore, the way instructions given to participants and nature of modality of paradigms may bring about different pattern in amygdala activities. Therefore, future fMRI studies should look into using a more task-independent approach, such as investigation of resting-state conditions, that can potentially reconcile the nature of amygdala and PFC activity, as well as shed light on other networks such as the default mode network and salience networks relating to mindfulness and anxiety. This may be helpful when comparing manipulations of mindfulness and/or anxiety to investigate the brain regions associated with better emotion regulation ability.
1.5 Molecular biomarkers
Reports have suggested consequential impact of stress and anxiety on the body (Fava, Cosci, & Sonino, Reference Fava, Cosci and Sonino2017), which are typically known as psychosomatic disorders (Walker, Reference Walker1956). For example, Fink (Reference Fink2011) suggested that post-traumatic stress disorder is a form of anxiety disorder that leads to high arousal with concurrent activation of the autonomic nervous system, principally sympathetic adrenomedullary (SAM) pathway. It was described as stress, prompting an activation response from a neuroendocrine system that is regulated by the hypothalamic–pituitary–adrenal (HPA) axis and the SAM. The hyperactivity of the two systems (HPA and SAM) may trigger a cascade of abnormal responses in behavior, hormonal secretions, immune system, and genetics (biological aging) (Black & Slavich, Reference Black and Slavich2016; Fink, Reference Fink2011). Previous review articles have already addressed the behavioral implications of anxiety and mindfulness in the above sections. Therefore, the following section summarizes the literature principally relating to three biomarkers: neuroendocrine, immunological and genetic that further strengthen the proposed linkage between mindfulness and anxiety.
1.5.1 Neuroendocrine biomarkers
One primary neuroendocrine product of the HPA axis is cortisol, a steroid hormone which under normal condition maintains the homeostasis of the body (Viau, Reference Viau2002). However, in response to stress or anxious condition, cortisol leads to increase in blood pressure, suppression of immune function in addition to several other bodily changes mediated by SAM (Hannibal & Bishop, Reference Hannibal and Bishop2014; Tsigos & Chrousos, Reference Tsigos and Chrousos2002). Conversely, mindfulness has been suggested to act as a buffer that protects from pathological effects of stress (Brown et al., Reference Brown, Weinstein and Creswell2012; Rosenkranz et al., Reference Rosenkranz, Davidson, Maccoon, Sheridan, Kalin and Lutz2013, Reference Rosenkranz, Lutz, Perlman, Bachhuber, Schuyler, MacCoon and Davidson2016). In their study, Brown et al. (Reference Brown, Weinstein and Creswell2012) showed that individual differences in mindfulness level could predict cortisol responses. Participants performed Trier Social Stress Test (Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993), a standard task in which participants need to deliver a speech for minutes in front of two critical evaluators and, consequently, induced stress response. Participants with high mindfulness showed lower cortisol reflecting lower stress responses than was seen in low mindfulness individuals (Brown et al., Reference Brown, Weinstein and Creswell2012). The role of mindfulness in stress buffering was further strengthened by the results from a short-term meditation study (Tang et al., Reference Tang, Ma, Wang, Fan, Feng, Lu and Posner2007) that employed integrative body–mind training (IBMT) with mindfulness training as a critical component. The control and meditating groups initially had no baseline differences in their cortisol levels. After 5 days of IBMT training, meditators showed lower cortisol responses than a control group after stress induction as well as in the condition after additional training (Tang et al., Reference Tang, Ma, Wang, Fan, Feng, Lu and Posner2007). Brown et al. (Reference Brown, Weinstein and Creswell2012) explained the neural basis of the mindfulness and cortisol responses as a high level of mindfulness leading to a reduction in amygdala activity in response to stressors (Creswell et al., Reference Creswell, Way, Eisenberger and Lieberman2007). Since the amygdala is connected to the HPA pathway through hypothalamic projections (Sullivan et al., Reference Sullivan, Apergis, Bush, Johnson, Hou and Ledoux2004), attenuation of amygdala activity will reduce the HPA axis secretion of cortisol. Therefore, cortisol may serve as a promising biomarker for future mindfulness studies investing stress and anxiety, being directly sensitive to variability in mindfulness and anxiety either at the trait or at the state level.
1.5.2 Immunological biomarkers
The interaction between two stress axes, HPA and SAM, interferes with normal activities of the immune system (Black & Slavich, Reference Black and Slavich2016), which can be downregulated by buffering action of mindfulness. Creswell and Lindsay (Reference Creswell and Lindsay2014) proposed that mindfulness may alleviate the activity of SAM either directly or indirectly via upregulation of the parasympathetic pathway. This alleviated activity of the SAM axis may lead to reduction in peripheral stress responses, including immune functions. Immunoglobin A (IgA) is a secretory antibody that is generated in response to a stressor in saliva (Deinzer, Kleineidam, Stiller-Winkler, Idel, & Bachg, Reference Deinzer, Kleineidam, Stiller-Winkler, Idel and Bachg2000). Tang et al. (Reference Tang, Ma, Wang, Fan, Feng, Lu and Posner2007) in their study showed that a meditating group showed a higher concentration of salivary IgA than the control group, indicating a better response to stress in the meditating group. The effect of mindfulness on secretory immune responses was further complimented by mucosal immunity (Davidson et al., Reference Davidson, Kabat-Zinn, Schumacher, Rosenkranz, Muller, Santorelli and Sheridan2003). A meditating group and waitlist control group were vaccinated with influenza vaccine after the end of 8 weeks of mindfulness training. The mindfulness group showed higher antibody levels than did the control group, suggesting they had better immunoreactivity (Davidson et al., Reference Davidson, Kabat-Zinn, Schumacher, Rosenkranz, Muller, Santorelli and Sheridan2003).
There are immunoreactive responses triggered by stress that may have adverse health effects. For instance, tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8), which are regarded as pro-inflammatory cytokines, are modulated by stress (Glaser et al., Reference Glaser, Kiecolt-Glaser, Marucha, MacCallum, Laskowski and Malarkey1999). For example, Rosenkranz et al. (Reference Rosenkranz, Davidson, Maccoon, Sheridan, Kalin and Lutz2013) showed that individuals with mindfulness training showed a larger reduction in levels of TNF-α and IL-8 than that a matched control group, indicating less of an inflammatory response after being exposed to stress in the mindfulness group. Additionally, C-reactive protein (CRP), another pro-inflammatory protein, which was reduced after mindfulness training in normal individuals (Malarkey, Jarjoura, & Klatt, Reference Malarkey, Jarjoura and Klatt2013) and in ulcer colitis patients (Jedel et al., Reference Jedel, Hoffman, Merriman, Swanson, Voigt, Rajan and Keshavarzian2014). CD4+ T lymphocyte cell count is another important index of better immune function, and chronic stress may lead to a decline in its count. This reduction in CD4+ T lymphocyte cell count may lead to the absence of defense line against disease onset and progression often observed in cancer and HIV patients (Mellors et al., Reference Mellors, Munoz, Giorgi, Margolick, Tassoni, Gupta and Rinaldo1997; Reiche, Nunes, & Morimoto, Reference Reiche, Nunes and Morimoto2004). Mindfulness intervention has been shown to either increase the count of or attenuate the decline in count of CD4+ T lymphocyte cell in breast cancer patients (Lengacher et al., Reference Lengacher, Kip, Post-White, Fitzgerald, Newton, Barta and Klein2013) as well as in HIV patients (Creswell, Myers, Cole, & Irwin, Reference Creswell, Myers, Cole and Irwin2009). In sum, mindfulness seems to offer a comprehensive way to protect the immune system either by promoting the humoral (IgA) and mucosal (CD4+ T lymphocyte) immune responses or by decreasing the levels of pro-inflammatory molecules (IL-8, TNF-α, CRP).
1.5.3 Genetic biomarkers
The stress response of the HPA axis not only affect the immune response at the humoral and mucosal levels, but also at a genetic level which is instrumental for the generation of an array of extracellular proteins (Slavich & Irwin, Reference Slavich and Irwin2014). For example, the production of pro-inflammatory cytokines is regulated by nuclear factor-kB (NF-kB), a DNA-binding protein, and upregulates the gene expression of these inflammatory proteins (Bharti & Aggarwal, Reference Bharti and Aggarwal2002). Therefore, an increase in NF-kB level has been proposed as a direct indicator of augmented responses to stress and upregulates the expression of proteins promoting inflammation (Bierhaus et al., Reference Bierhaus, Wolf, Andrassy, Rohleder, Humpert, Petrov and Nawroth2003). Conversely, mindfulness intervention has been shown to reduce the level of NFkB protein. For example, the protective effect of mindfulness was evident in two independent studies where they observed a reduced NF-kB transcription activity in older adults suffering from loneliness (Creswell et al., Reference Creswell, Irwin, Burklund, Lieberman, Arevalo, Ma and Cole2012) and in breast cancer patients (Bower et al., Reference Bower, Crosswell, Stanton, Crespi, Winston, Arevalo and Ganz2015).
Biological aging is another deleterious effect caused by chronic stress exposure leading to reduced telomerase activity and, consequently, facilitation of the cellular aging process (Epel et al., Reference Epel, Daubenmier, Moskowitz, Folkman and Blackburn2009). Telomerase is an enzyme that maintains the lengths of the telomeres (protective caps found at the terminals of the chromosomes) after every mitotic cell division (Blackburn, Reference Blackburn2005). Thus, telomerase activity prevents early cell death and thus promotes longevity or delays the aging process. In their study, Needham et al. (Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel2015) showed that women with high anxiety or panic disorder had shorter telomere length than non-anxious women, but no such effect was observed in men. Additionally, major depression patients treated with antidepressants showed shorter telomere length, which was suggested to be a possible side effect of the treatment (Needham et al., Reference Needham, Mezuk, Bareis, Lin, Blackburn and Epel2015). Furthermore, it was proposed by Epel et al. (Reference Epel, Daubenmier, Moskowitz, Folkman and Blackburn2009) that mindfulness facilitated protection against the cellular aging by positive cognitive states and positive arousal by downregulating the negative affect that brings about a stress response (Epel et al., Reference Epel, Daubenmier, Moskowitz, Folkman and Blackburn2009). A series of randomized controlled trial studies further strengthened this idea by showing mindfulness training led to an increase in telomere lengths in immune cells (peripheral blood mononuclear cells) (see review Schutte & Malouff, Reference Schutte and Malouff2014).
1.6 Summary of the dynamic relationships among indices of mindfulness and anxiety
Emotion regulation strategies tend to change the emotional state and, consequently, generate a cascade of psychological, behavioral, cognitive, physiological, immunological, and genetic responses. In a simplified model, we propose that a stressor or anxiogenic event (recent or past) increases amygdala activation. A hyperactive amygdala, in turn, upregulates the HPA axis pathway mediated by the SAM pathway (Fink, Reference Fink2011). Increased activity of the HPA axis negatively affects a broad spectrum of human characteristics (see Figure 1). Conversely, mindfulness downregulates the activity of the amygdala and attenuates the HPA axis response to stressful situations (Creswell et al., Reference Creswell, Way, Eisenberger and Lieberman2007). Consequently, mindfulness provides protection from harmful effects caused stress and anxiety. Another novel and potential biomarker to estimate efficacy of mindfulness programs objectively can be human microbiota assessed with stool samples (Househam, Peterson, Mills, & Chopra, Reference Househam, Peterson, Mills and Chopra2017). The gut microbiome is susceptible to being disturbed by stress (Santos et al., Reference Santos, Saunders, Hanssen, Yang, Yates, Groot and Perdue1999). The microbiota serve as protective barriers for the gut epithelium by producing anti-inflammatory short-chain fatty acids, while stress leads to an increase in gut barrier permeability and increased inflammatory immune responses (Groschwitz et al., Reference Groschwitz, Ahrens, Osterfeld, Gurish, Han, Åbrink and Hogan2009). Therefore, an investigation of how stress management through mindfulness may be reflected in human gut microbiota will insightful in understanding the mechanism of stress physiology.
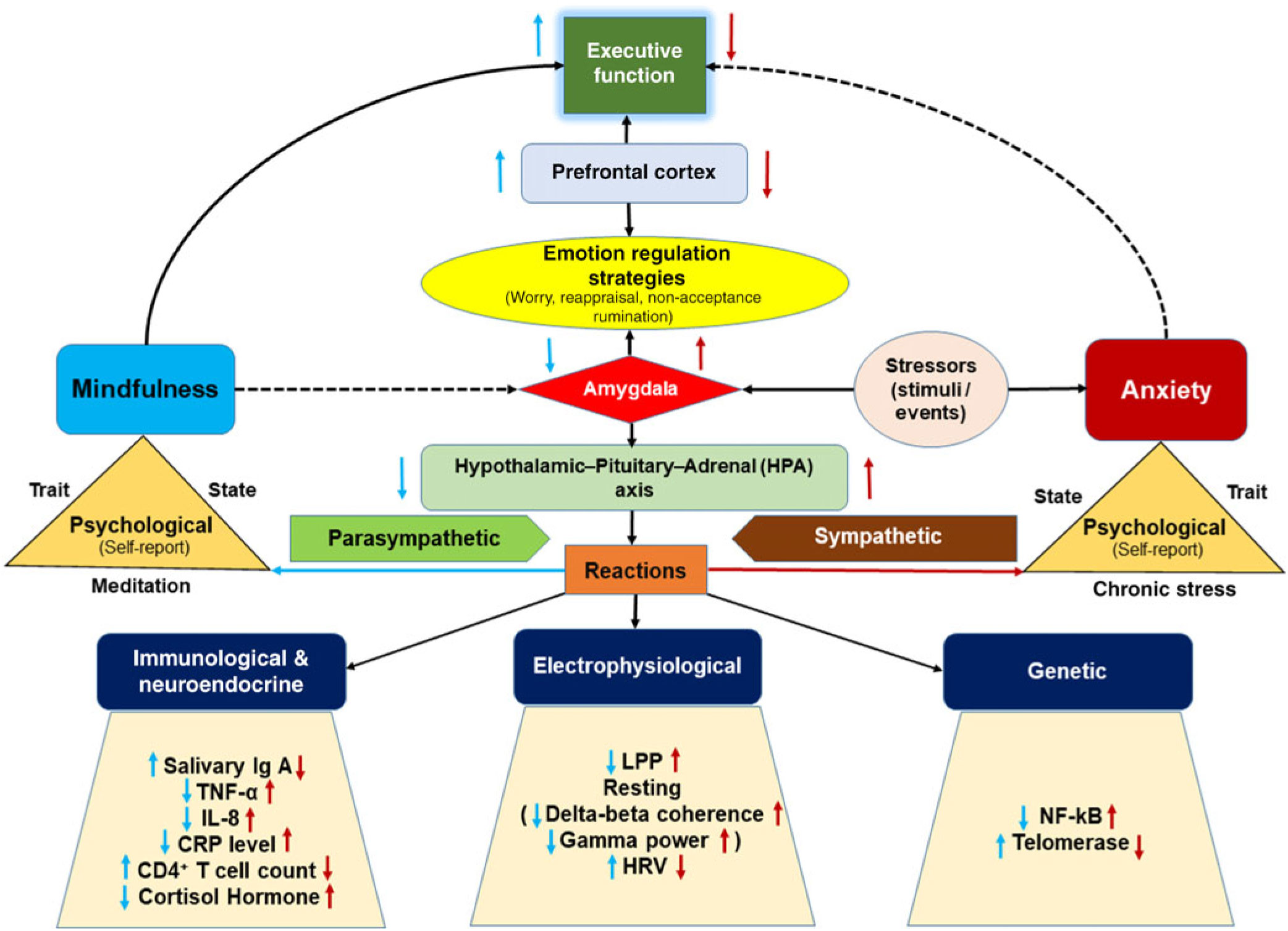
Figure 1. (Color online) A schematic overview of the indices of mindfulness and anxiety demonstrating their inverse relationship. A stressor in an anxious situation enhances the activity of the amygdala, the center of emotional reactions that trigger the hypothalamic–pituitary–adrenal (HPA) pathway. This hyperactive HPA axis leads to a cascade of reactions on different human characteristics mediated by the sympathetic nervous system. The immunological reactions show a decrease in salivary IgA, decrease in CD4+ T lymphocyte count/activity, increase in levels of tumor necrosis factor alpha (TNF-α), Interleukin-8 (IL-8), and C-reactive protein (CRP)), while endocrine reactions exhibit an increase in cortisol hormone levels. Electrophysiological effects are evident in the rise in late positive potential (LPP) amplitude in response to threatening visual stimuli, stronger delta–beta coherence, higher gamma power and a decrease in heart rate variability (HRV). In genetic effects, an increase in nuclear factor-kB (NF-kB) level and a decrease in telomerase activity are often observed. Behaviorally, anxiety impairs executive function. Conversely, mindfulness is proposed to act in the opposite direction to this with a cascade of reactions in response to a stressor mediated by the parasympathetic nervous system. Psychologically, chronic exposure to stress may lead to the more stable trait of anxiety, while the practice of mindfulness meditation may alter the mindfulness trait, suggesting a plastic nature of both traits which may be enhanced by continuous accumulation of these states. Neuroanatomically, emotion regulation is principally governed by functional interaction between amygdala and prefrontal cortex (PFC) regions, which are respectively involved in monitoring emotional reactions and higher order cognitive functions. It is proposed that mindfulness-related skills facilitate better emotion regulation strategies, such as by alleviating worry and rumination and enhancing acceptance and reappraisal abilities. This proposed schematic overview was adapted from concepts mentioned relating to the measurement indices (blue arrow: activity contributed by mindfulness, dark red arrow: activity contributed by anxiety, upward arrow: increase in activity, and downward arrow: decrease in activity).
1.7 Challenges, limitations, and future directions
There are some studies which have reported an absence of effect of mindfulness, while others showed positive effects. For instance, the results from two recent studies on trait mindfulness showed one reporting that dispositional mindfulness could successfully predict emotional reactivity (Brown et al., Reference Brown, Goodman and Inzlicht2013), while other reported that it could not (Cosme & Wiens, Reference Cosme and Wiens2015). These differences between the two studies might be due to dilution of effect sizes in psychologically healthy participants. This dilution effect may also influence the outcomes of mindfulness intervention programs, as such intervention programs may only produce large enough effects in a subgroup of a population under investigation (Flaxman and Bond., Reference Flaxman and Bond2010). The median split analysis is one common approach that has been frequently used to determine two subgroups based on mindfulness or anxiety levels of the participants (e.g., Bishop., Reference Bishop2009; Brown et al., Reference Brown, Goodman and Inzlicht2013). However, researchers have argued that it is inappropriate to consider that values just above or below the median, as would be the case for many individuals when a median split categorization is used, are meaningfully different from each other (MacCallum, Zhang, Preacher, & Rucker, Reference MacCallum, Zhang, Preacher and Rucker2002). Additionally, it has been noted as being quite challenging to delineate an effect that already exists using such a split (Aiken, West, & Reno, Reference Aiken, West and Reno1991; McClelland et al., Reference McClelland, Lynch, Irwin, Spiller and Fitzsimons2015). Therefore, it has been suggested that employing a mean ± standard deviation criterion rather than using median split analysis (MacCallum et al., Reference MacCallum, Zhang, Preacher and Rucker2002), will enable avoidance of the dilution effect that may result from participants with intermediate levels of mindfulness or anxiety.
Another alternative to avoid the dilution effect in mindfulness intervention programs may be employing “double selection criteria”, an approach to categorize groups based on high mindfulness-low anxiety and low mindfulness-high anxiety (Jaiswal et al., Reference Jaiswal, Tsai, Juan, Liang and Muggleton2018). This is an objective criterion based on the assumption of the inverse relationship between mindfulness anxiety and can provide two matched comparison groups. It is proposed that the low mindfulness-high anxiety group that shows poorer executive function than a high mindfulness-low anxiety group may have a stronger response to a mindfulness intervention program and subsequently have improved cognition and other health outcomes.
Despite accumulating evidence of a close relationship between mindfulness and anxiety, it is still not clear whether both or one of these is an emergent property of the brain. From an evolutionary perspective, anxiety is attributed as a conserved property in humans as part of a self-defense mechanism (Rozin & Royzman, Reference Rozin and Royzman2001) and its effects on cognition are entirely consistent with this (Eysenck et al., Reference Eysenck, Derakshan, Santos and Calvo2007). While emotions are indeed regarded as emergent processes of the brain (Knyazev, Reference Knyazev2007; Scherer, Reference Scherer2009), it appears anxiety may be better reflected as an emergent process than is the case for mindfulness. Therefore, it is necessary to understand how mindfulness is governed through brain processes to further proceed in developing an operational definition of mindfulness.
2. Conclusions
Considering the above self-report, behavioral, electrophysiological, fMRI, and biological markers evidence leads us to propose that mindfulness and anxiety are indeed mediated by emotion regulation strategies such as worry, reappraisal, nonacceptance, and rumination, through which they may interact in an antagonistic manner (Coffey & Hartman, Reference Coffey and Hartman2008; Desrosiers et al., Reference Desrosiers, Vine, Klemanski and Nolen-Hoeksema2013; Greeson & Brantley, Reference Greeson, Brantley and Didonna2009). One recommendation arising is that future studies on the effects of mindfulness (or anxiety) on cognitive processes should look at these in conjunction with anxiety (or mindfulness), rather than independently. Since longitudinal intervention programs can be resource-intensive and come with logistic constraints, one should consider to what extent these measures are feasible to estimate. It is often recommended that the greater the amount of information that can be collected, the richer the quality of the research will be. Therefore, combining mindfulness and anxiety into investigations may facilitate the efficacy of assessment of MBI programs by providing a broad spectrum of indices which may reveal the individual differences in responsiveness to the program. Additionally, it is suggested that future investigations should also investigate the neural mechanisms associated with the resting state in a task-independent manner related to mindfulness and anxiety.
Financial Support
This work was sponsored by the Ministry of Science and Technology, Taiwan (grant numbers: 107-2420-H-008-009-;108-2639-H-008-001-ASP; 108-2321-B-075 -004 -MY2;107-2628-H-008 -002 -MY3; 106-2410H-008-038-MY3; 106-2628-H-008-002-MY4; 107-2410-H-008 -040 -MY3) and sponsored by Taiwan Ministry of Education’s "Academic Strategic Alliance: Taiwan and Oxford University" project grant (MOE Oxford – NCU-BRC collaborative project).
Conflicts of Interest
The authors have nothing to disclose.




