INTRODUCTION
In neotropical rain forests, seed dispersal by rodents has been shown to play an important role in plant reproduction via scatterhoarding, seed caching and/or secondary seed dispersal (Forget Reference FORGET1990, Reference FORGET1992; Forget & Milleron Reference FORGET and MILLERON1991, Forget et al. Reference FORGET, HAMMOND, MILLERON, THOMAS, Levey, Silva and Galletti2002, Jansen et al. Reference JANSEN, HIRSCH, EMSENS, ZAMORA-GUTIERREZ, WIKELELSKI and KAYS2012). Rodents shown to disperse seeds of neotropical plants via these mechanisms are generally medium- to large-bodied, and consume fruit and/or seeds of canopy tree species (Adler & Kestell Reference ADLER and KESTELL1998, Dittel et al. Reference DITTEL, LAMBERT and ADLER2015, Forget Reference FORGET1990, Reference FORGET1991, Reference FORGET1992; Haugaasen et al. Reference HAUGAASEN, HAUGAASEN, PERES, GRIBEL and WEGGE2010, Hoch & Adler Reference HOCH and ADLER1997, Smythe Reference SMYTHE1989). Small-bodied rodents, on the other hand, are widely considered to be seed predators (Demattia et al. Reference DEMATTIA, CURRAN and RATHCKE2004, Denslow & Moermond Reference DENSLOW and MOERMOND1982, Grenha et al. Reference GRENHA, MACEDO, PIERES and MONTEIRO2010, Griscom et al. Reference GRISCOM, KALKO and ASHTON2007, Ostfeld et al. Reference OSTFELD, MANSON and CANHAM1997, Pinto et al. Reference PINTO, SANTOS and TABERELLI2009). Because of their influence on seed survivorship and seedling recruitment, several authors have called for inclusion of small rodents in guild/community studies of seed dispersal, but mostly to investigate their influence via seed predation (Demattia et al. Reference DEMATTIA, CURRAN and RATHCKE2004, Grenha et al. Reference GRENHA, MACEDO, PIERES and MONTEIRO2010). However, recent evidence for the neotropical rodent subfamily Sigmodontinae (Cricetidae) indicates that several species consume fruit and/or pass intact seeds in diverse neotropical habitats such as the montane forests in Peru (Noblecilla & Pacheco Reference NOBLECILLA and PACHECO2012, Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015), scrub habitats in Chile (Meserve Reference MESERVE1981) and the Atlantic forests in Brazil (Vieira et al. Reference VIEIRA, PIZO and IZAR2003). Despite these findings, only one published account for cricetid rodents (< 100 g) noted primary seed dispersal occurring via the digestive tract by Necromys lasiurus (formerly Bolomys lasiurus) after consumption of Miconia albicans fruit (Magnusson & Sanaiotti Reference MAGNUSSON and SANAIOTTI1987). Data on diet of small-bodied rodents and their potential for primary seed dispersal are therefore limited (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015).
Determining the role that small-bodied (<100 g) rodents might play in seed dispersal is crucial, given the importance of dispersal for plant reproductive success (Dalling et al. Reference DALLING, MULLER-LANDAU, WRIGHT and HUBBELL2002, Howe & Smallwood Reference HOWE and SMALLWOOD1982, Wunderle Reference WUNDERLE1997), re-establishment of plants in disturbed ecosystems (Medellin & Gaona Reference MEDELLIN and GAONA1999, Parrotta et al. Reference PARROTTA, HENRY KNOWLES and WUNDERLE1997, Tabarelli & Peres Reference TABARELLI and PERES2002, Wunderle Reference WUNDERLE1997), and the ubiquity of small rodents in neotropical habitats (Voss & Emmons Reference VOSS and EMMONS1996). During a study examining potential impacts on small-rodent populations due to construction of a natural gas pipeline through a montane tropical forest, Sahley et al. (Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015) found that seven species of sigmodontine rodent had intact seeds present in their faecal samples. In this study we tested the following hypotheses: (1) small rodents belonging to the subfamily Sigmodontinae (Cricetidae) have viable seeds in their faeces and function as seed dispersers; (2) based on a previous diet study (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015) the genus Thomasomys would disperse a greater diversity and abundance of seeds as well as seeds with a higher viability, therefore species belonging to this genus would be the most effective seed dispersers at the site; and (3) small rodent-dispersed plant families and genera would be more similar to those dispersed by birds than by bats.
METHODS
Study site
Our study site was located near Chiquintirca, department of Ayacucho, in the province of La Mar (13°03′34′′S, 73°42′25′′W), Peru. It is near the upper limit of montane forests of the Apurimac River valley ranging in altitude from 3200 to 3500 m asl. This area is categorized as pluvial montane subtropical forest (Instituto Nacional de Recursos Naturales 1995), upper montane pluvial forest of the yungas (Josse et al. Reference JOSSE, NAVARRO, COMER, EVANS, LANGENDOEN, FELLOWS, KITTEL, MENARD, PYNE, SCHULZ, SNOW and TEAGUE2003) and the Apurimac river valley montane forest ecotone (Langstroth et al. Reference LANGSTROTH, DALLMEIER, CASARETTO, SERVAT, Alonso, Dallmeier and Servat2013). Vegetation consisted of a mosaic of tropical forest dominated by Polylepis spp. co-occurring with tropical shrubs (Langstroth et al. Reference LANGSTROTH, DALLMEIER, CASARETTO, SERVAT, Alonso, Dallmeier and Servat2013, Servat et al. Reference SERVAT, FERIA, HURTADO, MENDOZA, ALCOCER, Alonso, Dallmeier and Servat2013). Rainfall at the site in 2011 and 2012 ranged from 30 mm in June to 388 mm in February 2012 (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015).
Rodent captures and sample collection
In 2011 and 2012, we used nine trapping grids 20 × 150 m in size to live-trap rodents, following the protocol outlined in Pacheco et al. (Reference PACHECO, SALAS, BARRIGA, RENGIFO, Alonso, Dallmeier and Servat2013). Each grid was made up of two parallel lines separated by 15–20 m each, and each line had 16 capture stations 10 m apart. Each station consisted of two Sherman traps that were 7.6 × 8.9 × 22.9 cm. When possible, one of the Sherman traps was placed on a branch or shrub of a tree, 1–2 m above ground. Traps were baited and opened in the late afternoon and checked the following morning. In 2011, one trapping session was conducted in October and another in November (just prior to the rainy season) and in 2012 one trapping session was conducted in May (just after the rainy season). Traps were left open for a total of four nights in each session, for a total capture effort of 6912 trap nights. Captured rodents were identified to species, age class and weighed before being tagged and released.
Faecal pellets in each trap were collected and placed in labelled aluminium paper for storage. All faecal samples were transported in a cooler with silica gel to reduce humidity and stored at 0°C.
Plant species richness and reference material
We collected leaves, stems, fruits and their seeds in 2012 to establish a reference collection for seed identification. All material was deposited in the Herbarium of the Universidad Nacional Mayor de San Marcos Natural History Museum. We used Servat et al. (Reference SERVAT, FERIA, HURTADO, MENDOZA, ALCOCER, Alonso, Dallmeier and Servat2013) and Sahley et al. (Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015) for plant identifications.
Seed identification and quantification
We placed 12 seeds from each aluminium packet in a 140 × 20-mm Petri dish. We used distilled water to disaggregate faecal pellets and locate seeds. We used a 20× Leica RX stereoscope to determine seeds to family, genus and morphospecies when possible using our reference collection and identification keys found in Caceres (Reference CACERES2004), Cornejo & Janovec (Reference CORNEJO and JANOVEC2010), Gentry (Reference GENTRY1993), Ponte (1988) and Rios et al. (Reference RIOS, GIRALDO and CORREA2004).
All seeds were photographed with a 1-mm grid paper placed below a Petri dish. Seed length was measured with a 1-mm grid paper placed below a Petri dish. We utilized the program Image J (www.ImageJ.nih.gov) to determine the length for seeds smaller than 1 mm. We calculated the mean and standard deviation for seed length in our samples.
Seed viability
We assessed seed viability using a 1% solution of tetrazolium. This test allows for determination of cellular respiration, which turns the seed embryo tissues a scarlet red colour and allows for an efficient estimate of germination capacity (ISTA 1996). In most cases, we determined viability for a minimum of 10 seeds per plant and per rodent species. We were unable to conduct viability test for Rubiaceae sp. 1 and we tested only one seed for Myrteola sp. To conduct viability tests, we made a small cut in the seed coat on the side opposite from the embryo. The seed was soaked in distilled water for 24 h. We then removed the distilled water and we added the tetrazolium solution and soaked the seeds in complete darkness for an additional 24 h. We then removed the seed coat to evaluate viability via embryo examination. We scored the embryo as viable only when it was scarlet red; if it was partially coloured, pink, or not coloured at all we classified the seed as non-viable. Because partially coloured or pink seeds can sometimes be viable (ISTA 1996), our viability analysis is conservative and may underestimate the proportion of viable seeds.
Analysis of data
We calculated the relative abundance of each rodent species by dividing the number of individuals captured per species by the total number of rodents captured for all species combined. We utilized 12 faecal pellets per individual rodent captured to quantify the total number of seeds for each species of plant and rodent; this allowed us to standardize the relative seed quantity estimates across rodent taxa. We calculated the mean and standard deviation of seed abundance in faecal samples per individual for each rodent species and the mean and standard deviation of seed number per sample (one sample = 12 faecal pellets) for each plant species.
We calculated the proportional abundance of seeds dispersed by each rodent species by dividing the total number of seeds found for each rodent species by the total number of seeds found for all rodent species combined. The proportion of plant species diversity for each rodent species was calculated by dividing the total number of plant species found for each rodent species by the total number of plant species in all rodent species combined. The proportion viability of plant species for each rodent species was calculated by dividing the total number of scarlet-coloured seeds for each plant species per rodent species by the total number of seeds evaluated for viability for that plant species. The total combined seed viability for each rodent species was calculated by adding the number of all viable seeds and dividing this value by the total number of seeds evaluated. Total seed viability for plant species was calculated by dividing the total number of viable seeds for each plant species by the total number of seeds evaluated.
A Kruskal–Wallis test was calculated to examine differences in frequency distributions among plant species abundance in faecal samples across rodent species and also for differences in frequency distributions among plant species abundance in faecal samples across plant species. We calculated a chi-square statistic to examine differences in proportion seed viability by plant species for which we had n > 10 viability tests, as well as a chi-square statistic to test for differences in proportion seed viability among rodent species.
We calculated an index of disperser effectiveness for each species of rodent by utilizing the following equation:
 $$\begin{eqnarray*}
$$\begin{array}{l} {\rm Disperser\, effectiveness}\\
\quad\quad\,=\,{\rm Relative\, \,abundance\, \,each\, \,rodent\, \,species}\\
\quad\quad \times\, {\rm proportion\, \,intact\, \,seeds\, \,in\, \,faecal\, \,samples}\\
\quad\quad \times\, {\rm proportion\, \,number\, \,plant\, \,species\, \,in\, \,samples}\\
\quad\quad \times\, {\rm proportion\, \,viable\, \,seeds\, \,\times\, 100}
\end{array}$$
\end{eqnarray*}$$
$$\begin{eqnarray*}
$$\begin{array}{l} {\rm Disperser\, effectiveness}\\
\quad\quad\,=\,{\rm Relative\, \,abundance\, \,each\, \,rodent\, \,species}\\
\quad\quad \times\, {\rm proportion\, \,intact\, \,seeds\, \,in\, \,faecal\, \,samples}\\
\quad\quad \times\, {\rm proportion\, \,number\, \,plant\, \,species\, \,in\, \,samples}\\
\quad\quad \times\, {\rm proportion\, \,viable\, \,seeds\, \,\times\, 100}
\end{array}$$
\end{eqnarray*}$$Statistical analyses were performed using SPSS version 21.
RESULTS
Rodent captures
We captured and released a total of 134 rodents from seven species belonging to the subfamily Sigmodontinae (Cricetidae) in 2011–2012. These were Akodon torques (Thomas, 1917) (n = 49); Calomys sorellus (Thomas 1900) (n = 20); Microryzomys minutus (Tomes, 1860) (n = 5); Oligoryzomys andinus (Osgood, 1914) (n = 3); Thomasomys kalinowskii (Thomas, 1894) (n = 34); T. oreas Anthony, 1926 (n = 21); and T. aureus (Tomes 1860) (n = 2).
Plant families and species
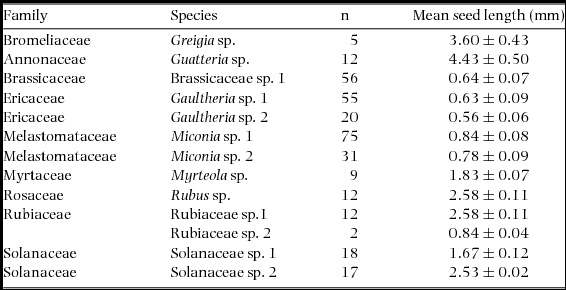
We found seeds of a total of eight plant families, nine genera and 13 morphospecies in faecal samples collected in 2011–2012. Plant families and genera recorded include Annonaceae (Guatteria sp.), Brassicaceae (Brassicaceae sp. 1), Bromeliaceae (Greigia sp.), Ericaceae (Gaultheria sp. 1 and G. sp. 2), Melastomataceae (Miconia sp. 1 and M. sp. 2), Myrtacae (Myrteola sp. 1), Rosaceae (Rubus sp. 1) and Rubiaceae (Rubiaceae sp. 1 and sp. 2). We found intact seeds and/or evidence of fruit pulp in faecal samples in all species of rodent. For this study, we only found fruit pulp in the faecal samples of T. aureus and could not conduct seed viability tests. Average seed length found in samples ranged from 0.56 mm to 4.43 mm, with Gaultheria sp. 2 having the smallest seeds and Greigia sp. having the largest (Table 1).
Table 1. Mean (± SD) length of seeds in rodent faecal samples for 2011–2012, Chiquintirca, Ayacucho, Peru. n = sample size. Seed size was very small in all cases.

Seed abundance and distribution across samples
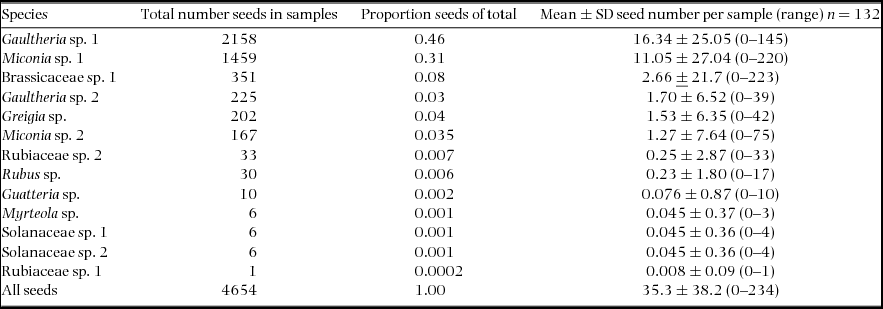
We found a total of 4654 seeds in rodent faecal samples, with a mean ± SD number of 35.3 ± 38.2 seeds per sample (Table 2). The distribution of seed abundance in samples by plant species was significantly different (Kruskal–Wallis test, χ2 = 24.2, df = 5, P < 0.05). The Ericaceae (n = 6 species of rodent), Melastomataceae (n = 3) and Rosaceae (n = 3) were the best represented families consumed by rodent species, followed by Myrtaceae and Solanaceae (n = 2), and Brassicaceae and Bromeliaceae (n = 1). Gaultheria sp. 1 (Ericaceae) not only had the highest total number of seeds in faecal samples, but also the highest mean number of seeds per faecal sample; in addition seeds were also present in all six rodent species examined (Table 2). Miconia sp. 1 (Melastomataceae) had the second highest abundance and mean number per sample, while Gaultheria sp. 2 had considerably lower total and mean abundance values (Table 2). Miconia sp. 1 and Gaultheria sp. 2 occurred in faecal samples from three species of rodent. Gaultheria sp. 2 and the remaining morphospecies have mean values of less than three seeds per sample and are found in one to two species of rodent.
Table 2. Total and mean seed abundance ± SD for plant species recorded in 12 faecal samples per rodent, Chiquintirca, Ayacucho Peru, 2011–2012. Plant species are listed in order of abundance.

Seed viability
We found that all plant species tested (except for Myrteola sp.) had viable seeds in faecal samples. Differences in proportion seed viability among plant species (excluding Myrteola sp., Solanaceae sp. 2 and Rubiaceae sp. 1 from the analysis due to n < 10) were significant (χ2 = 229, n = 1231, df = 9, P < 0.001). Greigia sp. (n = 110) and Guatteria sp. (n = 10), only found in T. kalinowskii, had the highest viability values, 80% to 84% respectively. Gaultheria sp. 1 was found in samples from every rodent species except for M. minutus. Overall viability was 30% (n = 535), with 21% (n = 200) viability for seeds found in A. torques, 70% (n = 110) for C. sorellus, 0% (n = 15) for M. minutus, 20% (n = 20) for O. andinus, 32% (n = 90) for T. kalinowskii, and 14% (n = 100) for T. oreas. Gaultheria sp. 2 seeds had a total viability of 27% (n = 90) with viability values of 27% (n = 30) for A. torques, 25% (n = 20) for C. sorellus, and 28% (n = 40) for T. kalinowskii. Seeds of this species were not found in samples from M. minutus, O. andinus or T. oreas. Miconia sp. 1 had a total seed viability of 15% (n = 374), with 4% viability (n = 164) for A. torques, 27% (n = 150) for T. kalinowskii, and 13% (n = 60) for T. oreas. Seeds of this species were not found in C. sorellus, M. minutus or O. andinus. Miconia sp. 2 were found only in T. kalinowskii and T. oreas, and had an overall viability of 7.5% (n = 40) with a viability of 0% (n = 10) for T. kalinowskii, and 10% (n = 30) for T. oreas. Rubus sp. had a total viability of 55% (n = 20), and seeds were found only for T. kalinowskii. Myrteola sp. had 0% viability but our very small sample size (n = 1) for T. kalinowskii precludes us from making reliable viability estimates. Seeds belonging to the Brassicaceae sp. 1 were found only in T. kalinowskii and had a total viability of 20% (n = 30). Rubiaceae sp. 2 seeds had a total viability of 9% (n = 11), with 1 seed out of 1 viable for T. kalinowskii and 0 seeds out of 10 for T. oreas. Solanaceae sp. 1 also had a total viability of 9% (n = 11), with 1 seed of 1 viable for T. kalinowskii and 0 seeds out of 10 viable for T. oreas. Solanaceae sp. 2 was found only in T. kalinowskii and had a total viability of 75% (n = 4). We were unable to conduct a viability test for Rubiaceae sp. 1.
Rodent species and seed disperser effectiveness
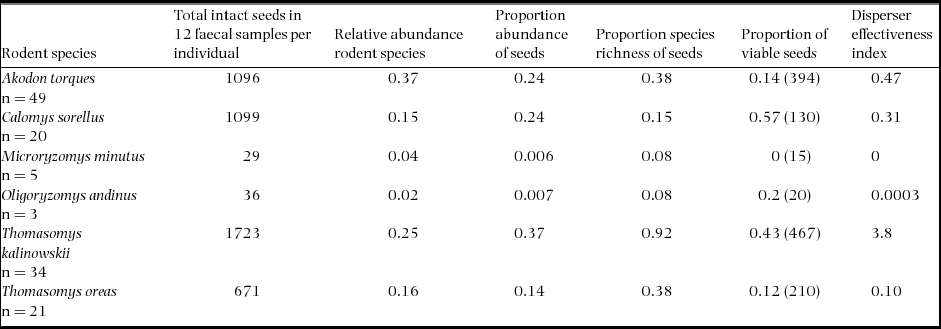
We found that the distributions of plant species in faecal samples varied significantly across rodent species (Kruskal–Wallis test, χ2= 24.2, df = 12, P < 0.01). Total seed abundance in faecal samples was greatest for T. kalinowskii (x ± SD = 50.7 ± 57.8), followed by C. sorellus (x ± SD = 55.0 ± 36.5), A. torques (x ± SD = 22.4 ± 18.7), T. oreas (x ± SD = 32.0 ± 20.1), O. andinus (x ± SD = 12.0 ± 4.0) and M. minutus (x ± SD = 5.8 ± 1.8) in descending order (Table 3). Thomasomys kalinowskii had the highest proportion plant species richness represented in faecal samples with 12 species of plants occurring in faecal samples (Table 3). Thomasomys oreas faecal samples contained five species of plants, followed by A. torques (3 species), C. sorellus (2 species), M. minutus and O. andinus (1 species each).
Table 3. Total intact seeds found in faecal samples, relative abundance of rodent species, proportion relative abundance of intact seeds, proportion species richness found in samples and proportion of viable seeds (total number of seeds tested) for each rodent species at Chiquintirca, Ayacucho, Peru 2011–2012. The disperser effectiveness index is the relative contribution of each rodent species to effective seed dispersal and is the product of the relative abundance of each rodent species × proportion abundance of seeds × proportion species richness of seeds × proportions of viable seeds × 100.

Although containing a relatively low seed species diversity in samples, C. sorellus had a relatively high seed abundance, and the greatest percentage of viable seeds in faecal samples (57%) followed closely by T. kalinowskii (43% viability; Table 3). The remaining species of rodents had from 12% to 20% viable seeds, except for M. minutus, which had no viable seeds in faecal samples. Differences in total seed viability among rodent species were statistically significant (χ2 = 177, n = 1236, df = 5, P < 0.001).
An index for seed disperser effectiveness for each rodent species, calculated using relative rodent abundance, proportional seed abundance, plant species diversity and seed viability, showed that the combination of these variables indicates that T. kalinowskii is the most effective seed disperser at this montane-forest site. Akodon torques and C. sorellus are the second and third most effective seed dispersers. Thomasomys oreas and O. andinus are the fourth and fifth most effective seed dispersers whereas M. minutus did not disperse viable seeds (Table 3).
DISCUSSION
We found that small sigmodontine rodents (<100 g) are primary seed dispersers in the high-elevation tropical montane forest studied. Five of six species of rodents passed intact and viable seeds of 13 morphospecies, eight families and nine genera of plants. Our estimate of primary seed dispersal occurrence is likely conservative because a longer-term study examining diet found seeds from 17 morphospecies and nine families in faecal samples of all seven species at the same study site (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015). Primary seed dispersal for small rodents of the cricetid family has been noted only once, for Necromys lasiurus, which passed viable Miconia albicans seeds through its digestive tract (Brewer & Rejmánek Reference BREWER and REJMÁNEK1999). Consumption of fruit and/or passage of intact seeds by small sigmodontine rodents has been noted for these and other species in various habitats such as the Brazilian Atlantic forest (Vieira et al. Reference VIEIRA, PAISE and MACHADO2006), Chilean temperate rain forest (Meserve et al. Reference MESERVE, LANG and PATTERSON1988) and montane forest in Peru (Noblecilla & Pacheco Reference NOBLECILLA and PACHECO2012, Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015), however except for the N. lasiurus study (Brewer & Rejmánek Reference BREWER and REJMÁNEK1999) seed viability and/or contribution to seedling establishment was not examined.
Our study site is comprised of approximately 172 species and morphospecies in 94 genera and 54 families of plants that comprise a mosaic of forest and shrub habitat near the upper limit of montane forest (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015, Servat et al. Reference SERVAT, FERIA, HURTADO, MENDOZA, ALCOCER, Alonso, Dallmeier and Servat2013). The families identified in faecal samples are known to produce berries and in one case, capsules. Small-seeded berries are produced by the Bromeliaceae (Benzing Reference BENZING2000, Will & Zizka Reference WILL and ZIZKA1999), Melastomataceae (Renner Reference RENNER1989), Rubiaceae (Bremer & Eriksson Reference BREMER and ERIKSSON2008), Myrtaceae (Pizo Reference PIZO, Levey, Silva and Galetti2002), Ericaceae (Eriksson Reference ERIKSSON2008, Stiles Reference STILES1980), Rosaceae (Stiles Reference STILES1980) and Solanaceae (Barbosa-Albuquerque et al. Reference BARBOSA-ALBUQUERQUE, VELÁZQUEZ and MAYORGA-SAUCEDO2006) while the Brassicaceae produce capsules (Hall et al. Reference HALL, TISDALE, DONOHUE, WHEELER, AL-YAHYA and KRAMER2011). Krebs et al. (Reference KREBS, COWCILL, BOONSTRA and KENNY2010) found that berries are important food resources for North American small rodents and influence their population dynamics; thus it is not surprising that small neotropical rodents would also feed on small-seeded fruits (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015).
Seed disperser effectiveness
Our study shows the genera Thomasomys, Akodon and Calomys as important seed dispersers in the tropical montane forest studied. In accord with our hypothesis, the genus Thomasomys was responsible for most seed dispersal, with T. kalinowskii being the most effective disperser for the studied plant community. Thomasomys spp. may exhibit semi-arboreal habits; nests for T. aureus have been found in trees (Brito et al. Reference BRITO, TESKA and OJALA-BARBOUR2012), and evidence of arboreal activity for T. oreas have been recorded (Pacheco unpubl. data), although such data do not exist as of yet for T. kalinowskii. Further research on the genus Thomasomys including effects of gut passage on seed viability, foraging behaviour and comparative dispersal effectiveness among species is warranted.
Akodon torques, reported as insectivorous (Noblecilla & Pacheco Reference NOBLECILLA and PACHECO2012, Solari Reference SOLARI, Kelt, Lessa, Bravo and Patton2007), consumed fruit and passed intact seeds at our study site, including a diverse and large quantity of insects. While it consumes fruits of fewer species than the genus Thomasomys, because of its high relative abundance and passage of viable seeds for Gaultheria spp. and Miconia sp. 1, it had the second highest effective disperser index of this rodent assemblage.
Calomys sorellus, a rodent often considered to be insectivorous and commonly found in high Andean grassland habitat (Pizzimenti & de Salle Reference PIZZIMENTI and DE SALLE1980) was found to include fruit in its diet at our study site (Sahley et al. Reference SAHLEY, CERVANTES, PACHECO, SALAS, PAREDES and ALONSO2015). The proportion of intact seeds (belonging to the genus Gaultheria) and seed viability in faecal samples was relatively high. This is significant as we found that C. sorellus was the only rodent to cross the 25-m-wide area cleared during pipeline construction during early restoration efforts before taller shrubs were re-established (Sahley unpubl. data). Thus, C. sorellus may have contributed to dispersing seeds to the 25-m-wide pipeline right of way during early stages of vegetation restoration.
Oligoryzomys andinus and M. minutus both had intact Gaultheria sp. 1 seeds in faecal samples. The relative abundance of both species was low in this rodent assemblage. For O. andinus seed viability was low to medium while for M. minutus seed viability was zero. Overall, these two species contribute the least to seed dispersal compared with others in this rodent assemblage.
In summary, T. kalinowskii was the most effective disperser in montane forest and shrub areas because of high seed abundance, diversity, and viability values; it also disperses seeds of two genera that are not found in other rodent species at our site. Akodon torques was important primarily because of its high abundance, and C. sorellus because of its high viability for one plant genus and its ability to cross the recovering pipeline right of way prior to the re-establishment of vegetation cover.
Fruit consumption and seed dispersal of plant families at the study site
The rodent assemblage at our site consumed fruits and dispersed seeds from families and genera that are also consumed and dispersed by other taxonomic groups, but these overlapped more with terrestrial mammals and birds than with bats. For example, Greigia sp. (Bromeliaceae) is a bromeliad that grows near the ground; its fruits are consumed by the Andean bear (Tremarctos ornatus; Troya et al. Reference TROYA, CUESTA and PERALVO2004) as well as by T. kalinowskii. Greigia fruits are also utilized for human consumption (Hornung-Leoni Reference HORNUNG-LEONI2006, Will & Zizka Reference WILL and ZIZKA1999). Seed viability of Greigia sp. was high for T. kalinowskii (84%), but we did not record Greigia sp. in faecal samples of other rodent genera.
Gaultheria spp. (Ericaceae) are shrubs that produce berries that are consumed by birds, the spectacled bear (Tremarctos ornatus) and Andean fox (Lycalopex culpaeus); Rivadeira-Canedo (Reference RIVADEIRA-CANEDO2008) showed that seeds of this genus were viable after bird and mammal consumption. We found Gaultheria sp. 1 seeds in all species of rodents studied and found viable seeds in four of the six rodent species examined, while Gaultheria sp. 2 was consumed by four of six rodent species examined.
Miconia spp. are primarily known for being consumed and dispersed by birds (Levey Reference LEVEY1990, Loiselle & Blake Reference LOISELLE and BLAKE1999, Wheelwright et al. Reference WHEELWRIGHT, HABER, MURRAY and GUINDON1984). Miconia spp. seeds were relatively abundant in samples from this rodent assemblage, with three of six rodent species found to pass viable seeds. The Miconia spp. have previously been reported to pass as viable through the digestive tract of the sigmodontine rodent Necromys lasiurus (Magnusson & Sanaiotti Reference MAGNUSSON and SANAIOTTI1987).
Guatteria spp. are generally found as small- to medium-sized trees and produce berries. These are consumed by birds (Snow Reference SNOW1981, Wheelwright et al. Reference WHEELWRIGHT, HABER, MURRAY and GUINDON1984) as well as spider monkeys (Ateles spp.) and the woolly monkey, Lagothrix lagotricha (Link & Di Fiore Reference LINK and DI FIORE2006, Stevenson Reference STEVENSON2000). We found Guatteria sp. (Annonaceae) seeds in relatively low abundance and only in T. kalinowskii. However, viability of these seeds was high.
Species belonging to the Rubiaceae in the neotropics have fruits that have been recorded as being consumed by small passerines (Loiselle et al. Reference LOISELLE, SORK, NASON and GRAHAM1995, Snow Reference SNOW1981, Tabarelli & Peres Reference TABARELLI and PERES2002). We recorded two unidentified morphospecies in the Rubiaceae samples from the genus Thomasomys.
Rubus spp. have been recorded as being consumed and dispersed by birds (Wheelwright et al. Reference WHEELWRIGHT, HABER, MURRAY and GUINDON1984), as have other species within the Rosaceae (Herrera & Jordano Reference HERRERA and JORDANO1981). One species belonging to the genus Rubus (Rosaceae) was found in samples, although relative seed abundance was low. While Rubus sp. seeds were viable in T. kalinowskii samples, sample size was too low to make any firm conclusion on the importance of T. kalinowskii to its reproductive ecology.
Both bats and birds are reported to consume and/or disperse fruits and seeds belonging to the family Solanaceae (Caceres & Moura Reference CACERES and MOURA2003, Galindo-Gonzales et al. Reference GALINDO-GONZALEZ, GUEVARA and SOSA2000, Loayza et al. Reference LOAYZA, RIOS and LARREA-ALCAZAR2006, Snow Reference SNOW1981, Wheelwright et al. Reference WHEELWRIGHT, HABER, MURRAY and GUINDON1984). Seeds belonging to Solanaceae sp. 1 and sp. 2 were found only in samples belonging to the genus Thomasomys.
The Brassicaceae were represented by one morphospecies found in faecal samples belonging to T. kalinowskii; viability of seeds was 20%, suggesting a role for T. kalinowskii as a seed disperser. The Brassicaceae, unlike the other families identified in samples, form two-valved jointed and non-jointed capsules instead of berries (Hall et al. Reference HALL, TISDALE, DONOHUE, WHEELER, AL-YAHYA and KRAMER2011) and have been reported to primarily utilize passive dispersal modes such as wind and water (Willis et al. Reference WILLIS, HALL, RUBIO, WANG and DONOHUE2014).
Implications for seed-dispersal ecology
In many studies of neotropical forests, primary or secondary seed-disperser taxa have been identified as bats, birds or medium to large rodents primarily belonging to the Dasyproctidae and Echimyidae. Our study suggests that in habitats where sigmodontine rodents consume small-seeded fruits in areas with shrubs or small to medium-sized trees, it is likely that they are serving as primary seed dispersers in montane forests, transition zones, as well as tropical montane habitats in early successional stages, and areas that are being restored or recovering from deforestation. Thus, we recommend that for a more complete understanding of plant reproductive ecology in montane forests, sigmodontine rodents should be included in frugivore and seed-dispersal studies.
While we identified the presence of intact seeds and seed viability in faecal samples of sigmodontine rodents, we recognize that additional components of seed dispersal, such as quality of deposition sites, germination rates and seedling establishment are necessary to gather a more complete picture of small-rodent contributions to seedling recruitment. We hope that additional studies will continue to elucidate the relationships between sigmodontine rodents, their food plants, and their role as seed dispersers in neotropical forests.
ACKNOWLEDGEMENTS
We thank the government of Peru (Dirección General Forestal y de Fauna, Ministerio de Agricultura) for granting us permits to conduct the study (No. 440-2009-AG-DGFFS-DGEFFS, No. 344-2010-AG-DGFFS-DGEFFS, No. 144-2012-AG-DGFF-DGEFFS). We are indebted to Edgar Rengifo, Cecilia Barriga, Maria Peralta, Oscar Centty, David Figueroa, Juan Tito and Wendy Calderon for assistance in the field, and to Edith Arias, Pamela Nina and Giovana Vadillo for assistance in the laboratory. Ornella Sissa and Karim Ledesma provided logistical support. We thank the Smithsonian Institution and PERU LNG for financial support. This is publication # 37 from the Peru Biodiversity Program, Center for Conservation and Sustainability, Smithsonian Conservation Biology Institute.