I. INTRODUCTION
Tissue engineering unites both engineering and life science principles to advance the development of biological substitutes that can restore or improve the function of tissue and organs. Reference Wobma and Vunjak-Novakovic1 The final goal is to create artificial three-dimensional (3D) scaffolds that sufficiently mimic the natural biological environments, thereby biological cells in the artificial environments can function as well as they would in the real tissue. For designing artificial biological scaffolds, structural geometry and morphology should be tuned for high functionality and extracellular matrix (ECM) mimicry at various length scales to recapitulate the tissue complexity and engineer the desired organ. As a result, the cell niche could be generated within such artificial environment with the interactions of different cell types, morphogens, growth factors, and mechanical cues. To successfully reproduce and control the local cellular microenvironment, microtechnology-based tissue engineering approaches provide the capacity of positioning and printing biomaterials and cells with high spatial and temporal resolution. Reference Ozbolat and Hospodiuk2 More recently, the miniaturization of tissue formation toward organ-on-a-chip system, Reference Zhang, Arneri, Dell’Erba, Shin, Aleman, Busignani, Bersini, Dokmeci, Annabi, Moretti, Rasponi and Khademhosseini3,Reference Zhang, Aleman, Arneri, Bersini, Piraino, Shin, Dokmeci and Khademhosseini4 aims to fabricate microphysiological systems that are favorably comparable with the functionality of real organs.
Cells in the tissue are responsive to their environment and receptive to the patterns of biochemistry as well as physical topography. However, conventional fabrication methods create relatively homogeneous scaffolds that cannot present the micro- and nanoscale instructive cues to maintain cell phenotype and behavior. To synergistically incorporate the scaffold architecture within the biological materials, several methods, such as nanoimprinting, Reference Lee, Lee, Kim, Yoo, Chung and Kim5 electron beam lithography, Reference Bhatnagar, Mark, Kim, Chen, Schmidt, Lipson and Batt6 electrospinning, Reference Joanne, Kitsara, Boitard, Naemetalla, Vanneaux, Pernot, Larghero, Forest, Chen, Menasche and Agbulut7–Reference Ahn, Ju, Takahashi, Williams, Yoo, Lee, Okano and Atala11 solvent casting/salt leaching, Reference Thadavirul, Pavasant and Supaphol12 gas foaming, Reference Salerno, Oliviero, Di Maio, Iannace and Netti13 phase separation Reference Janik and Marzec14,Reference Liu and Ma15 and solid freeform fabrication Reference Tran and Wen16 techniques, have been widely used. Nanoimprinting and electron beam lithography are able to precisely fabricate two-dimensional (2D) surface features, but they require long fabrication times, special apparatuses, and expensive instruments. Moreover, 2D microfabrication methods cannot fully mimic the real 3D tissue environments and properties. Other methods, such as electrospinning, solvent casting/salt leaching, and gas foaming, enable us to fabricate 3D scaffolds, but are difficult to use to control the physical geometries with high spatial precision less than 10 µm size.
Recently, laser-assisted techniques have been used to fabricate biological scaffolds with defined structures. Reference Selimis, Mironov and Farsari17–Reference Riggs, Dias, Schiele, Cristescu, Huang, Corr and Chrisey20 These techniques have shown unique properties for micromachining coating, patterning, and polymerization of various biomaterials for implantable medical devices and regenerative medicine. The optical nature of laser-assisted fabrication, such as noncontact, optically selective, high-throughput, and precise processing, provides the capability for high precision processing with submicron resolution (<1 µm). Laser-assisted biofabrication is compatible with a range of viscosities (1–300 mPa/s) and can print mammalian cells with negligible effect on cell viability and function. It can deposit cells at a density of upto 108 cells/mL with microscale resolution of a single cell per drop using a laser pulse repetition rate of 5 kHz, with speeds up to 1600 mm/s. Reference Guillotin, Souquet, Catros, Duocastella, Pippenger, Bellance, Bareille, Remy, Bordenave, Amedee and Guillemot21
Here, we review the current approaches for laser-assisted fabrication of biomaterials, which have great potential to incorporate biomimetic approaches in the material design and processing for complex artificial scaffolds. In this review, we focus on: (i) laser tweezers which harness the power of optical force to trap individual cells and pattern them with high spatial precision; (ii) laser-initiated polymerization which uses a laser beam to generate chemical bonds within multi-photon precursor material to fabricate 3D structure with resolution at submicron scale; (iii) laser induced forward transfer (LIFT) and matrix assisted pulsed laser evaporation (MAPLE) to transfer and deposit heat-sensitive organic or polymeric materials onto solid substrate; and (iv) laser ablation which selectively removes biomaterials with high precision to create holes, grids, or parallel lines at microscale in the biomaterials. Through these laser-assisted biofabrication technologies, we can control the spatial arrangement of biomaterials at the resolution less than 10 µm for fundamental understanding about cell–cell and cell–biomaterial interactions, which can be applied to regenerative medicine.
II. LASER-ASSISTED RAPID PROTOTYPING FABRICATION OF BIOMATERIALS
There are urgent needs for 3D biomaterial fabrication over a large area with relative high precision for more closely mimicking the in vivo tissue environments. However, conventional fabrication techniques cannot fulfill both the speed and precision necessary for large-scale biofabrication. For example, dip pen lithography is able to fabricate biomaterials with nanoscale precision (50–2000 nm) but requires a long processing time for large area patterning (0.1 nm/s). Reference Han, Hu, Genin, Lu and Xu22 In contrast, inkjet printing is adequate for high-speed large area patterning, but lacks precision to produce reliable detailed patterns. Reference Suntivich, Drachuk, Calabrese, Kaplan and Tsukruk23 In contrast, rapid prototyping fabrication techniques meet the requirements of both high speed and precise spatial arrangement, which make them promising for biomaterial patterning and tissue engineering.
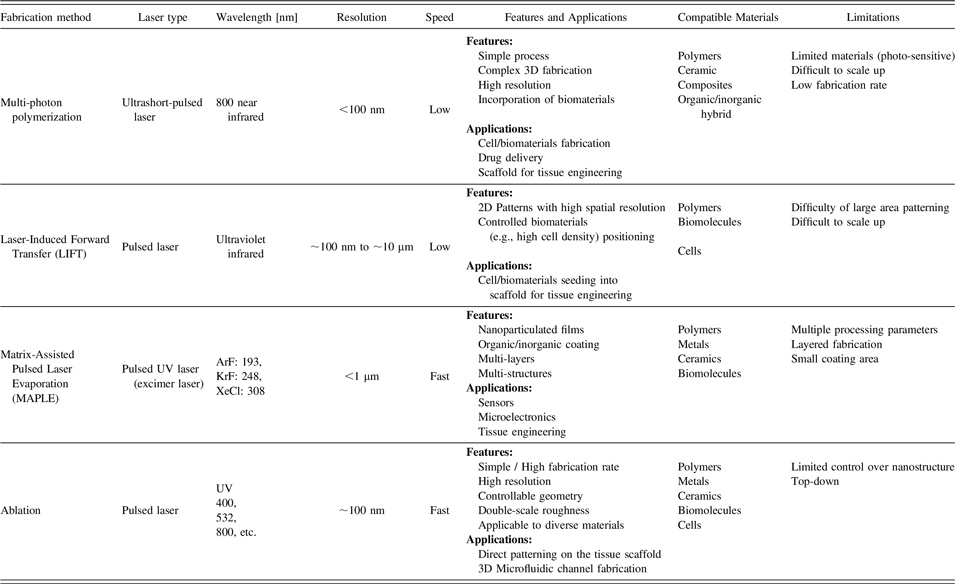
With the emergence of tissue engineering and regenerative medicine, rapid prototyping techniques are being applied to biomaterials and tissue scaffolds with controllable structural geometries, such as porosity, shape, and inter-connectivity. Reference Hendrikx, Kascholke, Flath, Schumann, Gressenbuch, Schulze, Hacker and Schulz-Siegmund24,Reference Guillotin, Catros, Keriquel, Souquet, Fontaine, Remy, Fricain and Guillemot25 In a variety of rapid prototyping techniques, laser-assisted fabrication methods have emerged as powerful technologies for the realization of arbitrary 3D structures with micron or sub-micron scale resolution (Table I). Due to the specific properties of lasers, such as high power density irradiation, coherence, defined wave length, polarization, monochromatic beam and directed optical energy emission, laser-assisted fabrication is a promising tool for precise fabrication. A scanned laser beam transfers energy into the target material to alter the material’s properties in a confined region. Numerous phenomena take place depending on the deposited net energy. Unlike continuous lasers, ultrafast lasers have short pulse duration (10–50 femtoseconds), which can deposit the energy on the target material within a time scale (100 femtoseconds) shorter than heat diffusivity (19 mm2/s in air), so that it can prevent the heat diffusion in the material.
TABLE I. Laser-assisted techniques for biomaterial fabrication and tissue engineering.

III. LASER TWEEZERS FOR SINGLE-CELL MICROPATTERNING
When light interacts with a particle, such as a biological cell, the momentum of light is changed due to refraction and reflection. According to Newton’s law of momentum conservation, the particle must undergo an equal and opposite momentum change, which results in a force that acts on the particle; that force is termed as optical force. Reference Ashkin26 Such optical force has been used to develop the laser tweezers technique, which was used in a wide-ranging series of experiments, including the cooling and trapping of neutral atoms, Reference Ashkin27 the manipulation of live bacteria and viruses, Reference Ashkin and Dziedzic28 and later thrived in biological sciences to study the mechanics of biomolecular interactions at the single-molecule level, such as actin–myosin interactions, Reference Ishijima, Kojima, Funatsu, Tokunaga, Higuchi, Tanaka and Yanagida29 kinesin’s motion along microtubules, Reference Kojima, Muto, Higuchi and Yanagida30 and DNA folding and transcriptions. Reference Brower-Toland, Smith, Yeh, Lis, Peterson and Wang31 With its high accuracy and spatial precision, laser tweezers have unlocked a new era in biofabrication as a result of their promising contributions to tissue engineering and regenerative medicine. Research using this technique aims to achieve accurate cell arrangement with minimal variation for the systemic and statistical study of cell–cell interactions at single-cell level with high spatial precision. Reference Odde and Renn32–Reference Nahmias and Odde34 Furthermore, as opposed to conventional cell patterning that constrains cells within the protein deposited area, this technique allows for natural cell migration after initial deposition thereby enabling cell–cell and cell–ECM interactions to be studied without specific cell or ECM confinement.
Taking advantage of optical force, Gao’s lab developed a laser-guided cell deposition microscope to micropattern rat mesenchymal stem cells (rMSCs) with neonatal rat cardiomyocytes (NRCMs) into a microfabricated substrate to form a single-cell co-culturing microenvironment. Reference Ma, Russell, Qin, Yun, Yuan, Peng, Borg and Gao35 During the co-culturing process, rMSCs exhibited gradual change in their electrophysiological properties depending on contact-mediated interactions with NRCMs Reference Ma, Yang, Liu, Xu, Runyan, Eisenberg, Markwald, Borg and Gao36 [Fig. 1(a)]. A continual study showed that this technique could be used to deposit a controlled number of cells (NRCMs, rMSCs, or fibroblasts) to form the cell bridges that connected the cardiac muscle fibers cultivated on the multielectrode arrays (MEAs) Reference Ma, Liu, Liu, Yang, Yun, Eisenberg, Borg, Xu and Gao37 [Fig. 1(b)]. Stem cell bridges demonstrated a strong ability to conduct electrical signals along microfabricated cardiac muscle fibers through gap junctions. These results indicated that stem cells had higher electrical compatibility with native cardiac muscle fibers than cardiac fibroblasts, thus a promising cell source for designing efficient cardiac cell therapies. Moreover, the precision of laser tweezers enabled investigators to study the inflection point, in which the length of cell bridge and the number of cells forming the bridge would no longer be conducive to electrical propagation.

FIG. 1. Laser tweezers for single-cell micropatterning (a) schematic of laser tweezers for single-cell micropatterning Reference Ma, Yang, Liu, Xu, Runyan, Eisenberg, Markwald, Borg and Gao36 ; (b) a mesenchymal stem cell bridge was created between two separated cardiac muscle fibers using laser tweezers technique Reference Ma, Liu, Liu, Yang, Yun, Eisenberg, Borg, Xu and Gao37 ; (c) single neurons were micropatterned by laser tweezers technique to create neural circuits within a pre-fabricated microstructure, Reference Pirlo, Sweeney, Ringeisen, Kindy and Gao38 and (d) 3D mouse embryonic stem cells were patterned using holographic optical tweezers and stabilized by PEG-based hydrogels. Reference Kirkham, Britchford, Upton, Ware, Gibson, Devaud, Ehrbar, Padgett, Allen, Buttery and Shakesheff40
Axon pathfinding plays an important role in both normal and pathogenic neurological development, the study of which has applicability to neurologic regenerative medicine as well as to understand the possible long-term or heritable effects of developmental neurotoxicity. In a study by Pirlo et al., PDMS-based biochips were created to investigate the influence of geometrical confinement on the polarity of axon growth and of glial cells on axon pathfinding Reference Pirlo, Sweeney, Ringeisen, Kindy and Gao38 [Fig. 1(c)]. Laser tweezers were used to pattern neurons and neuron–glial cell pairs onto the microwells of the biochip and monitor axonal growth and polarity along the microchannels connecting two microwells. The study demonstrated the differential efficacy of various microchannel geometries in influencing axonal growth polarity. A subsequent study by Wei et al. Reference Wei, Sweeney, Sheng, Fang, Kindy, Xi and Gao39 aimed at identifying the optimal microenvironment to investigate the impact of methylated mercury (MeHg) on axonal pathfinding demonstrated by elongation, turning, and branching. This study identified a biochip/laser cell-patterning model sensitive to MeHg at a 10-fold lower threshold than conventional assays, presenting a new avenue for developmental neurotoxicity research.
Research conducted by Kirkman et al. demonstrated that holographic optical tweezers (HOTs) in combination with other technologies enable nondestructive methods of cell micromanipulation to create stable, complex 3D in vivo-like microenvironments Reference Kirkham, Britchford, Upton, Ware, Gibson, Devaud, Ehrbar, Padgett, Allen, Buttery and Shakesheff40 [Fig. 1(d)]. With HOTs in combination with PEG-based hydrogel, researchers produced 3D, stable, large scale (simultaneously patterning 66 cells) and multi-cell type structures of mouse embryonic stem cells (mESCs), mouse mesenchymal stem cells (mMSCs), and mouse primary calvaraes cells (mPCs) co-cultures. This study verified the applicability of HOTs to not only pattern cells and polymeric materials, but also regulate controlled release to mimic the temporal influence of chemical factors. The enhanced HOTs technology coupled with the PEG-based hydrogel enabled a level of microenvironmental control at a scale, complexity, and resolution not previously demonstrated.
IV. 3D STRUCTURE FABRICATION USING MULTI-PHOTON POLYMERIZATION
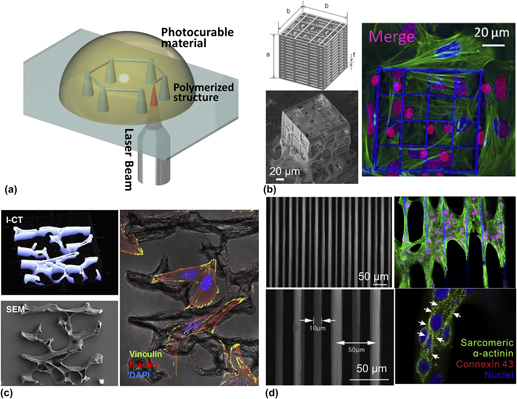
Multi-photon polymerization is a promising tool for 3D-structure fabrication with micro/nanoscale resolution. Irradiated by a tightly focused ultrafast laser beam at infrared wave length, nonlinear optical phenomena occur. Simultaneous absorption of multiple photons triggers the photo-induced polymerization within a selective small volume (voxel: ∼100 nm) through the radical generation upon interaction between photo-initiator and laser beam Reference Do, Nguyen, Li, Benisty, Ledoux-Rak and Lai41 [Fig. 2(a)]. This interaction has enabled the construction of a 3D biomimetic structure with high-resolution (from µm to nm) that could not have been obtained by traditional photolithography. Reference Ma, Koo, Finnegan, Loskill, Huebsch, Marks, Conklin, Grigoropoulos and Healy42

FIG. 2. Three-dimensional structures fabricated by multi-photon polymerization (a) schematic of multi-photon polymerization for 3D structure fabrication; (b) an engineered 3D structure was fabricated using multi-photon polymerization for hosting MG63 osteosarcoma cells (SEM image) and mesenchymal stem cells (confocal image) Reference Raimondi, Eaton, Lagana, Aprile, Nava, Cerullo and Osellame48 ; (c) a single Osteoprint based on 3D reconstruction of human trabecular bone was fabricated by two-photon polymerization and populated with SaOS-2 osteoblasts Reference Marino, Filippeschi, Genchi, Mattoli, Mazzolai and Ciofani58 ; and (d) multi-photon polymerized fibrous matrices were used to create a 3D human diseased cardiac tissue model with cardiomyocytes differentiated from human induced pluripotent stem cells derived from a patient with long QT syndrome. Reference Ma, Koo, Finnegan, Loskill, Huebsch, Marks, Conklin, Grigoropoulos and Healy42
However, multi-photon polymerization requires the laser beam to pass through the entire photo-curable material without attenuation, which limits its application to only transparent materials, such as SU-8, Reference Do, Nguyen, Li, Benisty, Ledoux-Rak and Lai41 ORMOCER®, Reference Ma, Koo, Finnegan, Loskill, Huebsch, Marks, Conklin, Grigoropoulos and Healy42,Reference Hidai, Jeon, Hwang and Grigoropoulos43 ORMOCOMP®, Reference Kapyla, Aydogan, Virjula, Vanhatupa, Miettinen, Hyttinen and Kellomaki44 ORMOSIL®, Reference Matei, Schou, Canulescu, Zamfirescu, Albu, Mitu, Buruiana, Buruiana, Mustaciosu, Petcu and Dinescu45,Reference Matei, Zamfirescu, Jipa, Luculescu, Dinescu, Buruiana, Buruiana, Sima and Petrescu46 zirconium based organic-inorganic hybrid material (SZ2080), Reference Rekstyte, Zukauskas, Purlys, Gordienko and Malinauskas47–Reference Stankevicius, Gertus, Rutkauskas, Gedvilas, Raciukaitis, Gadonas, Smilgevicius and Malinauskas49 IP-L780 Reference Mihailescu, Paun, Zamfirescu, Luculescu, Acasandrei and Dinescu50 and biocompatible hydrogels. Reference Zhang and Chen51 With custom-synthesized hybrid materials, 3D scaffolds can be fabricated with tunable geometries (e.g., porosity, pore size etc.) Reference Raimondi, Eaton, Lagana, Aprile, Nava, Cerullo and Osellame48 and mechanical properties (e.g., stiffness). Reference Kwok, Kuznetsov, Kim, Choi, Scarcelli and Yun52 For the purposes of tissue engineering, multi-photon polymerization of hydrogel would facilitate the development of biomimetic scaffolds for encapsulation of living cells in a defined manner. To extend the availability of biocompatible hydrogel for multi-photon polymerization, development of highly sensitive water-soluble photo-initiators is highly demanded.
Furthermore, the risk of cell damage during the multi-photon polymerization process should be considered for efficient cell encapsulation. It has been shown that cytoskeleton structure can be dissected by femtosecond laser pulse irradiation (790 nm, 1.5 W, pulse duration of 140 femtoseconds). Reference Kumar, Maxwell, Heisterkamp, Polte, Lele, Salanga, Mazur and Ingber53 However, pulse energy required for hydrogel polymerization and modification by multi-photon polymerization is lower than that for ablation of cytoskeleton structure. Reference Tibbitt, Kloxin, Dyamenahalli and Anseth54 Hence, multi-photon polymerization can be a low thermal process, and thus proteins, cells, and antimicrobial agents can be directly incorporated within the 3D biomaterial scaffold without significant thermal damage, which is essential for tissue engineering applications.
Using the laser uncaging technique for modification of chemical reactions, multi-photon absorption also allow for the rapid release of extremely small volume (less than 1 fL) chemical within tightly focused region. Reference Ellis-Davies55 Regulated by the multi-photon absorption in microscale focal volumes, this photo-reactive process also can generate complex microscale structures with different concentrations of biomolecules within the hydrogel scaffold. Reference Lee, Moon and West56 Using this technology, Lee et al. fabricated cell adhesive ligand (RGD)-modified hydrogels to control the cell adhesion and function. This chemically modified structure enhanced and guided the cell migration in collagenase-sensitive poly(ethylene glycol-co-peptide) diacrylate hydrogels. Reference Lee, Moon and West56
In the view of stem cell manipulation in artificial scaffolds, the maintenance of suitable cellular microenvironments is critical for stem cell self-renewal, niches formation, and differentiation. Reference Marino, Filippeschi, Mattoli, Mazzolai and Ciofani57 Laser-assisted multi-photon polymerization has been widely used for fabricating highly controllable structures with well-predefined geometry and porosity. For example, Raimondi et al., measured cell viability, migration and proliferation of the primary rMSCs seeded in the 3D structures fabricated using multi-photon polymerization. They observed that 3D mechanical niche played an important role on MSC colonization and differentiation Reference Raimondi, Eaton, Lagana, Aprile, Nava, Cerullo and Osellame48 [Fig. 2(b)]. In an application of bone tissue engineering, a 3D structure was fabricated by multi-photon polymerization composing of a micro-scale vertical tube and triangular lattice. Through assessment of osteoblast-like cells (MG63) in this 3D structure, researchers found that the physical geometry affected the alignment of the cell’s nucleus and potential for osteogenesis. By changing the micro-scale geometry of the vertical tube (height and circularity), the level of mineralization from osteoblasts also changed. Reference Mihailescu, Paun, Zamfirescu, Luculescu, Acasandrei and Dinescu50 A study by Marino et al. fabricated a trabecular-like structure with multi-photon polymerization, which was similar to the microstructure of the trabecular bone. By seeding the SaOS-2 osteoblasts to this structure, they observed the enhancement of osteogenic differentiation and hydroxyapatite production Reference Marino, Filippeschi, Genchi, Mattoli, Mazzolai and Ciofani58 [Fig. 2(c)].
Multi-photon polymerization has breakthrough applicability to the creation of human in vitro cardiac tissue models that enable the study of cardiac arrhythmias and related cardiovascular diseases. Native myocardial tissues are organized in complex 3D structures that are critical to the electro–mechanical function of the heart muscle. In a study by Ma et al., using multi-photon polymerization, a synthetic 3D filamentous model was created and populated with cardiomyocytes derived from healthy and long QT syndrome type 3 (LQT3) human induced pluripotent stem cells (hiPS-CMs) to mimic human ventricular myocardium. This artificial cardiac model enabled the study of contractility malfunctions associated with electrophysiological consequences of LQT3 as well as their response to a panel of drugs. In addition, since this technique allowed for tunable fiber diameter (5–10 µm) and spacing (25–100 µm), the impact of stiffness variability in the filamentous matrices on both contractility abnormality and susceptibility to drug-induced cardiotoxicity were observed Reference Ma, Koo, Finnegan, Loskill, Huebsch, Marks, Conklin, Grigoropoulos and Healy42 [Fig. 2(d)].
Fabrication of soft hydrogels is limited by their poor mechanical properties for supporting and guiding tissue formation. To solve this limitation, laser-assisted fabrication provides a possibility to temporally and spatially control cross-linking level of soft hydrogels. In a study by Kufelt et al., chitosan was crosslinked with synthetic poly(ethylene glycol) diacrylate (PEGDA) to modulate its mechanical and biochemical properties. By changing the chemical functional group of chitosan, synthesized solvent-free N-alkylation of chitosan could be used for multi-photon polymerization. Reference Akopova, Timashev, Demina, Bardakova, Minaev, Burdukovskii, Cherkaev, Vladimirov, Istomin, Svidchenko, Surin and Bagratashvili59 Successfully seeded with cells, the 3D multi-photon fabricated chitosan scaffolds were found to be biocompatible and promising for future tissue engineering applications. Multi-photon polymerization was also used to fabricate polylactic acid (PLA) scaffolds for neurological regenerative medicine. The biocompatibility of fabricated PLA scaffolds was evaluated through the viability and morphology of SH-SY5Y human neuronal cell line and primary cultured rat Schwann cells. These scaffolds sustained a high degree of Schwann cell purity (99%) and provided a suitable substrate for supporting Schwann cell spreading physiological morphologies. Multi-photon fabricated PLA scaffolds were found suitable for neural growth and valuable as potential platform for peripheral nerve repair studies.
One of the main drawbacks of 3D structure fabrication by multi-photon polymerization is the low processing speed (maximum scanning speed is 10 mm/s). Total processing time depends on the photosensitivity (reactivity) of the materials, laser intensity, scanning speed, and construct size. A combination of multi-photon polymerization with 3D printing technique has demonstrated rapid fabrication of diverse functional micro-featured and integrated devices. A tabletop thermal-based extrusion 3D printer was used for rapid fabrication of centimeter scale scaffolds. After the 3D printing process, the printed structures were subsequently immersed into a photocurable monomer solution for additional multi-photon polymerization process, which resulted in centimeter-scale structure with submicron-scale secondary structure inside the 3D printed scaffold. Reference Balciunas, Lukosevicius, Mackeviciute, Rekstyte, Rutkunas, Paipulas, Stankeviciute, Baltriukiene, Bukelskiene, Piskarskas and Malinauskas60 In terms of rapid fabrication, multi-foci methodologies based on a hologram patterning technology can significantly reduce the processing time. Reference Jiang, Shen and Wang61,Reference Stankevicius, Balciunas, Malinauskas, Raciukaitis, Baltriukiene and Bukelskiene62 Computer-generated hologram patterns were used to generate multiple laser beams in controlled positions from a single laser source. These multiple beams were used to simultaneously produce multiple microstructures, which suggests it has a great potential to improve the efficiency of multi-photon polymerization. Reference Gittard, Nguyen, Obata, Koroleva, Narayan and Chichkov63
V. BIOMATERIAL PRINTING USING LASER INDUCED FORWARD TRANSFER (LIFT)
Laser induced forward transfer (LIFT) addresses the challenges of printing cells and liquid materials with cell-level resolution and the anisotropy of living tissue. Reference Mezel, Souquet, Hallo and Guillemot64,Reference Barron, Wu, Ladouceur and Ringeisen65 In the LIFT process, energy from a pulsed laser (wave length 248 nm, pulse rate 2.5 ns, energy 5–10 µJ) is absorbed in a thin layer of absorbing material on a transparent substrate, and light–matter interaction takes place at the interface to generate a strong pressure at the region of laser irradiation. As a result, a small pixel of the thin film is ejected from the substrate, with velocities ranging from 200 m/s to 1200 m/s, and deposited on the target [Fig. 3(a)]. The size and shape of transferred materials can be controlled by the incident laser spot. To avoid cell or DNA damage, and preserve cell functionality for tissue engineering applications, multiple parameters of LIFT processing need to be optimized, such as laser pulse energy, bio-ink viscosity and processing time Reference Gruene, Deiwick, Koch, Schlie, Unger, Hofmann, Bernemann, Glasmacher and Chichkov66 [Fig. 3(b)]. Laser fluences need to be optimized to limit the heat diffusion into the film and avoid the generation of melted debris on the receiving substrates. High laser fluences will diminish the deposition quality, since they generate shockwaves that can be reflected back by the receiver and interfere with the ejected pixels (solid) or droplets.

FIG. 3. Laser induced forward transfer (LIFT) for biofabrication (a) schematic of LIFT technology; (b) mesenchymal stem cells were patterned into “BONE” using LIFT technology with high cell viability Reference Gruene, Deiwick, Koch, Schlie, Unger, Hofmann, Bernemann, Glasmacher and Chichkov66 ; and (c) human umbilical vein endothelial cells and mesenchymal stem cells were co-patterned by LIFT technology on a cardiac patch, which was implanted in vivo onto the area of blanched myocardium. Reference Gaebel, Ma, Liu, Guan, Koch, Klopsch, Gruene, Toelk, Wang, Mark, Wang, Chichkov, Li and Steinhoff70
Using modified LIFT with a sacrificial layer of metal (Ti or Au, ∼100 nm) for a rapid thermal expansion, cells showed near 100% viability and retained their genetic and phenotypic functionalities. Reference Ringeisen, Kim, Barron, Krizman, Chrisey, Jackman, Auyeung and Spargo67–Reference Schiele, Corr, Huang, Raof, Xie and Chrisey69 The repetition rate of the laser (5 kHz) has showed high cell density (108 cells/mL), high-speed (200 mm/s), and high-resolution (10 µm) patterning with multiple cell seeding on the scaffolds. Reference Guillotin, Souquet, Catros, Duocastella, Pippenger, Bellance, Bareille, Remy, Bordenave, Amedee and Guillemot21 Gaebel et al. compared the benefit of LIFT-based versus non-LIFT-based tissue engineered cardiac patches for the potential treatment of myocardial infarction. In this study, LIFT technology was used to seed HUVECs and human mesenchymal stem cells (hMSCs) onto polyester urethane urea (PEUU) cardiac patches with pre-defined features conducive to cardiac tissue regeneration. Patches were cultivated and transplanted to the infarcted zone of rat hearts. LIFT-based cell patterning affected the growth characteristics of HUVECs and hMSCs leading to an increase in vessel formation. Eventually, significant functional enhancement of infarcted hearts was observed following the transplantation of LIFT-engineered cardiac tissue patches. Because of the spatial patterning of HUVECs and hMSCs, the LIFT patches showed enhanced capillary density and integration of human cells into connected vessels of murine vascular system Reference Gaebel, Ma, Liu, Guan, Koch, Klopsch, Gruene, Toelk, Wang, Mark, Wang, Chichkov, Li and Steinhoff70 [Fig. 3(c)].
Recently, the LIFT technique has been moving forward to assemble the 3D structure of cells in the prospect of tissue construct, to create fully functionalized scaffolds for tissue replacement, and to fabricate in vivo-mimicked organoids via layer-by-layer approaches. In Wu and Ringeisen’s previous research, they fabricated the 3D branch/stem structures for co-culturing HUVECs and human umbilical vein smooth muscle cells (HUVSMCs) with small droplets (approximately 50 µm in diameter). Reference Wu and Ringeisen71 This study not only showed that LIFT technique can be used for initiating and guiding tissue self-organization, but also demonstrated that the microscale printing unit can be scalable into a macroscale 3D construct. In another LIFT application, Pirlo et al. fabricated the 3D structures by stacking the 2D PLGA/hydrogel bio-papers, on which HUVECs were printed with high resolution. Reference Pirlo, Wu, Liu and Ringeisen72 The stacking structures supported the development of HUVECs networks and retained their printed patterns. This research demonstrated that patterning capability of LIFT technology enabled the perfusion in the tissue constructs through 3D microvascular networks. Moreover, a proof-of-concept study by Ovsianikov et al. investigated the applicability of multi-photon polymerization in combination with LIFT as a novel dual fabrication technique for creating highly porous (pore size 92 µm) 3D scaffolds seeded with multiple cell types. Reference Ovsianikov, Gruene, Pflaum, Koch, Maiorana, Wilhelmi, Haverich and Chichkov73
VI. BIOMATERIAL DEPOSITION USING MATRIX ASSISTED PULSED LASER EVAPORATION (MAPLE)
Matrix-assisted pulsed-laser evaporation (MAPLE) is a natural extension of pulsed laser deposition (PLD). Because MAPLE uses a lower-powered laser operating in the UV region (wave length 193 nm and power ∼0.02 J/cm2), it is a less harsh approach for the transfer and deposition of heat sensitive organic or polymeric materials onto a solid substrate with minimized thermal/chemical decomposition, thereby having significant applicability to tissue engineering research. With a cryogenic composite target, the organic materials are dissolved in a laser absorbent solvent. A major part of laser energy is absorbed by the solvent, thus violent interactions between photons and active materials are diminished. Therefore, laser energy is converted into thermal energy, which helps fast evaporation of volatile solvent and deposits uniform thin films with maintained chemical properties and functionalities [Fig. 4(a)]. Evaporated solvents were pumped out by a vacuum system, to prevent them sticking on the receiving substrate. The receiving substrate is kept at room temperature or, in some cases, low temperature (∼200 K) to prevent degradation and damage of solute molecules.

FIG. 4. Matrix-assisted pulsed laser evaporation (MAPLE) for biofabrication (a) schematic of MAPLE technology; (b) MAPLE-deposited coating of MgOCP and SrOCP was analyzed by energy dispersive x-ray spectrometry (EDS), which showed homogeneous distribution of ions on the surface Reference Boanini, Torricelli, Fini, Sima, Serban, Mihailescu and Bigi75 ; and (c) keratinocyte stem cells exhibited preferential orientation on MAPLE-deposited PLGA/PU polymer substrates with micropatterned squares and channels. Reference Paun, Mihailescu, Calenic, Luculescu, Greabu and Dinescu79
MAPLE allows better control of thickness and surface morphology, enhancement of film/substrate adhesion, multi-layer deposition and patterning, and minimization of contamination than noncontact deposition. For the efficient MAPLE process, diverse parameters should be optimized, such as laser fluence, pulse repetition rate, substrate temperature, dynamic pressure, selection of solute–solvent mixture, and distance between target and collector. However, the compatibility of MAPLE is limited by restricted selections of solvents (e.g., methanol, chloroform), which must be chemically stable and inert. The solvents not only need to efficiently absorb the incident laser pulse and be easily evaporated but also must not interact with the solute during the laser irradiation.
Due to the strength of MAPLE in terms of deposition of either organic or inorganic material efficiently, it is possible to deposit organic–inorganic multilayer deposition in a single step. In bone tissue engineering, biomimetic nanocrystalline apatite thin film can be deposited on titanium substrates using MAPLE technology to not only preserve the structural and chemical nature of the nanocrystalline apatite, but also perpetuation of the nonapatitic environments. Reference Visan, Grossin, Stefan, Duta, Miroiu, Stan, Sopronyi, Luculescu, Freche, Marsan, Charvilat, Ciuca and Mihailescu74 Octacalcium phosphate (OCP) is a promising alternative to hydroxyapatite as biomaterial for hard tissue repair. OCP, Mg-doped (MgOCP) and Sr-doped OCP (SrOCP) were deposited by MAPLE onto titanium substrates, showing the enhancement of proliferation and differentiation of human osteoblast-like cells (MG63) on MgOCP and SrOCP coated surfaces comparing to OCT coated surfaces Reference Boanini, Torricelli, Fini, Sima, Serban, Mihailescu and Bigi75 [Fig. 4(b)].
MAPLE also provides great flexibility in selection and processing of scaffold materials. For example, MAPLE processing of zirconia and hydroxyapatite scaffold materials resulted in a medical device with nearly inert and bioactive implant–tissue interfaces. When hydroxyapatite, MG 63 cells and ECM were co-deposited using MAPLE, MG 63 cells maintained the viability and proliferation within this bio-ceramic scaffolds. This result suggested that MG 63-hydroxyapatite composites could be extended to develop integrated cell-scaffold structures for medical and dental applications. Reference Doraiswamy, Narayan, Harris, Qadri, Modi and Chrisey76 To treat bone metabolic disorders, MAPLE fabrication technology was used to create alendronate-hydroxyapatite (HA-AL) nanocrystal thin films on titanium (Ti) substrates to test the effects on osteoblasts and osteoclasts. In the presence of thin films, the osteoblast-like MG63 cells displayed normal morphology, increased proliferation, and differentiation compared to control. In contrast, human osteoclast cells showed significantly high apoptosis and reduced proliferation and differentiation. The data demonstrated MAPLE fabrication can be applied to synthesize coatings that synergistically couple the bioactivity of HA with the local availability of alendronate to more safely improve bone metabolism for the patients with osteoporosis or fibrous dysplasia. Reference Bigi, Boanini, Capuccini, Fini, Mihailescu, Ristoscu, Sima and Torricelli77
Ideally, biomaterials to be deposited by MAPLE should have minimal laser interaction. Doraiswamy et al. used triazine polymer as an intermediate absorbing layer and transferred viable B35 neuroblasts using MAPLE. Reference Doraiswamy, Narayan, Harris, Qadri, Modi and Chrisey76,Reference Doraiswamy, Narayan, Lippert, Urech, Wokaun, Nagel, Hopp, Dinescu, Modi, Auyeung and Chrisey78 After transferring viable B35 neuroblasts onto receiving substrates, the cell viability and proliferation were examined to show that the MAPLE deposition technique, coupled with an intermediate absorbing layer, was acceptable for creating patterns of viable cells at low fluency. Using MAPLE technology, researchers deposited PLGA/PU polymer to fabricate the substrate topography to study cell viability and preferential orientation of oral keratinocyte stem cells. Two sets of square micropatterns (one 50 × 50 µm2 and one 80 × 80 µm2) were fabricated of preferentially stem cell adherent [Fig. 4(c)]. This study suggested that MAPLE deposition of PLGA/PU is capable of preserving keratinocyte stem cell properties and that surface topography affected their preferential orientation. Reference Paun, Mihailescu, Calenic, Luculescu, Greabu and Dinescu79
Combining laser ablation and MAPLE technology, Paun et al. created a micropatterned two-layer substrate of biodegradable polymer blends comprised of polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), and polylactide-polyethylene glycol-polylactide (PPP) for potential use in tissue engineering research. Laser ablation was used to fabricate microchannels in the bottom of the two-layer polymer substrate. On top of the bottom layer, a thin layer film of the tri-polymer blend was deposited using MAPLE technology. These micropatterned substrates were used for the selective attachment of oral keratinocyte stem cells, and sponge-like microchannel structures provided the multiple anchoring points for the cell adhesion and proliferation. Reference Paun, Zamfirescu, Mihailescu, Luculescu, Mustaciosu, Dorobantu, Calenic and Dinescu80
VII. SELECTIVE BIOMATERIAL REMOVAL BY LASER-ASSISTED ABLATION
The laser ablation technique has been used for selective material removal to create precise patterning on biomaterials. Due to the Gaussian distribution of laser energy, the energy in the center of a laser focal point is high enough to generate the nonlinear optical phenomena and induce the ablation. Compared to conventional etching, femtosecond laser ablation allows for removing the biomaterial with minimized debris generation, high penetration and precision patterning [Fig. 5(a)]. Since pulse duration of femtosecond laser (wave length ∼400 nm and pulse duration ∼100 femtoseconds) is shorter than thermal diffusion time, the thermal effect of biomaterial can be minimized during the laser ablation process. Otherwise, the thermal effect from laser ablation may impair cell adhesion and proliferation.

FIG. 5. Laser direct-writing ablation lithography (a) schematic of laser ablation for selective biomaterial removal; (b) nanoscale craters were fabricated by laser ablation lithography, which could generate a repellent cell patterning Reference Jeon, Koo, Reese, Loskill, Grigoropoulos and Healy86 ; (c) an electrospun fibrous scaffold with laser-ablated micro-holes supported the infiltration of CD68+ pan macrophages Reference Lee, Jeon, Wang, Yan, Yu, Grigoropoulos and Li87 ; and (d) circular and line patterns were generated by laser ablation onto the collagen surface (DIC image), and mesenchymal stem cells seeded in the ablated collagen scaffolds were viable confirmed by fluorescent cell viability kit. Reference Liu, Sun, Singha, Cho and Gordon89
Therefore, laser ablation technique has been used not only to finely tune the micro/nano-scale geometries of the biomaterials, but also to functionalize the biomaterials by generating micro-grooves or micro-holes on the biomaterial surfaces. Reference Malinauskas, Rekstyte, Lukosevicius, Butkus, Balciunas, Peciukaityte, Baltriukiene, Bukelskiene, Butkevicius, Kucevicius, Rutkunas and Juodkazis81–Reference Abagnale, Steger, Nguyen, Hersch, Sechi, Joussen, Denecke, Merkel, Hoffmann, Dreser, Schnakenberg, Gillner and Wagner83 These artificial 3D substrates could be used as biomedical templates for cell culturing and growth, as well as biocompatible-biodegradable implants for tissue engineering purposes. Furthermore, laser ablation can also be used to modify the structure underneath the surface. For example, 3D guiding microchannels fabricated by femtosecond laser ablation could enhance the cell migration in guided direction. Reference Sarig-Nadir, Livnat, Zajdman, Shoham and Seliktar84,Reference Applegate, Coburn, Partlow, Moreau, Mondia, Marelli, Kaplan and Omenetto85 Laser ablation has been used to develop an artificial ECM that mimicked the native tissue with secondary surface morphology and topography Reference Jeon, Koo, Reese, Loskill, Grigoropoulos and Healy86 [Fig. 5(b)]. Using the femtosecond laser ablation, researchers were also able to fabricate the micro-grooves (50–200 µm) on the electrospun fibrous scaffolds Reference Lee, Jeon, Wang, Yan, Yu, Grigoropoulos and Li87 [Fig. 5(c). These engineered electrospun scaffolds with patterned hierarchical topographies regulated the focal adhesion distribution, adhesive cellular morphology, and cell migratory behaviors.
A study by Applegate et al. fabricated 3D multiple length scale patterns with high resolution in a bulk soft/transparent silk hydrogel with lower-energy ultrafast laser pulses. Reference Applegate, Coburn, Partlow, Moreau, Mondia, Marelli, Kaplan and Omenetto85 Using ultrafast laser (wave length 810 nm, pulse duration ∼100 femtoseconds), they fabricated 3D microchannel structures (200 µm diameter) that supported and guided cell growth within the hydrogels at a depth of nearly 1 cm. Moreover, they demonstrated the possibility of the laser ablation technique for 3D construct fabrication without damage of hMSCs in the cell-laden hydrogels. In the study of De Maria et al., S5Y5 neuroblastoma cells were first embedded in the hydrogels, which was then laser ablated to create 3D micro-topology similar to the nature tissues. After the laser ablation process, cells did not show damage caused by mechanical or thermal stress. This well-defined cell-embedded hydrogel was shown to guide the neural tissue formation. Reference De Maria, Grassi, Vozzi, Ahluwalia and Vozzi88 A study by Liu et al., researchers used femtosecond laser ablation to create 3D scaffolds in collagen gel as a substrate for viable cell growth [Fig. 5(d)]. The threshold fluence for ablation of the collagen scaffold was found to be 0.06 J/cm2, and the morphology of the ablation craters was measured as a function of the fluence. hMSCs seeded on the ablated scaffold were found to be viable for at least 10 days. The study concluded that collagen substrates sculpted by laser ablation in the intermediate fluence regime are hospitable to cell adhesion and growth in 3D. Reference Liu, Sun, Singha, Cho and Gordon89
Native tissues are composed of heterotypic cells with patterned architecture that enables intercellular interaction to perform specific physiological functions. In a study of Li et al., an ablation-based fabrication technique was used for fast heterotypic cell patterning with controllable topography. Poly(vinyl alcohol) (PVA)-coated glass showed a biphasic change in adhesion tendency with time. Although low for the first 24 h, cell adhesion eventually increased with time due to the serum protein adsorption. Using CO2 laser ablation, researchers selectively removed the coated PVA by fabricating the microwells. After seeding the keratinocytes with dermal papilla cells on the ablated patterns, they observed cell differentiation into a hair follicle fate, suggesting the epithelial–mesenchymal interaction. As a result, laser ablation could help the fast adjustment of heterotypic cell patterns and surface topography, therefore this technique can be applied to study cellular interaction and create tissue equivalent in vitro. Reference Li, Lin, Yen, Fan, Wu, Young, Cheng and Lin90
VIII. FUTURE PERSPECTIVES
Appropriate fabrication techniques that are being developed to create biomimetic patterns on biomaterials and facilitate engineered tissue functionality are still in infancy. To achieve the superior function of biomaterial scaffolds in tissue engineering, sophisticated control on different length scales (from nanometer to centimeter scale) is required for various biofabrication technologies. To overcome current limitations on multi-scale fabrication and functionalization, laser-assisted technologies emerge as new approaches for fabricating biomaterial-based tissue scaffolds with high resolution at multiple length scales. Laser-assisted biofabrication is advantageous for the material independent, noninvasive contactless processing, high precision and minimum damage, which enable precise patterning of living cells, creating highly-defined tissue architecture, and controlling subcellular features in the tissues.
These advantages offer great potentials for fabricating organ-on-chip systems, which allow us to more accurately answer basic cell biology questions, better predicting toxicity and efficacy of potential drugs in physiological-mimicked tissues, and increase our in-depth understanding of human diseases in complex and heterogeneous microenvironments. Toward multi-organ integration within one system, the laser-assisted biofabrication technologies showed their capability of direct-writing complex structures, so each organ compartment can be tailored according to the specific organ and tissue characteristics. Furthermore, with the advancement of patient-derived human iPSCs, we can fabricate “patient on a chip” system, which can help diagnose and design better treatment strategies for individual patients, taking into account individual variability in genes, environments, and lifestyle for each person.
Although laser-assisted biofabrication provides high spatial precision on biomaterial patterning and cell manipulation, these technologies suffer from low speed and limited scaffold size, which is difficult for scalable manufacturing or high-throughput studies. Since the scaffold sizes are limited to millimeter or micrometer scale, it seems impossible to use laser-assisted technologies to print the whole organ or large tissue for regenerative purpose. Another critical challenge in organ printing is to create bifurcated vascular network, which can integrate with printed organs for transporting culture media and supporting cell viability. 3D vascularized organs have been demonstrated by integrating vessel-like microfluidic channels with cellular spheroids Reference Ozbolat and Chen91 using an extrusion-based 3D bioprinting system. Semipermeable microfluidic channels allowed for the transportation and diffusion of media to the printed cellular assembly. Laser-based biofabrication of microvessels has been demonstrated, but their transport function is limited by their micro-scale size. Reference Lee, Moon and West56
In the biological aspect, another challenge is to improve the biological functions of printed tissues, ensuring either maturity for physiological mimicry or mechanical integrity for transplantation. Especially for stem cell-based tissue constructs, printed tissues only represent the embryonic or neonatal characteristics, instead of adult-like tissue functions. Proper design of bioreactors has been used to provide the optimal tissue environment and external stimuli to accelerate the tissue maturation process. Currently, effort focuses on dissecting the external stimuli, deciphering signaling pathways and harnessing this information for tissue maturation. Hereby, 3D bioprinting and bioreactors will play a crucial role to stimulate the in vitro processing of tissue maturation by providing relevant 3D tissue environmental motifs, such as in vivo mimicking morphology, external electrical stimulation, mechanical loading, and ECMs.
Development of novel ultrafast laser systems will lead to the technical breakthrough of laser-assisted biofabrication. The replacement of Ti:sapphire crystal with fibers or thin disks not only results in high average power (e.g., laser pulse intensity and repetition rates) that will increase the fabrication speed, but also makes the ultrafast laser systems more robust and cost-efficient. Reference Tünnermann, Schreiber and Limpert92 Recent interest of laser-assisted fabrication has shifted to simultaneous spatiotemporal manipulation of the femtosecond laser pulses, which enables modification of peak intensity distribution in the focal region and eliminates self-focusing for highly efficient 3D fabrication. Reference He, Xu, Cheng, Ni, Xiong, Xu, Sugioka and Midorikawa93–Reference Zeng, Chu, Gao, Liu, Li, Zhang, Yao, Ni, Chin, Cheng and Xu95 Integration of multi-laser beams through spatial light modulator have also been exploited for high-speed parallel processing. Reference Kim and So96 Furthermore, development of dynamic feedback-control mechanisms can also enhance the efficiency of laser-assisted fabrication.
Currently, researchers are integrating the laser-assisted biofabrication with other 3D bioprinting methods (e.g., inkjet or extrusion systems) for generating precise nano/microscale features and chemical patterning within a large scaffold. New biomaterial synthesis or localized functionalization will also broaden the applicability of laser-assisted biofabrication from fundamental biological research to engineering tissue scaffolds with high remedy efficiency. Continuous advancement in understanding the physical mechanisms, improvement of fabrication techniques, and development of new photo-curable biomaterials will help accelerate the widespread use of laser-assisted biofabrication technologies, especially in the applications of tissue engineering and regenerative medicine.
ACKNOWLEDGMENT
M.Z. acknowledges support from American Heart Association (AHA) postdoctoral fellowship (16POST27750031). S.K. and C.G. acknowledge support from National Science Foundation (NSF) Scalable NanoManufacturing (SNM) Award (1449305).