Major depressive disorder (MDD) is a common mental health concern, and more than 350 million people in the world experience the symptoms of MDD(1). MDD is also one of the major economic burdens worldwide(Reference Vigo, Thornicroft and Atun2). Mental health disorders will be projected to cost 5·0–17·0 trillion dollars worldwide by 2030(Reference Whiteford, Degenhardt and Rehm3). The past decade has seen promising developments in the field of nutritional neurology, from cross-sectional epidemiological studies to the exploration of biological mechanisms through the study of MDD and diet(Reference Berk and Jacka4). In an earlier cross-sectional epidemiological study, Jacka et al. suggested that a healthy diet was associated with a low risk of MDD(Reference Jacka, Pasco and Mykletun5). A recent RCT reported significant improvement in symptoms of MDD through the improvement of diet quality based on a dietary regimen(Reference Jacka, O Neil and Opie6). A study from the European Mood Food Consortium reported a clinical trial of an intervention to prevent MDD through the use of nutritional supplements (including n-3 fatty acids, selenium, folic acid, vitamin D3 and calcium); the study found that treatment using nutritional supplements reduced the incidence of MDD(Reference Bot, Brouwer and Roca7). In Australia, Natalie Parletta et al. reported that fish oil supplementation in a Mediterranean-style dietary intervention improved diet quality and mental health in depressed patients(Reference Parletta, Zarnowiecki and Cho8). Similarly, a meta-analysis also confirmed that dietary interventions could significantly reduce depressive symptoms(Reference Firth, Marx and Dash9). However, the current literature on the exploration of biological mechanisms consists mainly of preclinical animal studies.
The consumption of more vegetables is included in dietary recommendations to prevent MDD(Reference Opie, Itsiopoulos and Parletta10). McMartin et al. found that people with a high intake had a lower risk of MDD than those with a low intake of daily vegetables v. fruits(Reference McMartin, Jacka and Colman11). A new systematic review also reported that high intake of fruits and vegetables had a positive effect on women’s mental health(Reference Guzek, Gła Bska and Groele12). In addition, studies have reported that compared with processed fruit and vegetable intake, increased intake of raw fruits and vegetables predicts decreased depressive symptoms. Liu et al. analysed fruits and vegetables separately and reported that both fruits and vegetables were negatively associated with MDD(Reference Liu, Yan and Li13). Wickham et al. found that among the three major healthy lifestyle habits, including sleep, physical activity and diet, fruit and vegetable intake were dietary factors that predicted mental health and attainment of well-being in young people after controlling for covariates(Reference Wickham, Amarasekara and Bartonicek14). However, the above studies did not give recommendations for specific intakes. Early studies suggested that an intake of seven servings of fruits and vegetables per day was highly associated with good well-being and health(Reference Blanchflower, Oswald and Stewart-Brown15). Another systematic review reported that a daily intake of at least five servings of fruits and vegetables in adults may have beneficial effects on mental health(Reference Głąbska, Guzek and Groele16). The roles of fruits and vegetables in preventing MDD do not seem to be entirely the same. Mihrshahi et al. found that among middle-aged women, those who consumed more than two fruits per day had reduced odds of depressive symptoms, while depressive symptoms were associated with vegetable intake only at higher vegetable intakes(Reference Mihrshahi, Dobson and Mishra17). The authors recommended increased fruit intake, but vegetable intake was not addressed in the conclusions. A systematic review found that most cohort studies have supported a reduction in the risk of MDD with fruit intake, but when the effect of vegetable intake has been analysed separately, the results have differed from those when fruits were analysed alone and those when fruits were analysed together with vegetables(Reference Dharmayani, Juergens and Allman-Farinelli18). Another systematic review reported that vegetable consumption had a stronger positive effect on mental health than fruit consumption, but the effect of vegetable intake on mental health is unknown(Reference Tuck, Farrow and Thomas19). In addition, previous studies did not exclude the effect of confounding factors on the relationship between vegetable intake and MDD.
Mendelian randomisation (MR) is a powerful methodology of assessing the causal relationship between exposure and outcome using genetic instrumental variables(Reference Birney20). Because genetic variations are inherited randomly, MR is less susceptible to confounding factors and provides robust results. MR is considered to have a similar level of evidence to randomised controlled trials(Reference Emdin, Khera and Kathiresan21). Therefore, MR might be a useful tool to further evaluate the causal relationship between vegetable intake and MDD.
The purpose of this study was to analyse the relationship between vegetable intake and MDD in United States (U.S.) adults. We first conducted a cross-sectional analysis based on National Health and Nutrition Examination Survey (NHANES) to determine the observational association between vegetable intake and MDD. Then, we implemented bidirectional MR based on genome-wide association study (GWAS) database to detect the causal relationship between vegetable intake and MDD.
Methods
Overall study design
This study was conducted in two phases, as shown in Fig. 1. In phase 1, seven consecutive periods from NHANES (2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016 and 2017–2018) were all used to collect data on demographic, socioeconomic, intake of vegetables and MDD. Multivariate regression analysis was used to determine the relationship between vegetable intake and MDD. In phase 2, we performed bidirectional two-sample MR analysis on summary statistics from the publicly available GWAS database to evaluate the causal effects from genetically determined vegetable intake to MDD and from genetically determined MDD to vegetable intake.

Fig. 1 Flow chart of two-phase study. (a): phase I based on cross-sectional analysis. (b): phase II based on two-sample MR. MR: Mendelian randomisation; MDD: major depressive disorder; PGC: Psychiatric Genomics Consortium; IVW: inverse variance weighted
NHANES collected information on the health, nutrition and demographic characteristics of American households. A nationally representative sample of approximately 10 000 people was surveyed in each NHANES cycle, and the research was divided into two parts: interviews and physical examination data. The interviews included demographic, socioeconomic, diet and health-related issues; the physical examination included the collection of basic medical information, laboratory test data and some imaging data. The National Health Statistics Ethics Review Committee approved the NHANES survey protocol (No. 2018-01, effective beginning October 26, 2017), which complies with the Declaration of Helsinki(22). All participants gave written informed consent. Verbal consent was witnessed and formally recorded.
The GWAS data exposed in this study were selected based on three hypotheses: (1) a strong correlation between the instrumental variable and exposure (P < 5 × 10−8, linkage disequilibrium (LD) r2 < 0·001, F-statistics > 10); (2) no association between the instrumental variable and outcome (P for outcome ≥ 5 × 10−8) and (3) no correlation between the instrumental variable and confounding factors. Furthermore, we used PhenoScanner (https://www.phenoscanner.medschl.cam.ac.uk) to exclude SNP associated with confounding factors. Causal relationship analysis methods used for MR include inverse variance weighted (IVW), weighted median and MR-Egger. Based on different levels of horizontal pleiotropy, these three methods have different assumptions. IVW assumes that the SNP of the instrumental variables do not exhibit horizontal pleiotropy. This method combines the Wald ratio of each SNP to the outcome and obtains a summarised causal estimate. Weighted median and MR-Egger consider the existence of horizontal pleiotropy, which can provide more reliable estimates in a broader range of scenarios but with wider confidence intervals.
Research subjects and data collection
40 496 participants aged 18 or above who participated in the Patient Health Questionnaire (PHQ-9) were collected. We excluded 5488 participants with missing data on vegetable intake and 4114 participants who uncompleted the PHQ-9. The multiple imputation was used to account for missing data on BMI, education, marital status or poverty–income ratio. Thirty-three participants with very low energy intake (less than 800 kcal per day) were also excluded(Reference Churuangsuk, Hall and Reynolds23). Ultimately, 30 861 participants were included in this study.
The two sets of GWAS data related to vegetable intake, which are associated with cooked and raw vegetable intake, respectively, both originated from UK Biobank with sample sizes of 448 651 and 435 435. The GWAS data related to MDD were collected from psychiatric genomics consortium, consisting of 135 458 cases of MDD and 344 901 controls. The population was of European descent.
Evaluation of major depressive disorder and vegetable intake
The PHQ-9 was administered during face-to-face interviews at a mobile examination centre. The PHQ-9 was used to assess the severity of depressive symptoms over the last 2 weeks(Reference Kroenke, Spitzer and Williams24). The PHQ-9 questions are as follows: (1) How often have you had no interest or pleasure in doing things? (2) Are you feeling frustrated, depressed or desperate? (3) Is it difficult to fall asleep, or are you unable to fall asleep? Or are you getting too much sleep? (4) Are you feeling tired or having little energy? (5) Have you experienced loss of appetite or eating too much? (6) Do you feel that you are bad, feel that you are a failure or feel that you have let yourself or your family down? (7) Do you have difficulty focusing on things, such as being unable to concentrate when reading newspapers or watching TV? (8) Is your speed of action or speech so slow that others have noticed it, or just the opposite? (9) Do you have the idea of dying or hurting yourself in some way? The answer options include ‘not at all’, ‘a few days’, ‘more than half of the time’ and ‘almost every day’. Total scores on the PHQ-9 range from 0 to 27. PHQ-9 scores ≥ 10 demonstrate high internal consistency and maximised combined sensitivity and specificity in the diagnosis of MDD(Reference Hirschtritt and Kroenke25). A PHQ-9 score of 10 or above was considered to indicate MDD(Reference Levis, Benedetti and Thombs26).
Data on vegetable intake were derived from the Food Patterns Equivalents Database (FPED). The FPED converts food from the What We Eat in American (WWEIA) and NHANES to thirty-seven food pattern components. WWEIA is the dietary intake interview component of NHANES. Vegetable intake was measured in cups and reported by each participant on two days as the total per day. The FPED serves as a unique research tool to assess the food intake of Americans. See the FPED website (https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-overview/) for further details. Dietary guidelines for Americans recommend that adults consume the equivalent of 2–3 cups of vegetables per day(Reference Lee, Moore and Park27). Thus, low vegetable intake was defined as a daily vegetable intake of less than 2 cups per day.
Covariates
Covariates included sex, age, race, BMI, education level, marital status and poverty ratio. Sex was divided into ‘male’ and ‘female’. We divided the participants into two groups (‘< 65 years old’ and ‘≥ 65 years old’) according to their age. Race was classified as ‘white’, ‘black’, ‘Mexican’ and ‘other’, with white referring to non-Hispanic white, black referring to non-Hispanic black, Mexican referring to Mexican American and other referring to Hispanic or other races. BMI < 25 was classified as ‘underweight/healthy’, BMI between 25 and 30 was classified as ‘overweight’ and BMI ≥ 30 was classified as ‘obesity’. Education level was divided into ‘high school or below’ and ‘above high school’. The former included high school graduates or the equivalent and 12th grade or less (including 12th grade without a diploma), and the latter included a college degree or above (including an associate’s degree). Marital status was divided into ‘married or living with a partner’, ‘not married’ and ‘other’. Other included divorced, widowed and separated. The poverty income ratio refers to the ratio of household income to the poverty income line, and the purpose is to assess the degree of household poverty. The poverty income ratio ranges from 0 to 5, where a ratio equal to 1 indicates that the household income is the same as the poverty income line; a ratio greater than 1 indicates that the household income is not at the poverty level, and a ratio less than 1 indicates that the household income is at the poverty level.
Data analysis
The data from the seven periods were all weighted, and the number of US populations that could be represented in this study was calculated. All participants were divided into the low vegetable intake group and the normal vegetable intake group. Continuous variables are expressed as the mean (sd), and categorical variables are expressed as percentages (sd). Univariate logistic regression was used to analyse the relationship between low vegetable intake and MDD. Restricted cubic spline was used to analyse the nonlinear relationship between daily vegetable intake and MDD. Subgroup analysis was used to find the possible covariables influencing the relationship between vegetable intake and MDD. The interaction test was used for factors with inconsistent significance in the subgroup analysis (P > 0·05 and P < 0·05 in the same subgroup). Covariables were added to the logistic regression model for adjustment to analyse the relationship of vegetable intake with MDD.
The strength of individual SNP was evaluated by calculating the F-statistic using the formula F = R2 × (N − 1 − K)/[(1 − R2) × K], where R2 represents the proportion of exposure variance explained by genetic variation, N represents the sample size and K represents the number of instrumental variables(Reference Li, Wang and Guo28). SNP with an F-statistic less than 10 were excluded. We applied standard IVW evaluation to our main results, with weighted median and MR-Egger as supplementary analyses. If the estimated directions of these methods are inconsistent in our study, we set a tighter instrument P-value threshold. In addition, we used radial MR to exclude outliers and reevaluate with IVW, weighted median and MR-Egger. We used Cochran’s Q statistic, funnel plot, leave-one-out (LOO) analyses and MR-Egger intercept test to detect the presence of horizontal pleiotropy and assess the stability of our results. A P-value less than 0·05 for Cochrane Q test is considered to have heterogeneity. The funnel plot visually evaluates possible directional pleiotropy. LOO analysis involves iteratively dropping each exposure-related SNP, repeating the IVW analysis and determining whether the causal estimate is influenced by any one SNP. The intercept term of MR-Egger is used to assess horizontal pleiotropy.
All data were statistically analysed using RStudio software. P < 0·05 was considered statistically significant.
Results
Population characteristics of National Health and Nutrition Examination Survey
A total of 30 861 participants were included, and it is estimated that the representative population of the United States is 180 million. The number of participants with low vegetable intake was 23 249 (75·33 %), and the number of participants with a normal vegetable intake was 7612 (24·67 %). The baseline characteristics of the participants was shown in Table 1.
Table 1 Baseline characteristics of participants

M: mean; s e: standard deviation; BMI: body mass index.
Association between vegetable intake and major depressive disorder
Table 2 shows that, in the univariate regression analysis, vegetable intake was negatively correlated with the risk of MDD (OR = 0·73, 95 % CI (0·67, 0·79), P < 0·001). Compared with participants with normal vegetable intake, the risk of MDD was higher in participants with low vegetable intake (OR = 1·89, 95 % CI (1·65, 2·18), P < 0·001).
Table 2 Univariate analysis of the relationship between low vegetable intake, age, sex, ethnicity, BMI, education, marital status, poverty income ratio and major depressive disorder
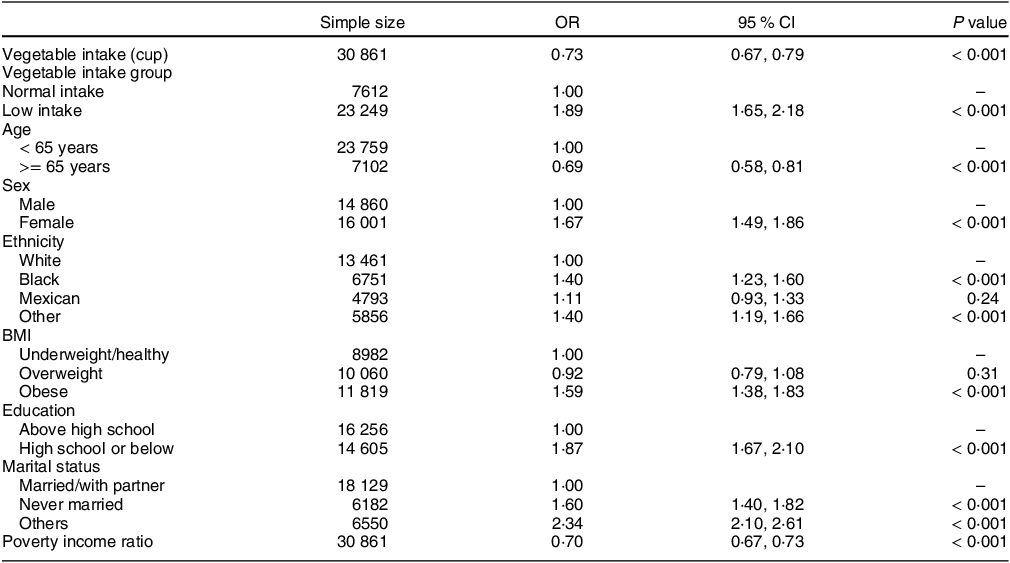
The first OR is per cup increment in vegetable intake; The second OR is low vegetable intake v. normal vegetable intake.
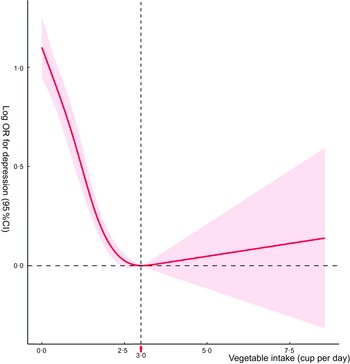
Figure 2 shows the nonlinear relationship between daily vegetable intake and the risk of MDD (P for nonlinearity < 0·001). A daily vegetable intake equal to 3·0 cups is the cut-off value for controlling the risk of MDD. When the vegetable intake increased from 0 to 3·0 cups per day, the risk of MDD increased rapidly; when the daily vegetable intake increased above 3·0 cups, the risk of MDD increased steadily.

Fig. 2 Restricted cubic splines show a non-linear relationship between vegetable intake and major depressive disorder
Table 3 shows the relationship between the low-vegetable diet and the risk of MDD by subgroup after adjusting for the covariates. Low vegetable intake was associated with a higher risk of MDD than normal vegetable intake in the age subgroups (< 65 years: OR = 1·48, 95 % CI (1·27, 1·71), P < 0·001; ≥ 65 years: OR = 1·91, 95 % CI (1·26, 2·89), P = 0·003), the male subgroup (male: OR = 1·49, 95 % CI (1·19, 1·87), P < 0·001; female: OR = 1·56, 95 % CI (1·31, 1·85), P < 0·001) and the education subgroups (above high school: OR = 1·37, 95 % CI (1·33, 1·66), P = 0·001; high school or below: OR = 1·74, 95 % CI (1·42, 2·12), P < 0·001).
Table 3 Subgroup analysis of the effects of covariates after adjustment other variables on the relationship between vegetable intake and major depressive disorder

Table 4 shows the effect of BMI, marital status and ethnicity on the risk of MDD associated with low vegetable intake diet. In non-adjusted and adjusted model, marital status and ethnicity had an interaction with low vegetable intake on the risk of MDD (non-adjusted P value for interaction: ethnicity = 0·002, marital status = 0·004; adjusted P value for interaction: ethnicity = 0·016, marital status = 0·032).
Table 4 The effect of BMI, marital status and ethnicity on the risk of major depressive disorder associated with low vegetable intake
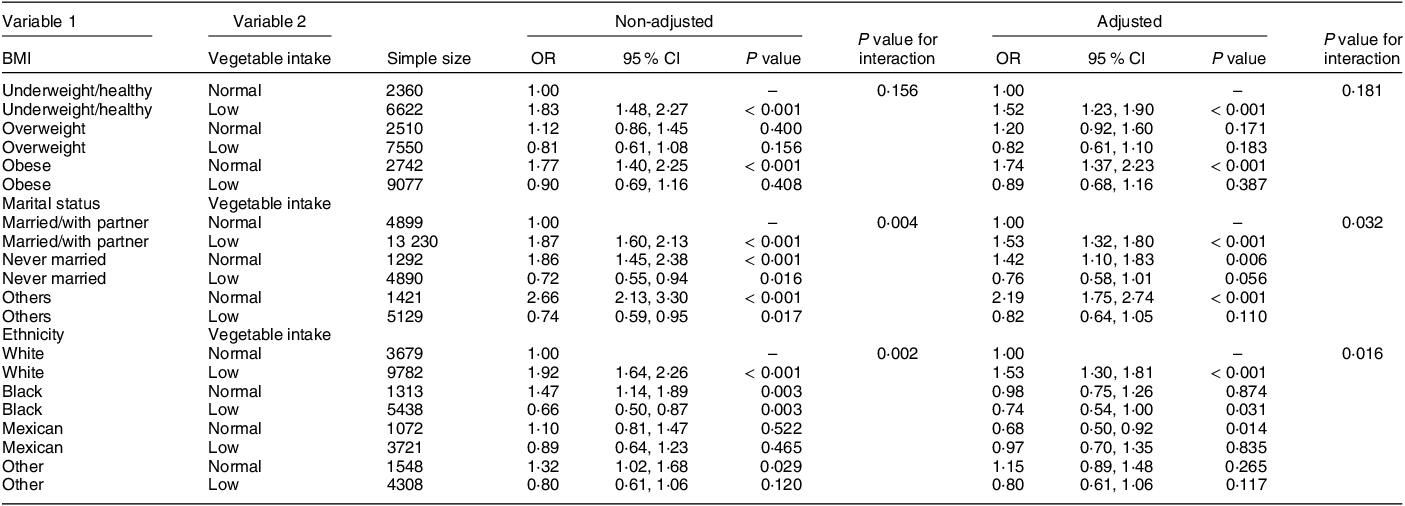
Table 5 shows the relationship between vegetable intake and MDD under different adjustment strategies. In the absence of adjustment variables, there was a negative correlation between vegetable intake and the risk of MDD (OR = 0·73, 95 % CI (0·67, 0·79), P < 0·001). Low vegetable intake increased the risk of MDD (OR = 1·89, 95 % CI (1·65, 2·18), P < 0·001); after adjusting for sex, age, education, BMI and poverty ratio (Adjust I), the above relationship did not change (OR = 0·83, 95 % CI (0·77, 0·89), P < 0·001; OR = 1·53, 95 % CI (1·33, 1·77), P < 0·001). After adjusting for sex, age, ethnicity, BMI, education, marital status and poverty ratio (Adjust II), the above relationship still did not change (OR = 0·83, 95 % CI (0·77, 0·90), P < 0·001; OR = 1·53, 95 % CI (1·32, 1·77), P < 0·001).
Table 5 Multiple regression analysis of the relationship between vegetable intake and major depressive disorder

Adjust I was adjusted for sex, age, education, BMI and poverty income ratio. Adjust II was further adjusted for Adjust I plus ethnicity and marital status
Mendelian randomisation of vegetable intake and major depressive disorder
Details of the SNP included in the MR analysis and their corresponding F-statistics are presented in online supplementary material, Supplemental Tables 1–4. The bidirectional MR results for vegetable intake and MDD are shown in Table 6.
Table 6 The bidirectional MR results for vegetable intake and MDD

MR: Mendelian randomisation; CVI: cook vegetable intake; RVI: raw vegetable intake; MDD: major depressive disorder; IVW: inverse variance weighted; WM: weighted median.
The MR analysis using vegetable intake as exposure showed no significant causal relationship with MDD. The P-values of the Cochrane Q test were 0·10 and 0·31, indicating no heterogeneity. The P-values of MR-Egger intercept test were 0·58 and 0·43, indicating no horizontal pleiotropy. In addition, funnel plots were symmetrically distributed (see online supplementary material, Supplemental Figs 1 and 2). However, LOO analysis showed that the causal relationship might be influenced by single SNP (see online supplementary material, Supplemental Figs 3 and 4).
The MR analysis using MDD as exposure showed no significant causal relationship with vegetable intake. The P-values of both the Cochrane Q test and MR-Egger intercept test were greater than 0·05, indicating no heterogeneity or horizontal pleiotropy. In addition, funnel plots were symmetrically distributed (see online supplementary material, Supplemental Figs 5 and 6). LOO analysis conducted with cooked vegetable intake as an outcome revealed that the causal relationship might be influenced by single SNP (see online supplementary material, Supplemental Fig. 7). However, LOO analysis conducted with raw vegetable intake as an outcome showed that the causal relationship was not influenced by any single SNP (see online supplementary material, Supplemental Fig. 8).
Discussion
To our knowledge, this is the first study to combine large-scale observational study data and large-scale MR gene data to analyse the relationship between vegetable intake and the risk of developing MDD. The results of the observational study showed a non-linear correlation between vegetable intake and MDD, with low vegetable intake being associated with an increased risk of MDD. However, the bidirectional MR results indicated a lack of a causal relationship between vegetable intake and MDD. This absence of causal evidence was further supported by our sensitivity analysis and genetic tools.
Some studies found no clear association between vegetable intake and MDD(Reference Gianfredi, Koster and Odone29,Reference Choda, Wakai and Naito30) , while two other high-quality studies demonstrated a clear association between vegetable intake and a reduced risk of developing MDD(Reference Ocean, Howley and Ensor31,Reference Akbaraly, Sabia and Shipley32) . A systematic review found that although fruit and vegetable intake was associated with a reduced risk of MDD, the results varied when the effect of vegetable and fruit intake alone on the risk of MDD was analysed separately(Reference Dharmayani, Juergens and Allman-Farinelli18). Mihrshahi et al. found that fruits and vegetables had different effects on the incidence of MDD, that the effects were not superimposed and that the intake of fruits was more important than the intake of vegetables(Reference Mihrshahi, Dobson and Mishra17). In contrast, Tuck et al. found that the intake of vegetables had a greater effect on mental health than the intake of fruits and that the effects were more consistent and stable for both men and women(Reference Tuck, Farrow and Thomas19). This finding is consistent with the results of this paper, which showed that vegetable intake was inversely associated with the occurrence of MDD for both men and women. Moreover, we found a curvilinear relationship between vegetable intake and the risk of MDD. Similar studies have also reported a non-linear relationship between vegetable intake and the risk of MDD(Reference Saghafian, Malmir and Saneei33).
Based on the NHANES data, we found a cut-off value of vegetable intake per day for controlling the risk of MDD. This result is close to the daily vegetable intake of at least 2–3 cups in the dietary recommendations for adults issued by the United States Department of Agriculture(Reference Lee, Moore and Park34). Blanchflower et al. reported that a daily intake of seven fruits and vegetables was highly related to high well-being and health(Reference Blanchflower, Oswald and Stewart-Brown15). A recent systematic review reported that it may be beneficial for mental health for adults to eat at least five portions of fruits and vegetables every day(Reference Guzek, Gła Bska and Groele12). Sagha fian et al. reported that a vegetable intake of 100–400 grams per day was associated with a reduced risk of MDD, while more than 400 grams of vegetables per day would increase the risk of MDD(Reference Saghafian, Malmir and Saneei33). Our results may help to recommend people to quantitate vegetable intake per day to decrease the risk of MDD. Either high or low vegetable diet may be unrecommended.
Our cross-sectional study found that the association between vegetable intake and MDD was influenced by marital status and ethnicity. Previous studies have shown that the mental health level of married individuals is higher than that of unmarried, divorced, widowed or separated individuals(Reference Hald, Ciprić and Øverup35). In our study, marital status played a similar effect on the results. In the low-vegetable intake group, people who were married or lived with a partner had a lower risk of MDD. Early studies showed that different ethnic groups had different rates of MDD(Reference Riolo, Nguyen and Greden36). A recent study also showed that the prevalence of MDD had significant racial differences, with the prevalence among other Hispanics being the highest, the prevalence among Asian Americans being the lowest and the prevalence among blacks being 1·2 % higher than that of whites(Reference Lim, Davis and Chen37). We found that white people had the highest risk of MDD in the low-vegetable intake group, while Mexicans had the lowest risk of MDD. In the normal-vegetable intake group, Mexicans had the lowest risk of MDD, and other ethnic groups had a higher risk of MDD. However, cross-sectional studies have limitations in discovering the causal relationship between exposure and outcome and controlling selection bias. In the second phase of our study, we analysed the potential causal association between vegetable intake and MDD through bidirectional MR.
An association between vegetable intake and MDD was found in our observational study, but the bidirectional MR results indicated no causal relationship between vegetable intake and MDD. Hoare et al. reported that the intake of fruits and vegetables could prevent adult MDD, but after adjustment for other related factors, this association weakened(Reference Hoare, Hockey and Ruusunen38). Differently, in our cross-sectional study, we found that low vegetable intake was still associated with an increased risk of MDD after adjustment for covariates. However, this association was unsupported by our bidirectional MR results. Bidirectional MR analysis is a powerful methodology that leverages genetic variation as an instrumental variable to establish causal relationships between risk factors and diseases. It helps overcome inherent biases present in observational studies, providing robust and representative results(Reference Birney20). Previous studies analysed the relationship between possible risk factors and MDD by MR and identified causal risk factors, such as chronic gastritis, gut microbiota and opioid use(Reference Rosoff, Smith and Lohoff39). An association between vegetable intake and MDD was reported by previous studies(Reference Jacka, Pasco and Mykletun5). But to our knowledge, there has been no MR analysis to further validate this relationship. In addition, previous MR analysis show null evidence for the association between vegetable intake and cardiovascular diseases(Reference Feng, Grant and Yang40). Hao Zhao et al. analysed the relationship between food-derived antioxidants and mental disorders through MR, and the result was negative(Reference Zhao, Han and Zhang41). Our MR results did not support a potential causal relationship between vegetable intake and MDD, implying that there might be some unpredictable confounding factors untaken into account in observational studies, which might explain why the associations between vegetable intake and MDD differ in previous observational studies.
This study has several main limitations. Firstly, the observational study included multiple races and the corresponding GWAS data on vegetable intake for only the European population was not available from public databases. This may lead to a biased interpretation of the results from observational studies. Future research should focus on studying populations of the same ethnicity to eliminate potential confounding factors due to population heterogeneity. Secondly, we did not obtain individualised data for MR analysis, which may lead to a biased interpretation of the non-linear relationship discovered in the observational study. A single genetic variation may not fully reflect the complexity of certain biological indicators. Further research with individualised data is needed to explain the potential non-linear relationship between vegetable intake and MDD.
Conclusion
Cross-sectional analysis identified a significant relationship between vegetable intake and MDD, whereas the results from bidirectional two-sample MR did not support a potential causal relationship.
Acknowledgements
The authors acknowledge the National Health and Nutrition Examination Survey (NHANES).
Financial support
No financial support.
Authorship
D.Y. conceived the study and collected the data. J.C. analysed and interpreted the data. Q.W. and Z.O. wrote the manuscript. L.L. and Y.C. revised the manuscript. All authors contributed to the article and approved the submitted version.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the National Health Statistics Ethics Review Committee. Written informed consent was obtained from all subjects/patients. Verbal consent was witnessed and formally recorded.
The data that support the findings of this study are openly available in the National Health and Nutrition Examination Survey (NHANES) database at https://www.cdc.gov/nchs/nhanes/index.htm
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980024001691