Introduction
Psychotic symptoms, as highlighted by recent research, are experienced not just by patients with psychiatric disorders but also by a substantial proportion of the general healthy population. In the absence of illness, these symptoms are also referred to as psychotic-like experiences (PLEs) or subclinical psychotic symptoms. A recent meta-analysis by van Os et al. (Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009) reported a median prevalence of 5–8% for these symptoms in the general population. Data on adolescents suggest that rates of PLEs are even higher among this age group (Poulton et al. Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000; Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2003; Yoshizumi et al. Reference Yoshizumi, Murase, Honjo, Kaneko and Murakami2004; Laurens et al. Reference Laurens, Hodgins, Maughan, Murray, Rutter and Taylor2007). Clinical cases of psychosis, then, represent only a small proportion of the total phenotypic continuum of psychosis, with the major proportion made up of a much commoner non-clinical psychosis phenotype (individuals who experience PLEs).
PLEs share criterion validity with clinical psychosis
A clinical continuum between PLEs and psychotic disorder was demonstrated in an influential paper from the Dunedin birth cohort study (Poulton et al. Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000). Children aged 11 years who reported psychotic symptoms were shown to be at a 5- to 16-fold increased risk of schizophrenia-spectrum disorder in adulthood. This finding was replicated in an Australian sample by Welham et al. (Reference Welham, Scott, Williams, Najman, Bor, O'Callaghan and McGrath2009), who showed that self-reported auditory hallucinations at age 14 years were associated with increased risk for psychotic disorder at age 21. Similarly, in the general adult population, Hanssen et al. (Reference Hanssen, Maarten, Rob, Vollebergh and van Os2005) found that 8% of those who experienced PLEs were clinically psychotic 2 years later. Individuals who report PLEs (the non-clinical psychosis phenotype) can therefore be considered to represent a high-risk group for psychotic illness. As with research involving the offspring of parents with psychotic disorders (the ‘genetic’ high-risk approach), research with persons who report PLEs also provides a unique high-risk paradigm for studying the developmental trajectory to psychosis (the ‘symptomatic’ high-risk approach).
PLEs share construct validity with clinical psychosis
In the elegant paper by Mackie et al. (Reference Mackie, Castellanos and Conrod2010), in addition to identifying two discrete developmental trajectories for PLEs (‘persistent’ and ‘increasing’), the authors identify several risk factors associated with these symptoms that have previously been recognized as risk factors for schizophrenia; namely, victimization (with associated elevated depressive and anxiety scores) and substance use. These findings add to the growing body of information on PLEs and the overlap with risk factors for schizophrenia. A wide range of risk factors for schizophrenia has now been investigated in individuals who report PLEs and some striking similarities have emerged between the non-clinical and clinical populations, demonstrating construct validity between the clinical and non-clinical phenotypes.
Familiality and heritability
Hanssen et al. (Reference Hanssen, Krabbendam, Vollema, Delespaul and van Os2006) investigated PLEs among 257 members of the general population and reported familial clustering of the symptoms, in line with findings in family and adoption studies in schizophrenia. Furthermore, Polanczyk et al. (Reference Polanczyk, Moffitt, Arseneault, Cannon, Ambler, Keefe, Houts, Odgers and Caspi2010) have shown familial covariation of PLEs with maternal schizophrenia-spectrum disorder, and also with family psychiatric hospitalizations and family suicide attempts. Twin studies have also established that PLEs are heritable, with studies showing greater concordance for PLEs among monozygotic than dizygotic twins (Lataster et al. Reference Lataster, Myin-Germeys, Derom, Thiery and van Os2009; Polanczyk et al. Reference Polanczyk, Moffitt, Arseneault, Cannon, Ambler, Keefe, Houts, Odgers and Caspi2010). Research on contributing genes or loci, however, is lacking.
Schizophrenia-related social risk factors
An elevated incidence of schizophrenia has been consistently demonstrated among migrant and ethnic minority groups, particularly African-Caribbeans in the UK (Fearon et al. Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006). Johns et al. (Reference Johns, Nazroo, Bebbington and Kuipers2002) found a similar pattern among individuals who report PLEs, with persons of African-Caribbean descent 2.5 times more likely to admit to hallucinations than the white British population. Similarly, Laurens et al. (Reference Laurens, West, Murray and Hodgins2008) found that PLEs were more commonly reported by British children of African-Caribbean ethnicity than by white British children. Although they did not find a significant association with migration status, a larger representative Australian study of more than 10000 people showed that migrants from non-English-speaking backgrounds were more likely to report PLEs on interview (Scott et al. Reference Scott, Chant, Andrews and McGrath2006). Further social risk factors for schizophrenia, including unemployment, lower socio-economic background and being unwed or divorced, were also replicated in this large Australian sample. Similarly, a higher rate of urbanicity, one of the most frequently reported findings in schizophrenia (Krabbendam & van Os, Reference Krabbendam and van Os2005), was also demonstrated in association with PLEs.
Schizophrenia-related adverse childhood experiences risk factors
The prevalence of PLEs has been shown to be greater in adolescents who have had traumatic experiences, including physical abuse (Kelleher et al. Reference Kelleher, Harley, Lynch, Arseneault, Fitzpatrick and Cannon2008), unwanted sexual experiences (Lataster et al. Reference Lataster, van Os, Drukker, Henquet, Feron, Gunther and Myin-Germeys2006) and exposure to domestic violence (Kelleher et al. Reference Kelleher, Harley, Lynch, Arseneault, Fitzpatrick and Cannon2008). An association has also been reported between peer victimization and risk of PLEs, with higher rates of PLEs reported by victims of bullying (Campbell & Morrison, Reference Campbell and Morrison2007; Schreier et al. Reference Schreier, Wolke, Thomas, Horwood, Hollis, Gunnell, Lewis, Thompson, Zammit, Duffy, Salvi and Harrison2009; Mackie et al. Reference Mackie, Castellanos and Conrod2010), but also, conversely, by perpetrators of bullying (Kelleher et al. Reference Kelleher, Harley, Lynch, Arseneault, Fitzpatrick and Cannon2008; Nishida et al. Reference Nishida, Tanii, Nishimura, Kajiki, Inoue, Okada, Sasaki and Okazaki2008) and, most strikingly, by children who are both victims and perpetrators of bullying (‘bully/victims’) (Kelleher et al. Reference Kelleher, Harley, Lynch, Arseneault, Fitzpatrick and Cannon2008). Mothers of children with PLEs have also been demonstrated to show increased levels of negative expressed emotion, although no difference was found in terms of maternal warmth (Polanczyk et al. Reference Polanczyk, Moffitt, Arseneault, Cannon, Ambler, Keefe, Houts, Odgers and Caspi2010).
Schizophrenia-related substance use risk factors
The well-established relationship between psychotic disorder and cannabis use has been replicated in adolescents with PLEs (Miettunen et al. Reference Miettunen, Tormanen, Murray, Jones, Maki, Ebeling, Moilanen, Taanila, Heinimaa, Joukamaa and Veijola2008; Harley et al. Reference Harley, Kelleher, Clarke, Lynch, Arseneault, Connor, Fitzpatrick and Cannon2009). In their paper, Mackie et al. (Reference Mackie, Castellanos and Conrod2010) show that among adolescents who follow a trajectory of increasing PLEs over 2 years, a rise in cannabis use preceded a sharp increase in symptoms. Henquet et al. (Reference Henquet, Krabbendam, Spauwen, Kaplan, Lieb, Wittchen and van Os2005), similarly, showed that the risk for PLEs among adolescents and young adults increased in a dose–response manner relative to the frequency of cannabis use over a 4-year period. Harley et al. (Reference Harley, Kelleher, Clarke, Lynch, Arseneault, Connor, Fitzpatrick and Cannon2009) demonstrated an interesting synergistic interaction between cannabis use and traumatic childhood events, with exposure to both variables associated with a greater risk of PLEs than the summed risk that each variable accounted for individually. Both alcohol dependence and tobacco use have also been shown to be more common among individuals who report PLEs (Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004; Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006).
Schizophrenia-related obstetric and developmental deficits
Adverse prenatal and perinatal events, including maternal infection and obstetric complications, are well documented in schizophrenia (Clarke et al. Reference Clarke, Harley and Cannon2006, Reference Clarke, Tanskanen, Huttunen, Whittaker and Cannon2009). These findings have been replicated in persons who report PLEs (Zammit et al. Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes, Gunnell, Hollis, Wolke, Lewis and Harrison2009). Neuromotor deficits have also been demonstrated among adolescents who report PLEs (Cannon et al. Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002; Blanchard et al.,Reference Blanchard, Jacobson, Clarke, Connor, Kelleher, Garavan, Harley and Cannonin press). However, associations with advanced paternal age and with winter and spring births have failed to be shown (Zammit et al. Reference Zammit, Horwood, Thompson, Thomas, Menezes, Gunnell, Hollis, Wolke, Lewis and Harrison2008; Polanczyk et al. Reference Polanczyk, Moffitt, Arseneault, Cannon, Ambler, Keefe, Houts, Odgers and Caspi2010).
Schizophrenia-related neuroanatomical abnormalities
Neuroimaging research has demonstrated significant overlaps between the clinical and non-clinical psychosis phenotypes. The typical functional magnetic resonance imaging (fMRI) profile of hypofrontality in schizophrenia has been replicated in adolescents who report PLEs, particularly in relation to the right cerebral hemisphere, and the classic frontotemporal disconnection now well established in clinical psychosis (Friston & Frith, Reference Friston and Frith1995) has also been demonstrated (Jacobson et al. Reference Jacobson, Kelleher, Harley, Murtagh, Clarke, Blanchard, Connolly, O'Hanlon, Garavan and Cannon2010). Deficits in white matter integrity have been identified, in addition to grey matter abnormalities, although, of interest, adolescents who reported PLEs demonstrated larger grey matter volumes in contrast to the grey matter deficits typically seen in schizophrenia (Jacobson et al. Reference Jacobson, Kelleher, Harley, Murtagh, Clarke, Blanchard, Connolly, O'Hanlon, Garavan and Cannon2010).
Schizophrenia-related deficits in IQ, cognition and language
Lower IQ scores have been demonstrated in the non-clinical psychosis population, as in schizophrenia (Cannon et al. Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002; Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004; Horwood et al. Reference Horwood, Salvi, Thomas, Duffy, Gunnell, Hollis, Lewis, Menezes, Thompson, Wolke, Zammit and Harrison2008). To date, however, there have been few studies of cognition for the non-clinical phenotype. Deficits in receptive, though not expressive, language have been reported among adolescents with PLEs (Cannon et al. Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002; Blanchard et al., Reference Blanchard, Jacobson, Clarke, Connor, Kelleher, Garavan, Harley and Cannonin press). Blanchard et al. (Reference Blanchard, Jacobson, Clarke, Connor, Kelleher, Garavan, Harley and Cannonin press) also demonstrated deficits in speed of processing in the non-clinical phenotype. Men who report PLEs have been shown to perform worse in tests of verbal fluency (Krabbendam et al. Reference Krabbendam, Myin-Germeys, Hanssen and van Os2005).
Schizophrenia-related co-morbid psychopathology
Nishida et al. (Reference Nishida, Tanii, Nishimura, Kajiki, Inoue, Okada, Sasaki and Okazaki2008) assessed a general population sample of more than 5000 adolescents for both the presence of PLEs and a range of psychopathologies. Adolescents who reported PLEs showed increased levels of anxiety, suicidal ideation and self-harm behaviours. Scott et al. (Reference Scott, Martin, Bor, Sawyer, Clark and McGrath2009) found that adolescents who reported PLEs scored higher in symptoms of depression and were 2.7 times more likely to receive a diagnosis of depressive disorder. PLEs were also associated with child psychopathology on parent-rated measures. Polanczyk et al. (Reference Polanczyk, Moffitt, Arseneault, Cannon, Ambler, Keefe, Houts, Odgers and Caspi2010) replicated the findings for depressive and anxiety symptoms and self-harm and suicidal behaviour, and also demonstrated an increase in antisocial behaviour among adolescents who reported PLEs.
Discussion and implications
The past decade of research summarized above illustrates a non-ambiguous continuum between the clinical and non-clinical psychosis phenotypes in the general population. PLEs are familial, heritable, confer increased risk for schizophrenia-spectrum disorder and covary with maternal psychotic disorder. Furthermore, these symptoms share an extensive range of social, environmental, substance use, obstetric, developmental, anatomical, motor, cognitive, linguistic, intellectual and psychopathological risk factors with schizophrenia (see Table 1).
Table 1. Aetiological and risk factor continuity between clinical and non-clinical psychosis phenotypes
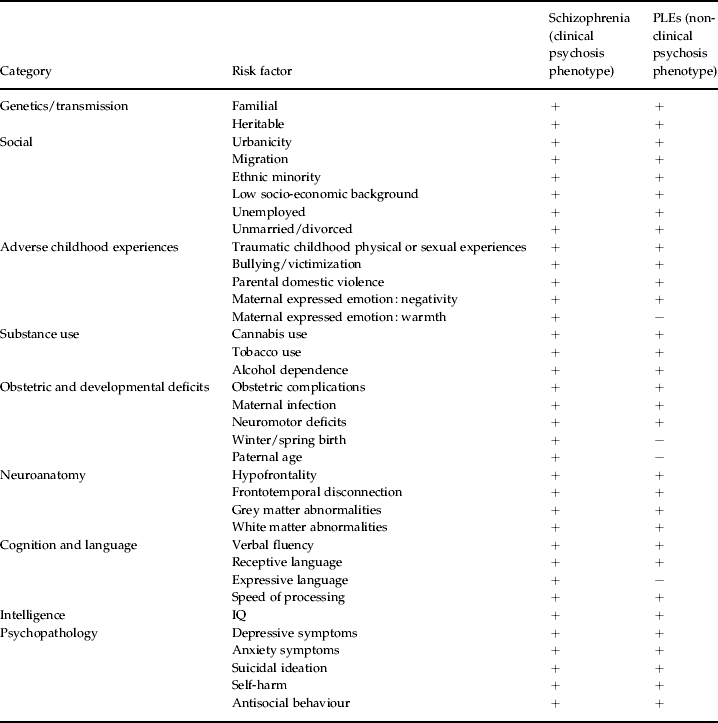
PLE, Psychotic-like experience.
+, Positive findings; −, negative findings.
Although the rapid nervous system changes that take place in adolescence are recognized to be of great significance in the development of clinical psychosis, the low disease incidence hinders attempts to study the developmental trajectory in large numbers prior to the onset of illness (given that only about one in every hundred children studied will develop schizophrenia). The findings presented here, however, demonstrate that the non-clinical psychosis phenotype represents a valuable, and valid, population in which to study the aetiology of psychosis, with the further advantages that this non-clinical phenotype (i) is more prevalent than the clinical (disease) phenotype, thus increasing the population pool for study, (ii) can be screened for using a validated brief questionnaire (Kelleher et al. Reference Kelleher, Harley, Murtagh and Cannon2009) and (iii) facilitates research into early (pre-morbid, pre-medication) neurodevelopmental changes in psychosis.
Much has been learned about this population, but more remains to be discovered, especially in terms of neuro-genetics, -cognition and -anatomy. Genetic research may be particularly valuable in elucidating the molecular mechanisms underlying psychosis because the construct and criterion validity between the clinical and non-clinical psychosis phenotypes suggests that genetic risk may be shared between both. That is, genetic risk for psychosis may relate to the broader (clinical and non-clinical) psychosis phenotype rather than specifically to psychotic disorder. Genetic research to date has used the simple dichotomy of persons with psychotic disorder compared to the general population. However, the reasonably common non-clinical psychosis phenotype in the general population may have masked genes of importance.
The identification of a non-clinical psychosis phenotype raises interesting evolutionary questions (Kelleher et al., Reference Kelleher, Jenner and Cannonin press). Psychotic disorders are highly heritable and exert strong negative fitness effects but persist despite selective disadvantage. The identification of a non-clinical psychosis phenotype, however, opens up the possibility that there may be selective advantages associated with genes that contribute to this phenotype but that also increase the risk for psychotic disorder. This biological compromise is not unique to psychosis; for example, genes that have been positively selected due to their protective effects against malarial infection bring with them the risk of sickle cell disease. Further investigation of this non-clinical population may help us to finally uncover an evolutionary basis for the persistence of psychosis genes in the general population.
Conclusions
Previous views of psychotic symptomatology as intrinsically pathological have been replaced by the idea that, in many cases, psychotic symptoms may fall within a spectrum of normal experience. Nonetheless, these symptoms are associated with increased risk for psychotic disorder and show criterion and construct validity for clinical psychosis. PLEs are familial, heritable, confer increased risk for schizophrenia-spectrum disorder, covary with maternal schizophrenia-spectrum disorder and share an extensive range of risk factors with schizophrenia. These findings indicate that the non-clinical psychosis phenotype represents a valid population for studying the aetiology of clinical psychosis and suggest a shared genetic aetiology between the clinical and non-clinical phenotypes. Much remains to be learned about psychosis by broadening the scope of research to include the non-clinical psychosis population.
Acknowledgements
M.C. is supported by a Health Research Board (Ireland) Clinician Scientists Award and a NARSAD Independent Investigator Award.
Declaration of Interest
None.



