Background
Pertussis (whooping cough) is a disease with very high morbidity that is still persistent in countries with broad (>95%) vaccination coverage (VC), with epidemic periods (~3–5 years) in spite of the introduction of vaccination with a diphtheria-tetanus-pertussis-whole-cell (DTwP) or with a diphtheria-tetanus-acellular pertussis (DTaP) vaccines [Reference Chambers1].
Pertussis mortality rate in children has increased from 195 000 worldwide in 2008, to 600 000 2018, with a higher incidence in unvaccinated children [Reference Black2]. Pertussis affects all age groups, but is most common in children under 1 year of age [Reference Black2, Reference Paksu3]. Despite the high primary VC (>90%) in some Central and Eastern European countries, the disease distribution has shifted towards other age groups, affecting between 9% and 40% of adolescents and adults, respectively [Reference Díez-Domingo4–6].
In Spain, the current practice uses various acellular vaccines constituted with Bordetella pertussis antigens. These are combined trivalent, pentavalent or hexavalent vaccines bearing antigens that confer immunity to other diseases. Depending on the amount of antigen used, they can have high antigen load DTaP, which are used in primary vaccination series, or reduced-antigen-content tetanus-diphtheria-acellular (dTap), which provides a booster vaccination against pertussis [7]. Since 2002, Spain has been administering the acellular vaccine in a 2–4–6 month primary vaccination schedule, followed by two boost doses, the first at 18 months and the second between ages 4 and 6 years [8].
There is evidence that management strategies and protocols applied in the health centres can have a direct effect on VC [Reference Briss9]. Sending regular reminders in the form of mobile phone text messaging to patients (or to patients’ tutors) to get vaccinated achieved a 5–20% increase in VC [Reference Briss9]. In addition, improvements in immunisation registries in the health care centres have also been associated with higher VC in adolescents [Reference Szilagyi10, Reference Fiks11]. For example, immunisation registries in Spain allow researchers to use individual data to conduct studies of vaccine effectiveness and to efficiently monitor VC.
According to the recommendations from the World Health Organization (WHO), there should be >90% coverage with primary series and booster vaccinations with DTaP/dTap. However, the data on VC from Spain showed that reinforcement coverage reached only 82% in 2016 [8].
Since the distribution of pertussis among younger people has progressed towards older age groups, it has now become necessary to evaluate and improve current vaccination strategies and the available acellular vaccines [12]. The objective of our study was to evaluate VC in patients aged 8–16 years who had been diagnosed with pertussis, and to compare these results to VC in healthy control subjects.
Methods
Study design
We designed a case–control study of 8- to 16-year-old patients with confirmed pertussis who had attended one of 52 Barcelona primary care centres (PCCs) in the Catalan Health Institute (ICS) between 2011 and 2015. Vaccination status was verified from the cases' and controls' digital clinical records e-CAP/MEAP (these acronyms refer to the digital system used in Catalan PCCs to record patients' medical data).
Case definition
All confirmed diagnoses of pertussis among 8- to 16-year-olds during the study period that were recorded in the e-CAP/MEAP (ICD10 codes: A37.0, A37.1, A37.8, A37.9). A case was considered confirmed if infection with B. pertussis was positive by laboratory diagnostic tests. The laboratory diagnostic methods used in Catalonia are: the identification of B. pertussis in culture (Bordet–Gengou or Regan–Lowe) and a reactive polymerase chain reaction (PCR) [13, Reference Guiso14]. Pertussis biological detection was carried out by many clinical laboratories in Spain and in other European countries. These tests generally used the IS481 sequence and its isoform IS1002, since they are specific for B. pertussis and have multiple copies, which increase the sensitivity of the test [Reference Espy15, Reference Guiso16]. According to the National Epidemiological Surveillance Network (RENAVE in Spanish), a confirmed case of pertussis is defined as a laboratory-confirmed disease (microbiological isolation or genome detection by amplification techniques of B. pertussis in nasopharyngeal swabs), plus the presence of clinically compatible disease symptoms (cough ⩾2 weeks, and at least one of the following symptoms: paroxysmal coughing, inspiratory stridor, vomit after coughing, ⩾37 °C body temperature or apnoea) [13].
Control definition
For each case, we selected three controls from among the healthy patients visiting the same PCCs during the same study period, according to the e-CAP/MEAP registry. Controls had the same age distribution as cases (±6 months) and sex was balanced between the two groups. Only individuals who had attended the PCCs for reasons other than cough were included in the study. Given that pertussis is not associated with any underlying diseases, this variable was not collected for either of the two groups, and was not analysed.
Exclusion criteria
We excluded patients who had previously shown symptoms compatible with pertussis (e.g. cough ⩾2 weeks, paroxysmal cough or vomit after coughing) and subjects from private PCCs. Cases and controls who had previously been diagnosed with pertussis in e-CAP/MEAP were excluded from our study.
Variables collected from the e-CAP/MEAP
Demographic: sex and age were divided into two groups: 8–12 years and 13–16 years resulting in 50% of the patients in each group.
Clinical: date of onset of symptoms, date of diagnosis, clinical manifestations registered by professionals in the e-CAP/MEAP.
Complications: pneumonia, seizures, encephalopathy, bronchitis, tachypnoea, respiratory syncytial virus, hospital admission. Before assigning people to the study group or control group, the e-CAP/MEAP was verified to check that the patient had been given the differential diagnosis of bronchitis and viral respiratory infections. Laboratory diagnostic tests included culture tests and/or positive/negative PCR tests.
DTaP/dTap VC status was recorded as either proper vaccination status (i.e. individuals fully immunised with five doses recorded) or improper vaccination status (i.e. individuals partially immunised with <5 doses recorded).
Statistical methods
We conducted a descriptive comparative analysis of the socio-demographic characteristics and the number of doses of pertussis vaccines administered to cases and controls, using percentages, mean scores and measures of statistical dispersion. In cases with small samples sizes, we used the Fisher's exact test.
We used a logistic regression model to estimate the odds ratios (OR) and 95% confidence intervals (95% CI) for the confirmed pertussis cases and the controls, based on the number of doses of pertussis vaccine administered. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS® version 18.0, Chicago, IL, USA) for Windows (Microsoft Corp., Redmond, WA, USA). Assuming a case:control ratio of 1:3, we compute the sample size, using GRANMO software (Version 7.12, IMIM, Barcelona, Spain) [17].
Ethical considerations
e-CAP/MEAP data for the selected patients were entered into a new, password-protected database. Subjects were later identified using an anonymous encrypted code, which was maintained throughout the study. The researchers guaranteed the confidentiality of the results. The study has been approved by the National Clinical Research Ethics Committee Jordi Gol i Gurina.
Results
A total of 52 PCCs of ICS attended 87 938 patients of 8–16 years old from 2011 to 2015. We included 229 cases and 576 controls. A total of 52.4% of the cases were female. In our study, 68.6% of the cases had received DTaP primary immunisation after 2–5 years, and of these, 31.4% had received the dTap booster immunisation after 7–10 years. The following clinical symptoms compatible with pertussis were observed in the cases: coughing for ⩾2 weeks (38.9%), vomiting after coughing (22.7%), paroxysmal coughing (19.2%), body temperature >37 °C (15.3%), inspiratory stridor (11.8%) and cyanosis (4.4%). The incidence of inspiratory stridor, a symptom associated with pertussis, was significantly higher in the study group than in controls (P < 0.001). In addition, people in the case group were more likely to suffer clinical symptoms compatible with pertussis than people in the control group (Table 1).
Table 1. Analysis of demographic and clinical variables associated with pertussis in cases and control subjects
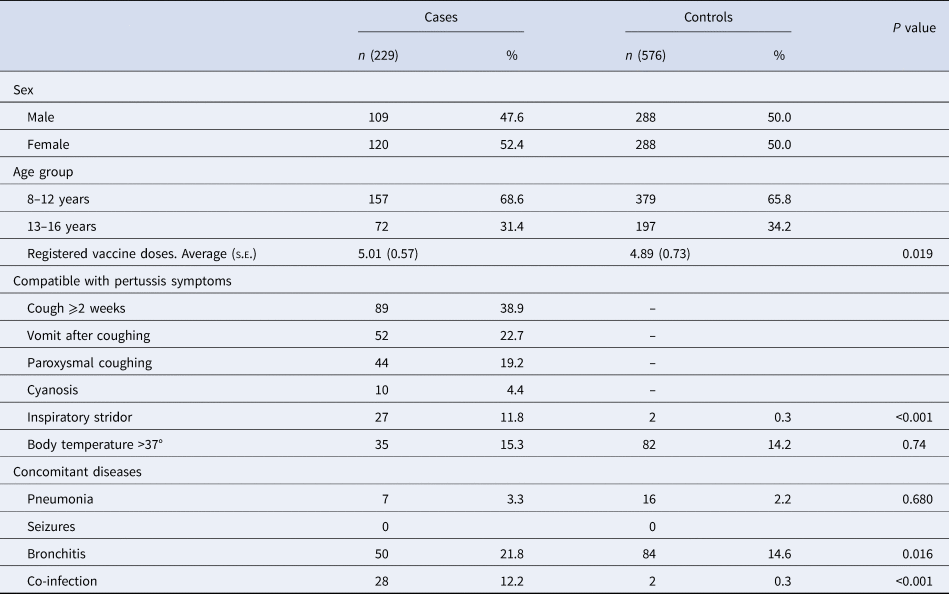
s.e., standard error.
During the study period, 21.8% of cases presented with bronchitis, 12.2% presented with co-infection and 3.3% presented with pneumonia. Bronchitis was more common among cases than controls (21.8% vs. 14.6%, P = 0.016). A negative diagnosis of pertussis was confirmed for people in the control group who had been diagnosed with bronchitis in the e-CAP/MEAP.
Twelve per cent of cases had co-infection diagnosed by laboratory tests. While only 20.0% of cases had a positive culture test for pertussis, 98.4% had shown positive genome detection of B. pertussis in the nasopharyngeal smear. Twenty-eight per cent of cases were referred to the hospital to be seen by another specialist. DTaP/dTap VC was higher in cases (mean 5.01, s.e.: 0.57) than in controls (4.89, s.e.: 0.73) (P = 0.02). VC among 14-year-old children in Catalonia was 96.4%. Eighty-seven per cent of children aged 5–9 years received complete vaccination with five doses of the DTaP/Tdap vaccines.
Ninety per cent of cases had a proper vaccination status compared to 81.3% of controls (OR 1.87, 95% CI 1.22–2.85; P = 0.03; Table 2). According to e-CAP/MEAP data, patients were vaccinated at the appropriate time points according to the corresponding vaccination schedule, as well at the appropriate time points according to age. The time at which a vaccine was administered to a patient was recorded. Among women, cases were more likely than controls to have proper vaccination status (OR 2.60, 95% CI 1.33–5.07; Table 2); in contrast, these differences in VC were not statistically significant between male cases and controls (OR 1.41, 95% CI 0.81–2.43).
Table 2. Vaccination coverage according to sex and age group in cases and control subjects, Barcelona PCCs, Spain
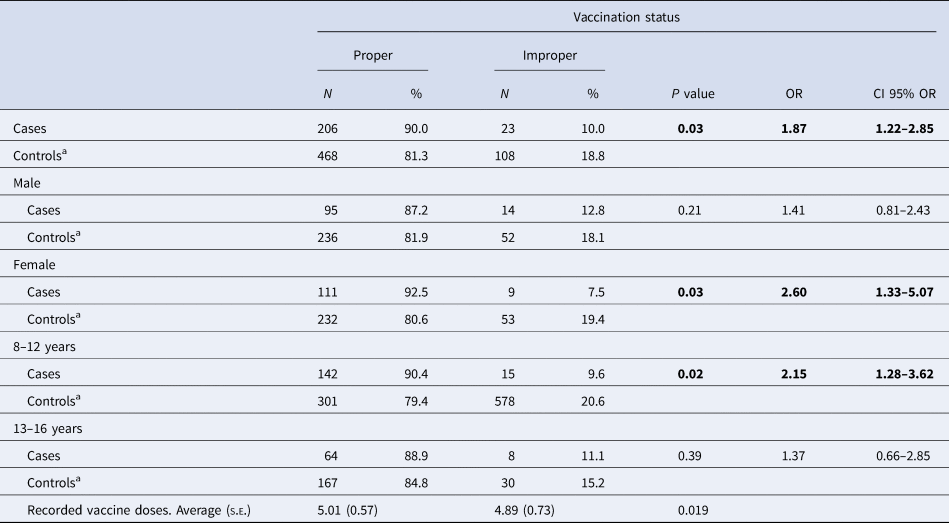
s.e., standard error. Bold indicates the variable is statistically significant p < 0.05.
a Referent group.
In terms of age, we observed a significant association between vaccination status and pertussis among 8- to 12-year-olds (P = 0.02; OR 2.15, 95% CI 1.28–3.62) but not among 13- to 16-year-olds (P = 0.39; OR 1.37, 95% CI 0.66–2.85), indicating that younger controls are more likely to have been properly vaccinated than older controls (Table 2).
Discussion
Since its introduction in 2012, the boost vaccines (dTap/dT) have had low VC nationally, resulting in a high percentage of pertussis cases among ⩾15-year-olds [Reference Campins18]. This finding is consistent with the results of our study; where the VC among cases was lower in 13- to 16-year-olds (88.9%) than among 8- to 12-year-olds (90.4%). In all clinical trials, DTaP/dTap vaccines were described as slightly less effective and as being protective for a shorter time period (<2 years after the third injection) [Reference Taranger19, Reference Simondon20].
In our study, VC was higher in the cases than in the controls, likely due to the more careful monitoring of vaccination status among children with confirmed pertussis, and higher VC in this group. There is evidence that immunity against pertussis after vaccination is quite high for the first year of life, when anti-pertussis vaccines are administered for primary immunisation in infancy at 2, 4 and 6 months of age, which was the vaccination schedule followed by individuals in our case and control groups. Similarly, another study showed that DTaP vaccines have 84% protective efficacy in the first 2 years of life [Reference Salmaso21].
It is now well known that the immunity induced by the DTaP vaccine lasts around 5–10 years according to the epidemiological situation report (WHO position paper). This is supported by a recent report on the data paediatric ambulatory surveillance of confirmed pertussis in France [Reference Guiso22], and a recent publication based on 16 years of disease-specific surveillance in Massachusetts [Reference Domenech de Cellès23]. It is well known that immunity from pertussis after vaccination reaches high levels during the first year, and then declines thereafter [Reference Radke24, Reference Rigo-Medrano25]. While in this study we did not analyse the time elapsed since vaccination and the duration of immunity provided by the vaccine, the data for the cases indicated that the time elapsed for the primary immunisation and booster vaccination was 2–5 and 7–10 years, respectively.
Our findings suggest that vaccination does not protect from developing pertussis disease. This is consistent with the findings of another study showing that vaccination with a fifth dose of dTap appeared to have a shorter protection period and did not reduce the risk of becoming ill or getting infected [Reference Rigo-Medrano25].
VC as one indicator of the effectiveness of vaccination programmes should be thoroughly evaluated in pertussis cases. When vaccination records are not available, a periodic evaluation report should be carried out to monitor the shortcomings in VC and provide corrective actions [Reference Robert26].
In this study, we observed a high proportion of correct vaccination among pertussis cases (90.0%), compared to a study conducted in Minnesota which showed a lower VC with dTap (58.2%) [Reference Barber27]. Nonetheless, these studies are essential for identifying other relevant socio-demographic variables, which we did not include in our analysis (e.g. characteristics of the population attending the PCCs, urbanity, socio-economic deprivation index, educational level and parents' country of origin), which can influence the coverage and effectiveness of vaccines, and can provide valuable input for developing more successful vaccination strategies [Reference Hill28].
The most common symptoms consistent with pertussis were cough for ⩾2 weeks (38.9%), vomiting after coughing (22.7%) and paroxysmal coughing (19.2%). These results are similar to those observed in a US case–control study conducted in 1998–2014 to evaluate the risk factors associated with infant death from pertussis [Reference Winter29]. A recent study showed that while pertussis acellular vaccines (DTaP or dTap) prevent disease symptoms, they do not prevent B. pertussis infection/colonisation and the subsequent chain of disease transmission [Reference Warfel30].
Although immunity declines over time, immunised people who develop pertussis later on tend to suffer from less severe symptoms. As highlighted by another study, serious pertussis symptoms and complications were less common among pertussis patients who had received an age-appropriate number of pertussis vaccines, thus demonstrating that the positive impact of pertussis vaccination extends beyond decreasing the risk of disease [Reference McNamara31].
Bronchitis was the most common disease complication in our study (21.8%). B. pertussis infection can cause life-threatening complications and exacerbate concomitant chronic diseases, especially in vulnerable groups such as children and adolescents who have not received booster vaccines, patients with immunodeficiency or pulmonary complications, and health care professionals exposed to contagious diseases [Reference Zycinska32]. Norway and Spain have reported a high anti-pertussis VC; disease complications have been widely reported, including co-infection with respiratory pathogens other than B. pertussis [Reference Moreno Samos33, Reference Reinton34].
In this sense, protection from pertussis infections following booster vaccination with dTap has a limited duration, regardless of the type of anti-pertussis vaccines received during childhood [Reference Koepke35, Reference Klein36]. In our study, VC among cases with confirmed pertussis was higher than among healthy controls, showing that proper pertussis vaccination will probably not increase VC. This could be interpreted as if humoral and cell-mediated immunity persisted, regardless of how antibodies deteriorate. As other authors have stated, the DTaP vaccine may enhance the cell-mediated immune response, although additional studies are needed to verify this [Reference Vermeulen37, Reference Edelman38].
We found that VC was limited in 8- to 16-year-old patients, highlighting the need for effective vaccination programmes in this age group. We recommend that this need be met through preventive, health promotion and educational programmes at the PCCs [Reference Guiso22, Reference Lu39].
Several limitations to the present study should be noted. First, the information bias and registration errors in e-CAP/MEAP. Second, laboratory tests for confirming pertussis cases may have been carried out in a sporadic manner at the PCCs, such that some cases with an atypical clinical symptomatology could have remained undetected, which complicates the estimations of the precise incidence of pertussis. However, we checked that the controls did not have a previous pertussis diagnosis to minimise selection bias. The e-CAP/MEAP records were carefully analysed for specific case definitions, to ensure that erroneous or false-positive cases were not included in the analysis.
In conclusion, we observed an overall VC of >81% among patients with pertussis and healthy controls aged 8–16 years, and even higher among the cases. Despite the fact that PCCs recommend booster vaccination for 8- to 16-year-old population, the VC for primary and secondary anti-pertussis boosters was <90%. This could be attributed to the presence of one or more potential unobserved confounders. We conclude that the findings in this study are unusual and require further studies to find a plausible explanation. Performing the same study in different countries could help to explain our observations.
Acknowledgements
The authors would like to thank the Outpatient and Primary Care Paediatricians of the Barcelona PCCs and the Health Technicians of the Evaluation and Quality Unit of the Catalan Health Institute (Numància). The study team also acknowledge the support and assistance reviewing the study protocol from Dr Angela Domínguez and Dr Joan Caylà for reviewing the work protocol for the development of this project. The authors also thank Dr Carmen Figueroa (WHO) and Dr Gavin Lucas for reviewing the English language.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.




